Gastric cancer is the second leading cause of cancer death worldwide, which leads to a substantial burden of morbidity, mortality, and healthcare costs (Reference Crew and Neugut4;Reference Kelley and Duggan18). Helicobacter pylori (H pylori) infection has been recognized as an important risk factor for cancer of gastric body and antrum (distal cancers) (5;13;15;24). Approximately 50 percent of the world population has been affected by H pylori (31). Although less than 1 percent of the infected will develop gastric cancer, H pylori screening in high-risk populations has been proposed as a cost-effective strategy in the long-term in Western countries (Reference Fendrick, Chernew and Hirth10;Reference Parsonnet, Harris, Hack and Owens25;Reference Roderick, Davies and Raftery26).

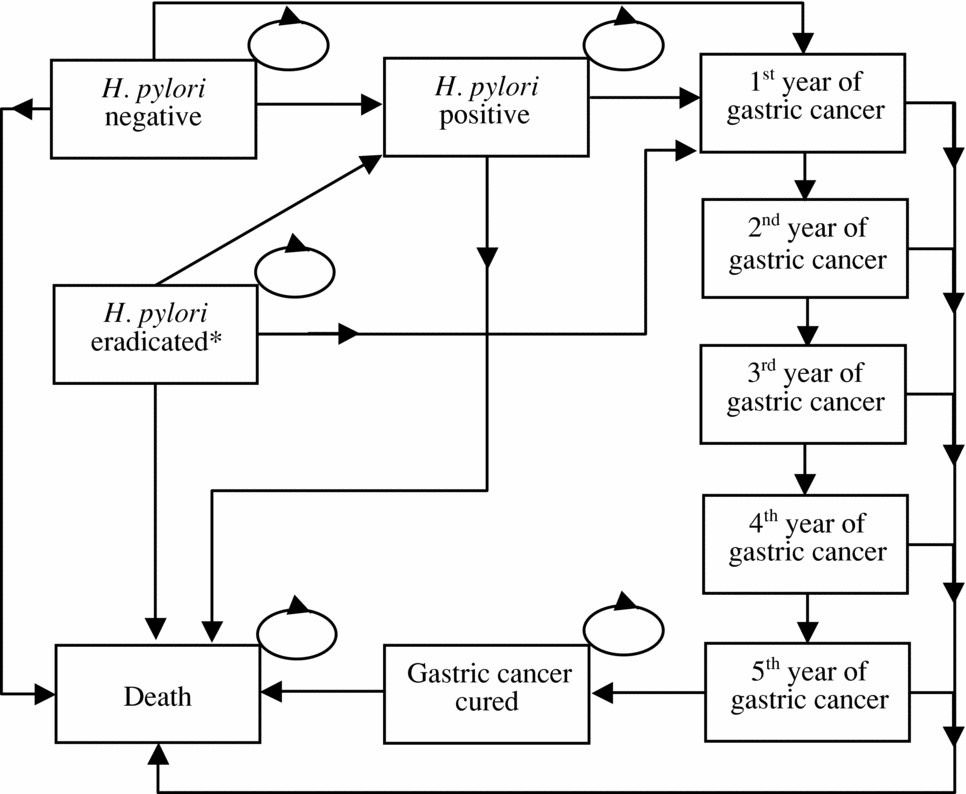
Figure 1. Markov model schematic. The asterisk at “H pylori eradicated” referred to the state of persons with positive screening test and the infection was successfully eradicated by the triple therapy.
East Asian countries such as China and Japan have the highest incidence of distal gastric cancer, which is twice as common in men as in women (Reference Kelley and Duggan18). H pylori infection was also found to be strongly linked to increased risk of gastric cancer in ethnic Chinese and Japanese people (Reference Miwa, Go and Sato23). Early detection and eradication of H pylori infection might be a useful way to reduce the risk of gastric cancer in Asian populations where the prevalence of H pylori infection and gastric cancer is significantly higher than that in Western populations (Reference Kelley and Duggan18). However, evidence is lacking on whether it is cost-effective to implement H pylori screening in high-risk Asian populations. Moreover, as several screening programs demonstrated acceptable sensitivity and specificity in detection of H pylori infection in Chinese (Reference Kang, Yeoh and Ho17;Reference Lee, Gwee and Teng21), which one is more cost-effective?
