Published online by Cambridge University Press: 05 December 2003
The host–parasite interaction between the rainbow trout Oncorhynchus mykiss and the fish louse Argulus japonicus was investigated by administering low levels of dietary cortisol before infecting the fish with low numbers of the parasite. After 24 h, the dietary cortisol treatment elevated blood cortisol and glucose levels and stimulated the synthesis of secretory granules in the upper layer of skin cells. Infection with 6 lice per fish caused skin infiltration by lymphocytes, also in areas without parasites. The lymphocyte numbers in the blood at 48 h post-parasite infection were reduced. Other changes, typical for exposure to many stressors and mediated by cortisol, were also found in the epidermis of parasitized fish, although neither plasma cortisol nor glucose levels were noticeably affected. Glucocorticoid receptors were localized immunohistochemically and found in the upper epidermal layer of pavement and filament cells, and in the leucocytes migrating in these layers. Cortisol-fed fish had reduced numbers of parasites and the changes in the host skin are likely involved in this reduction. Thus a mild cortisol stress response might be adaptive in rejecting these parasites. Further, the data suggest that this effect of cortisol is mediated by the glucocorticoid receptor in the skin epidermis, as these are located directly at the site of parasite attachment and feeding in the upper skin cells that produce more secretory granules in response to cortisol feeding.
The Branchiuran fish lice belonging to the genus Argulus are responsible for disease outbreaks in a wide variety of fish species throughout the world (Rahman, 1995; Rushton-Mellor & Boxshall, 1994). With some exceptions, these lice occur in fresh water and are a major problem for fish farmers in developing countries (Jafri & Ahmed, 1994; Rahman, 1996; Singhal, Jeet & Davies, 1990), but are also a problem in European fish culture (Buchmann & Bresciani, 1997; Buchmann, Uldal & Lyholt, 1995; Grignard, Melard & Kestemont, 1996). For example, a rainbow trout (Oncorhynchus mykiss) farm in Portugal (Menezes et al. 1990) and a Scottish rainbow trout and brown trout (Salmo trutta) stillwater fishery (Northcott, Lyndon & Campbell, 1997) were decimated by A. foliaceus.
Despite the economic losses from crustacean ectoparasites in fisheries and aquaculture, little is known about the biology of the parasite, including the nature of the relationship between the lice and their hosts. Since the early seventies, it has been recognized that the host fish can affect several aspects of lice biology, but the underlying mechanisms are poorly understood. Rejection of the cyclopoid copepods Lernaea cyprinacea and Lernaea polymorpha by their host has been reported for both naïve and previously infected fish (Shields & Goode, 1978; Woo & Shariff, 1990) and it was thought that the rejection was at least in part due to cellular responses of the host. With respect to the salmon louse Lepeophtheirus salmonis, naïve hosts of different species of salmon showed variability in their susceptibility to infection (coho<chinook<Atlantic; Johnson & Albright, 1992 a). Coho salmon were the most resistant to parasite establishment and mounted strong tissue responses, including inflammation and epithelial hyperplasia leading to parasite loss. Naïve Atlantic salmon, however, were highly susceptible to infection and failed to mount any probably significant tissue responses to any of the developmental stages. Cortisol administered in cocoa butter implants, which strongly elevated cortisol levels in the blood, suppressed the inflammatory response and the development of epithelial hyperplasia in the normally resistant coho salmon, and resulted in the inability of the fish to shed L. salmonis (Johnson & Albright, 1992 b).
