Introduction
Obsessive compulsive disorder (OCD) is characterized by recurrent, unwanted and intrusive thoughts or images (obsessions) and excessive ritualistic behaviors or mental acts (compulsions) typically performed in response to the obsessions (American Psychiatric Association, 2000). However, OCD demonstrates heterogeneous clinical presentation, with four consistent subtypes identified by factor analytic studies: contamination/washing, aggression/checking, symmetry/ordering and hoarding (Mataix-Cols et al., Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004). While OCD is broadly characterized by high levels of anxiety, its distinct symptom and treatment response profiles suggest distinct pathophysiology, leading to its re-classification as its own spectrum of anxiety disorders in the DSM-V (Association, Reference Association2013). Much is still unknown regarding the neural processes underlying OCD pathophysiology, a better understanding of which may improve treatment and outcomes.
Perhaps the most dominant neural account of OCD is the cortico-striato-thalamo-cortical (CSTC) model, in which symptoms are supported by two neural pathways: a direct pathway that excites processing of information from the thalamus to cortex, and an indirect, inhibitory pathway that operates on the same network (Saxena and Rauch, Reference Saxena and Rauch2000). It is suggested that in OCD, an imbalance between the two pathways leads to improper thalamic gating, resulting in the hyperactivity of the orbitofrontal cortex (OFC), caudate nucleus and the anterior cingulate (ACC). These regions demonstrate abnormally low gray matter volume in OCD (Rotge et al., Reference Rotge, Langbour, Guehl, Bioulac, Jaafari, Allard, Aouizerate and Burbaud2010), but are functionally hyper-activated by emotion provocation, a pattern that normalizes following successful treatment (Pauls et al., Reference Pauls, Abramovitch, Rauch and Geller2014). It is yet unclear whether this hyper-activation supports OCD symptoms, or represents a compensatory activation aimed at regulating stimulus-evoked anxiety (Maia et al., Reference Maia, Cooney and Peterson2008). Understanding the significance of altered neural reactivity in OCD therefore requires further study, with a particular focus on assessing the direction of association between stimulus-evoked neural reactivity and reported symptom burden.
A further challenge to neurobiological models of OCD lies in the heterogeneous nature of its symptomatology (Nakao et al., Reference Nakao, Okada and Kanba2014). While the differentiation of neuronal circuitry between OCD subtypes is still evolving, several neuroimaging studies have reported promising subtype distinctions (Leckman et al., Reference Leckman, Denys, Simpson, Mataix-Cols, Hollander, Saxena, Miguel, Rauch, Goodman, Phillips and Stein2010; Mataix-Cols et al., Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004; van den Heuvel et al., Reference van den Heuvel, Remijnse, Mataix-Cols, Vrenken, Groenewegen, Uylings, van Balkom and Veltman2009). Functional activation studies linked hyper-activation in the ventromedial prefrontal cortex (PFC) and the right caudate nucleus with washing, subcortical regions such as the putamen/globus pallidus and thalamus with checking, and the OFC with hoarding (Mataix-Cols et al., Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004). Structurally, abnormally low gray matter volume was noted in the caudate nucleus for the washing subtype, but in the temporal cortex for the checking subtype (van den Heuvel et al., Reference van den Heuvel, Remijnse, Mataix-Cols, Vrenken, Groenewegen, Uylings, van Balkom and Veltman2009). Such findings support neuroimaging approaches' potential for aiding in the development of a multidimensional model of OCD that accounts for distinct symptom subtypes (Kwon et al., Reference Kwon, Jang, Choi and Kang2009).