This study thus aimed to evaluate the costs and effectiveness associated with no screening, H pylori serology screening, and the 13C-urea breath test (UBT) in Singaporean Chinese at 40 years of age using a Markov model.
MATERIALS AND METHODS
Markov Model
The present study compared three strategies: strategy 1, no screening; strategy 2, single serology screening for H pylori and treating those tested positive with eradication therapy; and strategy 3, single screening for H pylori using the UBT and treating those tested positive with the same eradication therapy as used in strategy 2. After screening and treatment, both costs and outcomes associated with each strategy were evaluated using a Markov model (Figure 1) (Reference Briggs and Sculpher2;Reference Sonnenberg and Beck28), which estimated costs, life-years saved, and quality-adjusted life-years (QALYs) gained from the screening age to death (either died of gastric cancer or other causes or attained full life expectancy) (34). The distribution of the study cohort in different Markov states before the simulation started (i.e., cycle 0) was determined by the sensitivities and specificities of the screening strategies, prevalence of H pylori infection and the relative risk of cancer for H pylori infected. A separate health state was used to identify those infected by H pylori but the infection was successfully eradicated. Transition probabilities and corresponding plausible ranges in the model were obtained from a critical review of published literature on the target population wherever available (Table 1). Probabilities were converted from available rates using the recommended formula (Reference Sonnenberg and Beck28).
Table 1. Parameters Used in the Markov Model

a All costs were estimated from the records of local public hospitals.
H pylori, helicobacter pylori; Triple therapy: Rabeprazole 20 mg, amoxicillin 1000 mg, and clarithromycin 500 mg, twice a day for 7 days.
Clinical and Epidemiological Parameters
We evaluated all Singaporean Chinese at 40 years of age because the prevalence of H pylori infection for this age group increased substantially compared with younger groups (Reference Kang, Yeoh and Ho17;30). Age-specific mortality rates were applied when the cohort aged in the model (8). The relative risk of developing gastric cancer in the H pylori–infected persons compared with the uninfected was obtained from published literature (Reference Forman, Newell and Fullerton13;Reference Forman, Webb and Parsonnet14). The proportion of gastric cancer deaths among deaths from all causes was derived from local reports (Reference Seow, Koh, Chia, Shi, Lee and Shanmugaratnam27). The 1- to 5-year survival rates were estimated from a large prospective cohort study in Chinese patients (Reference Tian, Wang and Chen32). Persons who survived for more than 5 years after diagnosis of gastric cancer were assumed to be cured and therefore achieved full life expectancy as the 5-year survival rate adequately reflected curative success of gastric cancer treatment (Reference Koga, Kaibara and Kishimoto19;Reference Parsonnet, Harris, Hack and Owens25).
Screening and Treatment-Related Parameters
The screening strategies included one single serology screening using the enzyme-linked immunosorbent assay with sensitivity and specificity of 93 percent and 79 percent in Chinese, respectively (strategy 2) (Reference Kang, Yeoh and Ho17), and one single UBT using the simple gas chromatograph–mass selective detector with sensitivity and specificity of 98 percent and 96 percent in Chinese, respectively (strategy 3) (Reference Lee, Gwee and Teng21). In both strategies, persons with positive test results (including both true- and false-positive) for H pylori were treated with a triple therapy (i.e., rabeprazole 20 mg, amoxicillin 1,000 mg, clarithromycin 500 mg, all twice a day for 4 days) with an eradication rate of 91 percent (Reference Gambaro, Bilardi and Dulbecco16;Reference Yang, Wang and Chen35). This regimen was chosen because it is safe and effective, well accepted by patients, and is recommended by the Asia–Pacific consensus conference (Reference Danese, Armuzzi and Romano7;Reference Lam and Talley20;Reference Stack, Knifton and Thirlwell29). Persons who stopped the triple therapy due to side effects or did not comply with the regimen were considered as treatment failure and thus remained infected. Persons who remained infected despite attempts at eradication had life expectancy and other outcomes identical to those infected who did not undergo treatment. The reinfection rate of the persons whose infection had been successfully eradicated was assumed to be identical to the persons who had never been infected (i.e., 1 percent annually in the base-case analysis) (Reference Fendrick, Chernew and Hirth10;Reference Wang, Jin and Lin33). Once reinfection occurred, a gastric cancer risk was considered the same as that of an untreated, infected person.