Infestation with copepodid parasites is known to evolve a stress response in fish, involving a series of compensatory and/or adaptive behavioral and physiological responses, which are collectively referred to as the integrated stress response (see reviews by Wendelaar Bonga, 1997 and Tully & Nolan, 2002). In the case of ectoparasite infestations, these may protect the host by reducing parasite settlement and eliminating attached parasites, through stimulating non-specific immune responses, epithelial hyperplasia, mucous cell discharge, and by altering the composition of the mucous. The primary stress response of fishes involves the activation of the higher brain centres, which trigger a massive release of catecholamines and corticosteroids. The secondary stress responses are the manifold immediate actions and effects of these hormones and occur at all levels of organization. Cortisol has a broad activity spectrum in fishes and has both mineralocorticoid and glucocorticoid activities. Short-term high circulating levels of cortisol, or chronic but moderately elevated cortisol levels also impair hydromineral balance, as a result of catecholamine-induced increase of the permeability of the gills to ions (Wendelaar Bonga, 1997). Cortisol also mediates many of the stress-related changes in the skin of rainbow trout, where it stimulates mucous discharge, mitosis, apoptosis of epidermal cells, synthesis of granules in the cells of the upper epidermis, and the infiltration of the epidermis by leucocytes (Iger et al. 1995; Nolan, Van der Salm & Wendelaar Bonga, 1999 b). Infection of salmonids with low numbers of the salmon louse, L. salmonis generally has not resulted in significant increases in plasma cortisol levels (Bjorn & Finstad, 1997; Johnson & Albright, 1992 b; Mustafa & MacKinnon, 1999; Ross et al. 2000). However, heavy infections typically elevate plasma cortisol to levels that cause immunosuppression (Mustafa et al. 2000). Johnson & Albright (1992 b) reported similar plasma cortisol values in S. salar experimentally infected with copepodids of L. salmonis. The response of parasitized fish to an additional stressor has been investigated, especially in relation to blood cortisol levels in O. mykiss (Ruane et al. 1999, 2000). The rainbow trout infected with adult A. japonicus (Ruane et al. 1999) or with juvenile stages of L. salmonis (Ruane et al. 2000) showed increases in blood cortisol levels after net confinement to levels that were significantly higher than those in confined, but unparasitized, fish. As cortisol modulates the stress response in fish, parasites such as L. salmonis or Argulus sp. can also modulate the stress response in fish. Ruane et al. (1999) demonstrated that, in the host parasite interaction between O. mykiss and A. foliaceus, fish that were fed cortisol containing food prior to infestation showed signs of immunosuppression after 4 h net confinement 21 days post-infection. This indicated a long-term interaction between elevated cortisol levels and infestation on the response to additional stressor. Therefore the objectives of the present experiment were to measure the combined effect of low-level cortisol administration and infection with A. japonicus in O. mykiss in order to study the role of the hormone on the host–parasite interaction. Replicate groups of fish were fed cortisol in their diet, as a stress-free way of administration of the hormone, and were subsequently infected with 6 Argulus per fish. Fish were sampled after 14 days (24 h after the last cortisol feeding, and 48 h after infection with the parasites). Several parameters related to the stress response, hydromineral balance, immune function, and infestation levels were measured.
Six groups of 30 rainbow trout (approx. 73 g) were kept in 65 l black plastic circular tanks. The water was filtered with an Eheim power filter, aerated with an air stone and continuously refreshed with dechlorinated Nijmegen tap water. A partially flow-through system was used refreshing about 6 l per hour. The photoperiod was 12[ratio ]12 light[ratio ]dark, and the water temperature was 16 °C. Fish were fed with Pro-Aqua 20/4 (TrouwTM) at 1% of body weight once daily. The experiment began after a 14-day acclimatization period.
The design consisted of 3 experimental treatments, each performed in duplicate tanks: treatment with cortisol and treatment with A. japonicus, and sham treatment for cortisol and A. japonicus. Two groups of fish received food with cortisol, and 2 groups received food without cortisol. Afterwards these 4 groups were infected with A. japonicus. Two other groups served as controls and were sham treated for cortisol and parasite. For cortisol feeding, at 4 days and 2 days before infection, the normal food was substituted with the same ration of food treated with reagent grade ethanol (4 groups) or ethanol containing cortisol (2 groups). This food was prepared by mist spraying the pellets with absolute ethanol (Merck), or with the same volume of ethanol containing cortisol (hydrocortisone, Sigma) to give a cortisol content of 100 mg/kg food. The ethanol was allowed to evaporate for 5 days prior to feeding the treated pellets. Two days after the second cortisol containing meal, the 2 groups fed cortisol and ethanol-sprayed food and the 2 groups fed ethanol-sprayed food were each infected with 120 adult and subadult A. japonicus. This was accomplished by pouring beakers containing the required number of parasite into each tank. Control fish were sham infected by pouring beakers of water only into their tanks. The treatment groups are referred to as CONTROL (ethanol-sprayed food and sham infected), ARG (ethanol-sprayed food and A. japonicus infected) and CORT&ARG (cortisol containing food and A. japonicus infected).