Given the densely interconnected nature of the brain, abnormal interactions between brain regions may be as important as regional activation or gray matter density. To this end, functional connectivity analysis can provide important insights into neural communication, helping to elucidate the pathophysiology of neuropsychiatric disorders (Fox and Raichle, Reference Fox and Raichle2007). In OCD, functional connectivity studies have revealed dysregulated CSTC circuits, including the pathway running through the thalamus to the caudate and putamen, and terminating in the ACC and OFC (Anticevic et al., Reference Anticevic, Hu, Zhang, Savic, Billingslea, Wasylink, Repovs, Cole, Bednarski, Krystal, Bloch, Li and Pittenge2014; Beucke et al., Reference Beucke, Sepulcre, Talukdar, Linnman, Zschenderlein, Endrass, Kaufmann and Kathmann2013; Harrison et al., Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas, Deus, Alonso, Yücel, Pantelis, Menchon and Cardoner2009). For example, OCD patients examined at rest exhibited a loss of functional connectivity between ventral cortical and subcortical regions (Harrison et al., Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas, Deus, Alonso, Yücel, Pantelis, Menchon and Cardoner2009). However, no OCD study has examined functional connectivity in the context of stimulus-evoked distress. Given the established utility of stimulus-evoked studies of neural reactivity in OCD, combining such paradigms with functional connectivity analyses may further inform our understanding of disorder-related abnormalities in information processing.
To address this knowledge gap, we employed an emotion provocation paradigm during fMRI acquisition to investigate functional connectivity in OCD relative to a healthy control group. Furthermore, subtype-specific neural profiles were evaluated in the two most prominent OCD subtypes – washers and checkers.
Methods and materials
Participants
Participants were right-handed adults ranging in age from 19 to 57. Thirty one participants diagnosed with OCD (mean age = 34.00, s.d. = 8.50, 18 males) and 17 healthy volunteers (mean age = 32.65, s.d. = 9.19, 8 males) were included. After appropriate ethics approval, the study was conducted at the Centre for Addiction and Mental Health (CAMH), a teaching institution of the University of Toronto, Canada. For patients, inclusion criteria were: a primary diagnosis of OCD, with contamination or safety/harm as primary obsessions, and washing/cleaning (n = 12) or checking behaviors (n = 19) as corresponding primary compulsions. The diagnosis was confirmed using the structured clinical interview of the DSM-IV (SCID-I) by a trained interviewer. Exclusion criteria for all participants were: all current comorbid Axis I conditions, suspected organic pathology, substance abuse or dependence in the past 6 months, claustrophobia or the presence of metallic objects that contraindicate fMRI, and previous trials of Cognitive Behavioral Therapy (CBT), which was offered to patients following assessment and is described in a separate report. Patients with predominantly hoarding obsessions and compulsions, who often show poor response to conventional CBT (Saxena et al., Reference Saxena, Maidment, Vapnik, Golden, Rishwain, Rosen, Tarlow and Bystritsky2002), and may be considered a separate syndrome from OCD (Pertusa et al., Reference Pertusa, Fullana, Singh, Alonso, Menchón and Mataix-Cols2008), were also excluded from the study. No patients presented with predominant symptoms around symmetry.
Measures
Symptom severity and the change in OCD group were assessed using the Yale-Brown Obsessive Compulsive Scale (YBOCS) (Goodman et al., Reference Goodman, Price, Rasmussen, Mazure, Fleischmann, Hill, Heninger and Charney1989). In addition, several functional and behavioral measures were collected, such as Quality of Life Satisfaction and Enjoyment Questionnaire (QLESQ) (Burckhardt et al., Reference Burckhardt, Woods, Schultz and Ziebarth1989), Hassles and Uplifts Scale (Folkman and Lazarus, Reference Folkman and Lazarus1988), and the Disgust Sensitivity Scale (DS) (Haidt et al., Reference Haidt, McCauley and Rozin1994). Severity of depressive symptoms was assessed with the Beck Depression Inventory (BDI) (Beck et al., Reference Beck, Ward, Mendelson, Mock and Erbaugh1961).