An underlying assumption of the present study was that eradication of H pylori infection can reduce the excess risk of distal gastric cancer (Reference Eslick, Lim and Byles9;Reference Parsonnet, Friedman and Vandersteen24). We conservatively assumed that persons cured of H pylori infection would have a 30 percent excess risk reduction compared with those H pylori–infected persons in the base-case analysis. A wide range of excess risk reduction from 10 percent to 100 percent was used in probabilistic sensitivity analysis.
Costs
The present study was done from a public healthcare provider's perspective. Thus, the model included direct medical costs of the serology screening, the UBT, and the triple therapy. Costs associated with adverse effects of the triple therapy that necessitated medical intervention were also included (Table 1). Annual direct medical costs associated with treatment of gastric cancer were estimated at an average level across different stages of gastric cancer (Reference Dan, So and Yeoh6). Nonmedical direct costs and indirect costs were not included. All costs were accrued from the time of screening until death, reported in 2006 U.S. dollars, and annually discounted at 3 percent in the base-case analysis (Reference Lipscomb, Weinstein, Torrance, Gold, Siegel, Russell and Weinstein22).
Effectiveness
The two main health outcomes evaluated in this model were life-years saved and QALYs gained. All outcomes were annually discounted at 3 percent in the base-case analysis (Reference Lipscomb, Weinstein, Torrance, Gold, Siegel, Russell and Weinstein22).
Incremental Cost-Effectiveness Ratio
The incremental cost-effectiveness ratio (ICER) was expressed as U.S. dollars per QALY gained. It was calculated for the two screening strategies compared with no screening, as well as the UBT compared with the serology screening. The $50,000 per QALY was used as the ICER threshold.
Uncertainty Analysis
The point estimates of all parameters were used in the base-case analysis. To account for uncertainty surrounding these parameters’ values, a probabilistic sensitivity analysis was performed using the Monte Carlo simulation. Due to lack of information on distributions of these parameters, a triangular distribution was applied by using the point estimate, minimal and maximal values as inputs. Additionally, multiple cost-effectiveness acceptability curves, which by definition is the probability that an intervention is most cost-effective among all alternatives given a wide range of willingness-to-pay per QALY gained (Reference Briggs, Wonderling and Mooney3;Reference Fenwick and Byford11), were constructed for all three strategies.
To account for structural uncertainty, we explored the impact of different scenarios on ICERs, which included different target populations (all Chinese versus Chinese men only) and different levels of gastric cancer prevalence (high prevalence versus lower prevalence). The highest gastric cancer prevalence used in the scenario analyses was the prevalence in people older than 80 years of age based on the Singapore Cancer Registry report (Reference Seow, Koh, Chia, Shi, Lee and Shanmugaratnam27).
RESULTS
In the base-case analysis, compared with no screening, the serology screening strategy for all Chinese people at the age of 40 (n = 478,500) (8) saved 788 life-years or gained 763 QALYs by preventing 101 gastric cancer cases at an extra cost of $20 million. The UBT strategy saved 840 life-years or gained 814 QALYs by preventing 108 gastric cancer cases at an extra cost of $44 million (Table 2). The ICER of serology screening versus no screening was $25,881 per QALY gained. The ICER of UBT versus serology screening was $470,000 per QALY gained (Table 2).
Table 2. Costs, Effectiveness, and ICERs in the Base-Case Analysis

aThe ICER was calculated by comparing the serology screening with no screening.
bThe ICER was calculated by comparing the UBT with the serology screening.