Four fish from each tank were sampled at time zero (TZERO) i.e. after 14 days acclimatization. Five fish per tank were sampled 24 h after the second cortisol administration (TCORT) and another 5 fish were sampled 48 h post-A. japonicus infection (TARG). The 5 fish were netted into a solution of 2-phenoxyethanol (1[ratio ]1000; Sigma) in which they were rapidly and irreversibly anaesthetized. Blood was taken by needle from the caudal blood vessels and immediately discharged into pre-cooled Eppendorf tubes containing Na2EDTA/aprotinin (1·5 mg/3000 KIU per ml of blood; Sigma) for lymphocyte counts and plasma analysis. The fish were weighed, measured, and the number of attached parasites was counted.
For histological analysis, skin biopsies (10×10 mm) from 4 fish from each tank were taken from the anterior part of the head and fixed in Bouin's fixative for light microscopy (LM). For electron microscopy (EM), smaller pieces of skin (5×5 mm) from the same 4 fish were fixed in Na-cacodylate buffered glutaraldehyde for 20 min on ice, and post-fixed in 1% osmium tetroxide in the same buffer (Iger et al. 1995; Nolan et al. 2000). Tissues were fixed at the sample points post-cortisol feeding and post-A. japopnicus infection.
Infestation level was recorded as the total number of parasites on each fish. Condition factor was calculated as 100 (body weight/fork length3), where body weight is in g and fork length is in cm.
Plasma cortisol was measured with radioimmunoassays developed and validated to measure the hormone in the blood of fishes. Na+ concentrations were determined with a flame-photometric auto-analyser (Technicon model IV) coupled to a spectrophotometer for determining Cl− by the formation of ferrothiocyanate in plasma diluted 200-fold. Plasma glucose was assayed using the Boehringer UV-test kit following the manufacturer's protocol (Boehringer, Mannheim, Germany). Lymphocyte numbers were quantified by diluting the blood 1[ratio ]20 with Türck liquid, then counting them in a Bürker haemocytometer in a light microscope (Ruane et al. 1999). For LM, skin samples were fixed for 24 h, processed through paraffin wax and sectioned at a thickness of 5 μm. Mucous cells were stained with the Alcian blue (pH 2·5) method, as described by Nolan, Reilly & Wendelaar Bonga (1999 a) and Van der Salm et al. (2000). The presence and localization of cortisol receptors in the epidermis were demonstrated with an antibody against O. mykiss glucocorticoid receptors raised in rabbits (Tujague et al. 1998), as described by Van der Salm et al. (2000). The primary antibody was used at a working dilution of 1[ratio ]32000. For the controls, primary and secondary antibodies were omitted. For transmission electron microscopy (TEM), 4 samples per group at each time-point were processed, examined in the electron microscope and photographed without knowledge of the treatments. Five low magnification areas from the upper epidermis of each fish were enlarged 4 times and printed. From these micrographs, the numbers of secretory granules were counted in 7–10 filament cells per fish (each filament cell representing a cytoplasmic area of circa 200 μm2). Mean values for each fish were calculated. Only data from mature filament cells with a visible nucleus, which were neither necrotic nor apoptotic, were used. The general ultrastructure of the epidermis was examined with particular attention being paid to cellular necrosis (characterized by nuclei with aggregations of chromatin, swelling of the cytoplasmic compartment and loss of apical microridges) and apoptosis (characterized by progressive densification of the nucleus, organelles and cytoplasm, leading to cell shrinkage and loss of contacts with surrounding cells), leucocyte infiltration and epithelial integrity (evaluated by intercellular swelling and cell–cell contacts). The observations were compiled and expressed semi-quantitatively following other studies (Iger, Balm & Wendelaar Bonga, 1994 a; Nolan et al. 2000; Nolan et al. 1999 a).