Procedure
Two hundred and fifty image stimuli were selected for the emotion provocation paradigm. Stimuli were selected to fill 5 categories: 3 categories provoked reactivity within 3 major OCD subtypes: contamination/washing, checking, and hoarding; a fourth category of disgusting images was included as a generalized stressor, and a fifth category of neutral/happy scenes was included as a control condition. Washing, checking, and hoarding stimuli were selected as they represent the most common subtypes of OCD, and while hoarding-primary participants were excluded from the study, hoarding stimuli acted as a useful condition for examining more general, i.e. not subtype-specific, emotion provocation. Stimuli were selected from a standard set from the International Affective Picture System (IAPS) (Lang et al., Reference Lang, Bradley and Cuthbert1999) and from a set of pictures obtained with a digital camera and rated with 100% agreement by 3 clinicians with experience in OCD to be anxiety-provoking in patients with OCD, whereas the neutral images were rated to be relatively innocuous. Thus, 50 images from each of the 5 categories were available for the study, with each category divided into 10 category-specific blocks of 5 pictures each. Each participant saw a subset of 25 blocks sampled equally from each category, to reduce the risk of image-specific effects impacting study results. The emotion provocation paradigm consisted of 2 runs, of 12 and 13 pseudorandomized blocks respectively. Each block was 25 s long, with each stimuli presented for 3 s. At the end of each block participants rated their anxiety and disgust on a 9-point Likert scale, with 5 s for each rating. Thus, including 16 s for initial instruction screens and scanner saturation, the first block had a duration of 10.53 min, and the second block had a duration of 11.40 min.
Imaging data
Imaging setup
Imaging data were collected on a Siemens Trio 3.0 Tesla (Signa, General Electric Medical Systems, Milwaukee, WI, software version LX 8.2.5, NV/i hardware platform) located at the Baycrest Centre for Geriatric Care. The block design experiment was designed and implemented using Presentation software (Neurobehavioral Systems, Albany, California). Before the scan, the participants were provided with instructions and given time to practice the fMRI task.
Functional imaging
For each subject, a T2*-weighted gradient-echo echo-planar image (EPI) pulse sequence was prescribed and higher order shimmed for the functional trials. The EPI parameters were as follows: TE = 30 ms; TR = 2000 ms; flip angle = 270°; acquisition matrix = 64 × 64; FOV = 200 mm. Thirty axial oblique slices of the brain were acquired at each time point, with a voxel resolution of 3.1 × 3.1 × 5 mm, no skip between slices. Two experimental runs were collected, measuring 316 and 342 time points in length. The first 4 time points of each run were discarded to account for scanner equilibrium effects, for a total of 650 time points.
Preprocessing
Functional activation was determined from the BOLD signal using Statistical Parametric Mapping software (SPM12, University College London, London, UK; http://www.fil.ion.ucl.ac.uk/spm/software/spm12). Following reconstruction (SPM12 DICOM import utility), the time series functional data were spatially co-registered and re-aligned to correct for motion within and between functional scans (translational motion parameters were less than 1.5 mm for all participants) and co-registered with the SPM EPI template. Warping parameters were obtained from the tissue segmentation procedure and subsequently applied to the time series data (resampling to 3 mm3 voxels). Images were warped to normalize the data into a common stereotactic reference space (MNI), and then spatially smoothed to a 6 mm3 full-width half maximum Gaussian kernel.
First level statistical models
Single-subject time series data were submitted to first-level general linear statistical models examining neural activity during the neutral/happy and emotionally-evocative picture viewing periods. Each picture type was modeled as a separate condition. Using the SPM12 design specification, the task-specific boxcar stimulus functions were convolved with the canonical hemodynamic response function scaled to film clip duration. Each model included 6 participant-specific motion parameters (Johnstone et al., Reference Johnstone, Ores Walsh, Greischar, Alexander, Fox, Davidson and Oakes2006), within-session global scaling, and the AR1 method of estimating temporal autocorrelation (Friston et al., Reference Friston, Holmes, Poline, Grasby, Williams, Frackowiak and Turner1995). For each participant, each of the four arousing picture conditions were contrasted against the neutral/happy picture condition to generate a contrast map that was then entered in second-level random effects analyses. To estimate the overall effects of negative arousal, the neutral/happy picture condition was weighted against the four negative picture conditions at the first level to create a negative arousal contrast map for each participant.