LYS, life-years saved; QALY, quality-adjusted life-year; ICER, incremental cost-effectiveness ratio; UBT, 13C-urea breath test.
If the screening strategies were only applied to Chinese men at the same age group, the ICER was $16,162 per QALY for serology screening versus no screening and $286,470 per QALY for UBT versus serology screening (Table 2). If the screening strategies were applied to the population with lower gastric cancer prevalence (p = 1.2 per 100,000), the ICER was $100,577 per QALY for serology screening versus no screening and $1,699,296 per QALY for UBT versus serology screening. If the screening strategies were applied to the population with the highest gastric cancer prevalence (p = 342 per 100,000), the serology screening was dominant to no screening and the ICER was $3,706 per QALY for UBT versus serology screening (Table 2).
Probabilistic sensitivity analyses demonstrated that the 95 percent confidence interval of the ICERs of serology screening versus no screening was $5,700 per QALY to $120,000 per QALY (Figure 2). If using $50,000 per QALY as a threshold, the probability that the serology screening was cost-effective compared with no screening was 75 percent.
The 95 percent confidence interval of the ICERs of UBT versus no screening was $16,000 per QALY to $230,000 per QALY (Figure 3). The probability that the UBT was cost-effective compared with no screening was 38 percent. As shown in Figure 4, almost all ICERs of UBT versus serology screening were higher than $50,000 per QALY.

Figure 2. Incremental cost-effectiveness ratios of the serology screening versus no screening. QALYs, quality-adjusted life-years.
Multiple cost-effectiveness acceptability curves are shown in Figure 5. If the willingness-to-pay was less than $30,000 per QALY, the probability of no screening being the most cost-effective strategy was higher than the other two. If the willingness-to-pay was more than $30,000 per QALY, the probability of the serology screening being the most cost-effective strategy was higher than the other two. The probability of the UBT being the most cost-effective strategy was extremely low and therefore ignorable despite variations in willingness-to-pay.

Figure 3. Incremental cost-effectiveness ratios of the 13C-urea breath test screening versus no screening. QALYs, quality-adjusted life-years.

Figure 4. Incremental cost-effectiveness ratios of the 13C-urea breath test screening versus the serology screening. QALYs, quality-adjusted life-years.

Figure 5. Multiple cost-effectiveness acceptability curves. QALY, quality-adjusted life-year; UBT, 13C-urea breath test.
DISCUSSION
This study estimated the life-time costs and effectiveness associated with population-based H pylori screening using a Markov model. The serology screening strategy was demonstrated to be a cost-effective strategy in the base-case analysis and all scenario analyses, with the exception of the scenario using relative low gastric cancer prevalence. In contrast, the UBT strategy was cost-effective only in the scenario using relatively higher gastric cancer prevalence. However, these results have not received strong support from the probabilistic sensitivity analysis. Therefore, it cannot be confidently concluded (at least at 95 percent level) that population-based H pylori screening is a cost-effective strategy in the Singaporean Chinese–based population on the currently available clinical and epidemiological evidence.
Singapore is a Southeast Asian country adopting a copayment healthcare system, where no population-based H pylori screening has ever been taken. Therefore, the present findings conveyed some useful and important messages for healthcare decision makers. First, serology screening has demonstrated certain potential to be a cost-effective population-based screening strategy, especially in subpopulations with higher gastric cancer prevalence, whereas UBT has added less health benefit at significantly higher costs compared with serology screening. This potential would be more prominent under circumstances for which costs for gastric cancer treatment keep rising due to advances in new and costly technologies. One-time expenditure on screening could be substantially offset by savings in treating cancer cases in the long-term. This strategy will reduce economic burden of both patients and government. Second, as the prevalence of H pylori infection and gastric cancer in Chinese men is higher than in Chinese women, it would be more cost-effective to carry out serology screening only in Chinese men (see scenario 2). However, it should be noted that incidence and prevalence of H pylori in Singapore are expected to decrease over time (Reference Ang, Fock and Dhamodaran1;Reference Fock12). This trend will make H pylori screening less cost-effective in the future.