As there were no statistically significant differences in the data between replicate tanks, the data were pooled giving n=8 at TZERO and n=10 at TCORT and TARG. For LM parameters n=8 and for EM n=4. For all parameters except condition factor and Na[ratio ]Cl ratio, effects of treatments were tested by ANOVA at each sample point and differences between treatment groups were identified with Bonferroni's multiple range test. Non-parametric data were identified with Bartlett's test and were log transformed before analysis to achieve homogeneity of variance. For condition factor and Na[ratio ]Cl ratio, differences were tested by non-parametric ANOVA. Differences between ARG and CORT&ARG lice numbers at TARG were tested with the Mann–Whitney U-test. Spearman Rank Correlation analysis was used to examine the relationship between the numbers of attached parasites and the circulating blood hormone levels at 24 h post-infection. All data are presented as mean values±S.E. Statistical significance was P<0·05.
No fish died during the experimental period; the fish fed well and remained disease free. The mean condition factor of the CONTROL fish was 1·055±0·024 and was not significantly different from the ARG or CORT&ARG groups at any sample point (data not shown). At TARG, the number of parasites attached to ARG fish was significantly higher than on CORT&ARG fish (Table 1).
Table 1. Plasma cortisol levels and numbers of attached parasites at sampling for rainbow trout (Oncorhynchus mykiss) at the beginning of the experiment (TZERO), 24 h after receiving the second of 2 cortisol-containing feeds (TCORT), and 48 h post-infection with 6 Argulus japonicus per fish (TARG) (Data given as mean±S.E. for n=8–10. For full details, see text. *P<0·05; **P<0·01 compared with control. Where 2 groups share the same superscript letter, significance between them is P<0·01.)
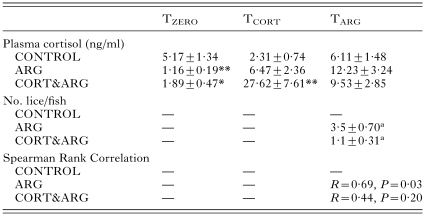
At TZERO, cortisol values were all well within levels considered basal for unstressed fish althrough they were higher in the CONTROL fish than in the ARG and CORT&ARG groups (Table 1). At TCORT, plasma cortisol was significantly higher in the CORT&ARG group. At TARG, the mean plasma cortisol values of ARG and CORT&ARG fish were not different from the CONTROL values (Table 1). There was a significant positive correlation between the numbers of parasites per fish and the levels of cortisol measured in the plasma of ARG fish, but there was no such correlation for the CORT&ARG fish (Table 1). The numbers of parasites were significantly higher in the ARG group than in the CORT&ARG group (Table 1). Table 2 shows that the plasma glucose values were significantly higher in the CORT&ARG group at TCORT, and that the numbers of circulating lymphocytes in the blood at TARG were significantly lower in ARG and CORT&ARG groups than in the CONTROL fish (Table 2). Plasma Na+ and Cl− concentrations of all fish groups sampled, as well as Na[ratio ]Cl ratios, remained similar throughout the experiment and were within the range of values reported for freshwater salmonids (data not shown).
Table 2. Plasma glucose and circulating lymphocytes in rainbow trout (Oncorhynchus mykiss) at the beginning of the experiment (TZERO), 24 h after receiving the second of 2 cortisol-containing feeds (TCORT), and 48 h post-infection with 6 Argulus japonicus per fish (TARG) (Data given as mean±S.E. for n=8–10. For full details, see text. **P<0·01; ***P<0·001 compared with control.)

There were no significant changes in the numbers of mucous cells over time or between treatments (data not shown). Cortisol receptor staining occurred mostly in the upper layer of the epidermis, primarily in the top few cell layers (pavement cells and underlying filament cells, illustrated in Fig. 1 from a CONTROL fish at TZERO). Staining was also observed in the lower layer, mainly in irregularly shaped leucocyte-like cells, identified as macrophages, and small regularly shaped lymphocytes. There was a weaker, discrete staining in the cytoplasm of mucous cells.

Fig. 1. Immunolocalization of the glucocorticoid receptor in the epidermis of the head skin of control Oncorhynchus mykiss at TZERO. Staining (arrows) is concentrated in the upper layer of the epidermis, primarily in the top few cell layers (pavement cells and underlying filament cells and in the lower layer, mainly in irregularly shaped leucocyte-like cells. L, leucocyte; m, discharging mucous cell; p, pavement cell; PCells, melanin-containing pigment cells in subepidermal layer; Derm, dermis.
The EM appearance of the skin epidermis of the CONTROL fish was normal and consistent with previous reports for this species (Iger et al. 1994a; Iger, Jenner & Wendelaar Bonga, 1994 b; Schmidt et al. 1999). The multilayered epithelium was composed of several cell types, with the uppermost layer of cells, those in contact with the water, differentiated into pavement cells with elaborated apical microridges. Below this were large populations of filament and mucous cells, within which low numbers of leucocytes, mainly macrophages, granulocytes and lymphocytes, were observed. Both the pavement and filament cells contained considerable numbers of small granules (Fig. 2A). This epidermal structure was altered in the CORT&ARG group at TCORT (Fig. 2B) when more discharging mucous cells were seen, granule numbers in the pavement cells increased, and the granules were located closer to the apical surface (Fig. 2D) than in control fish (Fig. 2C). Additionally, some limited microridge disruption was observed and the incidence of apoptotic and necrotic cells in the upper cell layers increased slightly. Intercellular spaces in the epidermis became evident (Fig. 2B) and they were often infiltrated by leucocytes, which were more frequent than in the epidermis of the CONTROL group. The epidermal condition of the ARG group at TCORT was comparable to that seen in CONTROL. A semi-quantitative overview of the condition of the epidermis is presented in Table 3.

Fig. 2. Transmission electron micrographs of the upper epidermis of head skin from Oncorhynchus mykiss. (A) The upper pavement cells (p) have apical microridges (arrow) and, together with the underlying filament cells (f), contain considerable populations of granules. m, mucous cell. (B) 24 h after receiving the second of 2 cortisol-containing meals (TCORT), the granule populations are extensive in pavement (p) and filament cells (f). (C) Upper pavement cell of control trout at TCORT, showing signs of active granule synthesis and normal granule density. (D) Upper pavement cell 24 h after receiving the second of 2 cortisol-containing meals (TCORT). Granule density is greater and the granule populations are located closer to the apical side of the cells.
Table 3. Semi-quantitative evaluation of the ultrastructural parameters observed in the head skin epidermis of rainbow trout (Oncorhynchus mykiss) after receiving the second of 2 cortisol-containing feeds and 48 h post-infection with 6 Argulus japonicus per fish (Data given as mean±S.E. for n=8–10. For full details, see text. For a given parameter, 0=unaffected, −=negatively stimulated and +=positively stimulated.)

In general, the A. japonicus infestation had a greater impact on the epidermis than did cortisol feeding. In the ARG group at TARG, discharging mucous cells were more common, granule numbers in some pavement cells were depleted, microridges were more seriously disrupted, intercellular spaces were more extensive and contained more leucocytes, and the numbers of necrotic and apoptotic cells in the upper epidermis was increased when compared to the CONTROL group at TARG. The changes seen in some parameters in the CORT&ARG group were less than in the ARG group. The main differences were reduced effects on pavement cell necrosis, granule numbers, and the extent of leucocyte infiltration. The numbers of granules in the upper epidermal cells of the cortisol fed fish were significantly higher at TCORT (Fig. 3). There were no significant differences in the numbers of granules between treatments at TARG. A summary semi-quantitative evaluation of these results is given in Table 3.
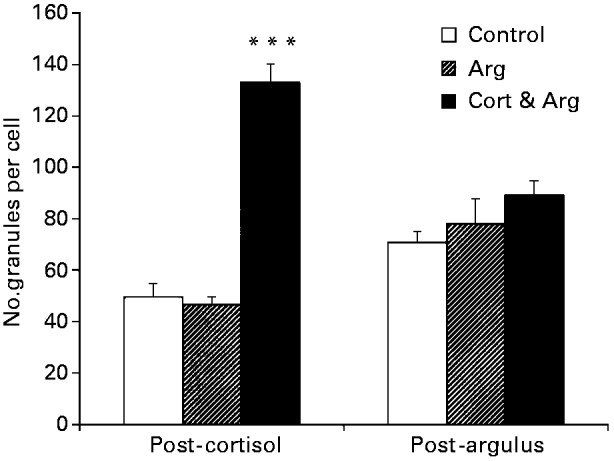
Fig. 3. Numbers of granules quantified (in the head skin epidermis of Oncorhynchus mykiss) after receiving the second of 2 cortisol-containing meals (post-cortisol), and 48 h post-infection with 6 Argulus japonicus per fish (post-argulus). The data represent counts per cytoplasmic area of upper epidermal cells. Data given as mean±S.E. for n=4. For full details, see text. *** P<0·001.