The finding in the present study was similar to the published studies using similar models to estimate the economic and clinical effects of H pylori screening (Reference Fendrick, Chernew and Hirth10;Reference Parsonnet, Harris, Hack and Owens25). These studies reported that one-time serology screening was a cost-effective strategy compared with no screening (Reference Parsonnet, Harris, Hack and Owens25) or serology screening with post-treatment confirmatory testing (Reference Fendrick, Chernew and Hirth10). However, both studies did not compare the serology screening with the UBT. Some improvements in modeling and estimation in the present study are worth noting. First, we had a health state to identify the persons who were H pylori–positive and whose infection was successfully eradicated by the triple therapy (i.e., “H pylori eradicated” in Figure 1). This was a health state in the Markov model that allowed for capturing economic and health benefits resulted from the screening strategies. Second, in line with an important assumption that persons who survived more than 5 years after diagnosis of gastric cancer were assumed to be cured (Reference Koga, Kaibara and Kishimoto19;Reference Parsonnet, Harris, Hack and Owens25), we used five tunnel states, instead of a single gastric cancer health state, to represent the status for each of the first 5 years since diagnosis with gastric cancer. Mortality rates for these tunnel states were different from each other based on epidemiological evidence (Reference Tian, Wang and Chen32). This refinement may better simulate the real progress of gastric cancer and thus obtain more accurate estimations of costs and effectiveness. Third, our model was a life-time estimation and every person remained in the model until death. Thus, the mortality rate varied over time. Instead of fixed-point estimates with plausible ranges, age-specific mortality rates might be more appropriate and accurate because of the aging of the study cohort. Last but not least, probabilistic sensitivity analyses, rather than one-way sensitivity analyses, was performed in the present study, which allowed for the examination of robustness of our conclusion by taking into consideration uncertainty of all parameters simultaneously.
Prevention of gastric cancer will reduce medical expenditure for treatment of cancer and increase life-years and QALYs. However, this health benefit could be associated with additional expenditure incurred during extended life-years (e.g., the expenditure on daily living in extended life-years), which will not occur in case of premature death. Because including this cost component remains controversial, we did not take it into consideration in the present study. We also acknowledge that the arbitrarily defined triangular distribution of parameters used in probabilistic sensitivity analyses may have certain influence on the results.
It cannot be confidently concluded that either H pylori screening was a cost-effective strategy compared with no screening in Singaporean Chinese at 40 years of age. Nevertheless, the serology screening has demonstrated the potentiality to be a cost-effective strategy, especially in the population with higher gastric cancer prevalence.
CONTACT INFORMATION
Feng Xie, MSc, PhD (fengxie@mcmaster.ca), Assistant Professor, Department Clinical Epidemiology & Biostatistics, McMaster University, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada; Faculty member, Programs for Assessment of Technology in Health, St. Joseph's Healthcare Hamilton, 25 Main Street West, Suite 2000, Hamilton, Ontario, Canada L8P 1H1
Nan Luo, PhD (medln@nus.edu.sg), Research Fellow, Centre for Health Services Research, Yong Loo Lin School of Medicine, National University of Singapore, BLK MD11, #01-10, 10 Medical Drive, Singapore 117597
Gord Blackhouse, MSc (blackhou@mcmaster.ca), Research Associate, Department Clinical Epidemiology & Biostatistics, McMaster University, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada
Ron Goeree, MA (goereer@mcmaster.ca), Associate Professor, Department Clinical Epidemiology & Biostatistics, McMaster University, 1200 Main Street West, Hamilton, Ontario L8N 3Z5, Canada; Director, Programs for Assessment of Technology in Health, St. Joseph's Healthcare Hamilton, 25 Main Street West, Suite 2000, Hamilton, Ontario L8P 1H1, Canada
Hin-Peng Lee, FFPHM (UK) (cofleehp@nus.edu.sg), Director and Professor, Centre for Health Services Research, Yong Loo Lin School of Medicine, National University of Singapore, BLK MD11, #01-10, 10 Medical Drive, Singapore 117597