This study is the first to examine a role of the stress hormone cortisol on the relationship between the fish louse, A. japonicus, and the trout O. mykiss in the short term. The results show that the administration of low levels of cortisol reduced the intensity of infestation. While we do not understand the exact mechanism of this beneficial response, it is possible that the elevation of plasma glucose in cortisol-fed fish provided more energy for dealing with the countering of the parasite. To obtain as comprehensive a picture as possible, we measured a variety of parameters in the host. In the short term, infestation with low parasite numbers resulted in the migration of lymphocytes out of the blood and into the epidermis, where the epithelial disruption was associated with an increased incidence of discharging mucous cells and moderately elevated levels of apoptosis. Infection with low numbers of the parasite did not affect hydromineral balance, nor did it cause an increase in plasma cortisol or glucose levels, which are primary stress parameters used to assess stress in fish. From the positive correlation between numbers of lice and blood cortisol levels, we predict that higher numbers of parasites would significantly increase the levels of circulating cortisol. Our observation that low levels of infestation by this fish louse do not disrupt the hydromineral balance is consistent with the results of other studies (Grimnes & Jakobsen, 1996; Nolan et al. 1999 a).
The formation of intercellular spaces in the teleost skin epidermis and infiltration of these spaces by leucocytes was reported for Atlantic salmon with an infestation of the sea louse L. salmonis (Nolan et al. 1999 a), as well as for a variety of other stressors (e.g. Iger et al. 1994 b; Iger & Wendelaar Bonga, 1994; Nolan et al. 1998, 2000). Extravasation of leucocytes from the blood vessels and their subsequent appearance in the peripheral tissues is normally observed in stressed fish (Wendelaar Bonga, 1997), where it is thought to be, in part, mediated by cortisol (Iger et al. 1995; Van der Salm et al. 2000). This is supported by the mammalian literature where, during acute stress, cell-mediated immunity in rats was enhanced in vivo by directing leucocytes to the skin, whereas chronic stress suppressed such movements. Additionally, the reaction to acute stress, and the beneficial aspects of both the stress response and immune function (principally increases resistance to viruses, bacteria and fungi), were positively related with circulating glucocorticoid levels in the blood of the rat (Dhabhar & McEwen, 1997). In the present study, low-level cortisol administration did not significantly reduce the number of circulating lymphocytes in the blood, but did increase their presence in the epidermis. This observation points to rapid replacement of the extravasating leucocytes by blood cells from the haematopoietic tissues. Moderately elevated cortisol levels during exposure to a stressor are thought to be adaptive, whereas high cortisol levels can have deleterious effects (Bury et al. 1998; Wendelaar Bonga, 1997). On the basis of plasma cortisol data from previous experiments in which rainbow trout were fed cortisol in their diet, we may assume that in the present experiments, the cortisol peaks would have been about 100–150 ng/ml within 2–4 h, and would have returned to basal levels within 24–36 h (Balm & Pottinger, 1995; Barton, Schreck & Barton, 1987). Cortisol is implicated in mediating the inhibitory effects of stressors on the immune response, thus decreasing disease resistance (Ellis, 1981; Wendelaar Bonga, 1997). For instance, the mortality of the brown trout Salmo trutta, due to bacterial and fungal disease, increases with chronic elevation of the plasma cortisol level (Pickering & Pottinger, 1989). Similarly, the increase in numbers of the ectoparasite Gyrodactylus salaris on several salmonids treated with cortisol is attributed to the immunosuppressive effect of the hormone (Harris, Soleng & Bakke, 2000). In our study, we observed a beneficial effect of cortisol feeding with the hormone reducing the number of parasites when rainbow trout O. mykiss were infected with the lice A. japonicus. In this experiment, the fish were not exposed to a chronic elevation of plasma cortisol, and thus serious suppressive effects on the innate immune system probably did not occur.
In the present study, stress-free cortisol administration via the diet increased the numbers of granules in the upper cell layers of the skin epidermis of trout. These secretory granules contain endogenous peroxidase activity (Iger et al. 1994b; Iger & Wendelaar Bonga, 1994). In rainbow trout their synthesis was also stimulated by cortisol (Iger et al. 1995), and many stressors have been reported to induce changes in the numbers of these granules in fish skin (Iger & Wendelaar Bonga, 1994; Iger et al. 1994b; Iger, Jenner & Wendelaar Bonga, 1994 c; Balm et al. 1995; Burkhardt-Holm, Escher & Meier, 1997). The contents of these granules are secreted out into the glycocalyx and mucous layer covering the fish. Peroxidase is considered to be an anti-microbial component of the non-specific defence system of fish and has been demonstrated in the mucous and glycocalyx on the surface of the skin (Brokken et al. 1998; Iger & Wendelaar Bonga, 1994). The secreted skin peroxidase is a biochemically distinct isoform from the peroxidase of the blood (Brokken et al. 1998). While the significance of the enhanced secretion of peroxidase during stress is unknown at present, the stimulation of granule synthesis by cortisol administration in the present study was associated with reduced establishment by A. japonicus on the host fish. Further, in another study, the granule populations in the cells of the upper epidermis of sea trout smolt (S. trutta) were depleted within 3 h of exposure of the fish to either Rhine water or acute temperature shock (Nolan et al. 2000). These observations are consistent with the hypothesis that these granules have a role in the integrated stress response to both toxic and non-toxic stressors.
Experimental infection of the Atlantic salmon with low numbers of the sea louse L. salmonis induced stress-related changes in the skin and gill epidermis and these occurred in a dose-dependent manner relative to the number of parasites introduced (Nolan et al. 1999 a). In the present experiments, fish infected with A. japonicus showed a statistically significant correlation between parasite number and plasma cortisol level, and this supports the view that increasing numbers of parasites are increasingly stressful for the fish. This relationship, however, was lost in the CORT&ARG group, possibly because exposure of the fish to cortisol before infestation had made the fish more resistant to the parasites than the fish fed cortisol-free food. This indicates that a high level of cortisol performs a functional role in the adaptive responses of the host to the parasite. Stimulation of leucocyte extravasation and their migration into the peripheral tissues might be such an adaptive response; stimulation of granule synthesis in the upper epidermis and their discharge might be another.
The positive correlation between plasma cortisol levels and parasite numbers in the ARG fish indicates that increasing numbers of A. japonicus induce stronger stress responses in the fish. Using a hyperbolic model, Poole, Nolan & Tully (2000) demonstrated that baseline cortisol levels in wild sea trout S. trutta naturally infested with L. salmonis were significantly higher than in uninfested fish. Furthermore, there was a positive correlation between infestation intensity and extrapolated cortisol levels. The cortisol response of trout to A. japonicus infestation and the effects of chronically elevated levels of cortisol in the blood remain to be demonstrated. Ruane et al. (1999) found no differences in plasma cortisol in O. mykiss 21 days post-infection with 6 Argulus per fish, indicating that adaptation to this number of parasites had occurred.
This is the first study to demonstrate cortisol receptors in the epidermis of the rainbow trout O. mykiss. Effects of cortisol administration on epithelial cells of O. mykiss include increased synthesis and secretion rate of granules of the upper epidermal cell layer, and increased infiltration of the epidermis by leucocytes (this study and Iger et al. 1995). These data support the view that these effects may be mediated via the cortisol receptor, as the cortisol receptor was found by immunohistochemistry to be located in the upper epidermis (consisting primarily of granule secreting filament cells just before or in the process of differentiation into pavement cells) and in leucocytes, cells known to be responsive to cortisol. Low levels of cortisol can enhance carp neutrophil function and proliferation in vitro, while higher levels induced apoptosis in B-lymphocytes (Weyts et al. 1998). The latter observations, together with those from the present study, support the hypothesis that cortisol levels slightly above unstressed basal levels are adaptive in teleost fish (Wendelaar Bonga, 1997).
In conclusion, this study confirms that, administration of low doses of cortisol induce cellular responses in the skin of rainbow trout that are also observed in response to a variety of stressors. Certainly, the increased synthesis and discharge of granules in the cells of the upper epidermis may be adaptive as it is associated with a lower infestation rate by the fish louse A. japonicus than found in fish that were not treated with cortisol before infestation. The localization of the glucocorticoid receptor in the pavement and filament cells, as well as the presence of leucocytes in the epidermis, suggests that the effects of cortisol on these cells are receptor mediated. Infestation increased the numbers of lymphocytes infiltrating the skin epidermis and indicated immunostimulation. The maintenance of hydromineral balance, in spite of moderate epithelial disruption, together with the absence of an infestation-related increase of cortisol or glucose in the plasma, indicates that the numbers of parasites used were not excessively or chronically stressful to the fish. However, the significant positive correlation between parasite number and plasma cortisol suggests that higher infestation levels would cause greater effects. A dose-response study to the parasite is required to examine this prediction.
The authors thank F. A. T. Spanings for design and provision of experimental set-up, fish care and sampling assistance, and J. C. A. van der Meij for analysing electron microscopy samples. The gift of the glucocorticoid receptor antibody from Dr B. Ducouret (INRA, Rennes, France) is gratefully acknowledged. This work was supported by the European Commission (Marie Curie Individual Fellowship: MCFI 2000–01280).
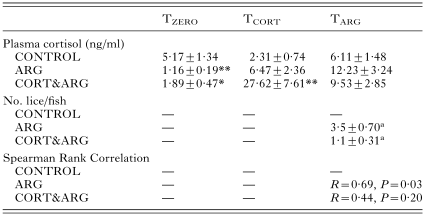
Table 1. Plasma cortisol levels and numbers of attached parasites at sampling for rainbow trout (Oncorhynchus mykiss) at the beginning of the experiment (TZERO), 24 h after receiving the second of 2 cortisol-containing feeds (TCORT), and 48 h post-infection with 6 Argulus japonicus per fish (TARG)

Table 2. Plasma glucose and circulating lymphocytes in rainbow trout (Oncorhynchus mykiss) at the beginning of the experiment (TZERO), 24 h after receiving the second of 2 cortisol-containing feeds (TCORT), and 48 h post-infection with 6 Argulus japonicus per fish (TARG)

Fig. 1. Immunolocalization of the glucocorticoid receptor in the epidermis of the head skin of control Oncorhynchus mykiss at TZERO. Staining (arrows) is concentrated in the upper layer of the epidermis, primarily in the top few cell layers (pavement cells and underlying filament cells and in the lower layer, mainly in irregularly shaped leucocyte-like cells. L, leucocyte; m, discharging mucous cell; p, pavement cell; PCells, melanin-containing pigment cells in subepidermal layer; Derm, dermis.

Fig. 2. Transmission electron micrographs of the upper epidermis of head skin from Oncorhynchus mykiss. (A) The upper pavement cells (p) have apical microridges (arrow) and, together with the underlying filament cells (f), contain considerable populations of granules. m, mucous cell. (B) 24 h after receiving the second of 2 cortisol-containing meals (TCORT), the granule populations are extensive in pavement (p) and filament cells (f). (C) Upper pavement cell of control trout at TCORT, showing signs of active granule synthesis and normal granule density. (D) Upper pavement cell 24 h after receiving the second of 2 cortisol-containing meals (TCORT). Granule density is greater and the granule populations are located closer to the apical side of the cells.

Table 3. Semi-quantitative evaluation of the ultrastructural parameters observed in the head skin epidermis of rainbow trout (Oncorhynchus mykiss) after receiving the second of 2 cortisol-containing feeds and 48 h post-infection with 6 Argulus japonicus per fish

Fig. 3. Numbers of granules quantified (in the head skin epidermis of Oncorhynchus mykiss) after receiving the second of 2 cortisol-containing meals (post-cortisol), and 48 h post-infection with 6 Argulus japonicus per fish (post-argulus). The data represent counts per cytoplasmic area of upper epidermal cells. Data given as mean±S.E. for n=4. For full details, see text. *** P<0·001.