Neural correlates of negative emotion provocation
Negative emotion provocation was modelled via a contrast between neutral and valenced, emotionally-evocative pictures (valence > neutral). Each of the valenced imaged categories (Hoarding, Contamination/Washing, Safety/Checking, Disgust) was contrasted separately against neutral images at the first level of analysis. At the second level of analysis, each valenced v. neutral image contrast was modeled as a within-participant condition factor, and the control group and two OCD subtype groups were modeled as a between-groups factor, for a total of 4 reactivity conditions (Hoarding, Contamination/Washing, Safety/Checking, Disgust) × 3 groups (Control, Washers, and Checkers) in a full-factorial ANOVA design.
Connectivity analyses
For each participant, psychophysiological interaction analyses (PPIs) were also performed using the PPI function included in the SPM12 software package. The PPI analysis tests if the correlation in activity between a designated Region of Interest (ROI) and the rest of voxels in the brain areas is affected by changes in experimental condition. Unlike using functional connectivity alone, PPI analysis identifies task-related changes in connectivity while controlling for the main effects of connectivity and experimental condition (Friston et al., Reference Friston, Buechel, Fink, Morris, Rolls and Dolan1997; Gitelman et al., Reference Gitelman, Penny, Ashburner and Friston2003).
In this case, the analysis examined whether the connection between ROI activity and the rest of the brain was altered by emotional reactivity. To model emotional reactivity, activation related to viewing pictures from the 4 emotion-provocation categories was contrasted against neutral picture viewing; emotion provocation was coded in the PPI analysis as the experimental condition regressor. The seed ROI was a 10 mm spherical area centered on the peak voxel that distinguished emotional reactivity between the OCD and Control groups. Signal from the ROI was extracted using the PPI function included in the SPM12 software. Then, for each participant, seed ROI signal, experimental condition, and the multiplicative interaction between them were entered as simultaneous regressors of brain activity.
Within-OCD subtype analyses
For the main effects of emotion provocation and for the connectivity analyses, an additional between-groups contrast was performed between the washing and checking subtypes of the OCD group, in order to identify subtype-specific patterns of neural reactivity and reactivity-related connectivity changes.
Correction for multiple comparisons
Main effects and group level t contrasts used a voxel height threshold of P unc < 0.005 (T ⩾ 2.68), and an SPM FWE-corrected voxel extent (cluster) threshold of K > 150. OCD Subgroup analyses were treated as exploratory, given the low sample size for comparing subgroups. We used the same voxel height threshold of P unc < 0.005 (T ⩾ 2.68), but an exploratory voxel extent (cluster) threshold of K > 50 for comparisons between the Washer and Checker subtypes.
Results
Clinical data
There were no significant differences between patient and control groups on demographic parameters (age, gender and education) (Table 1). For clinical indicators, YBOCS scores were obtained from all patients. However, complete BDI data was only obtained from 21 patients, and QLESQ and Disgust Sensitivity from only 22 patients due to time constraints during assessment. Patients had a mean YBOCS score of 24.74 (s.d. = 6.12) with a mean Clinical Global Impression Scale (CGI-S) score of 3.69 (s.d. = 0.79). The mean illness duration was 12.81 (s.d. = 7.79; range, 1–26 years). On the BDI, patients scored in the mild depressive symptoms range (M = 21.70, s.d. = 10.66), although none of them met DSM-IV criteria for major depressive disorder (MDD) at screening. Patients also reported a significantly lower quality of life compared to controls [t(37) = −6.35, p < 0.001, M Controls = 62.71 ± 7.45, M OCD = 43.09 ± 11.45]. YBOCS severity of illness was associated with lower QLESQ quality of life [r(20) = −0.62, p = 0.002], and greater BDI depressive symptoms [r(14) = 0.61, p = 0.020]. The washing and checking OCD subtype groups did not differ in symptom severity on any of these measures. Sixteen of the 31 OCD group participants were taking SSRIs, but controlling for medication status did not alter the findings reported below.
Table 1. Demographic and clinical characteristics of patients with OCD and controls
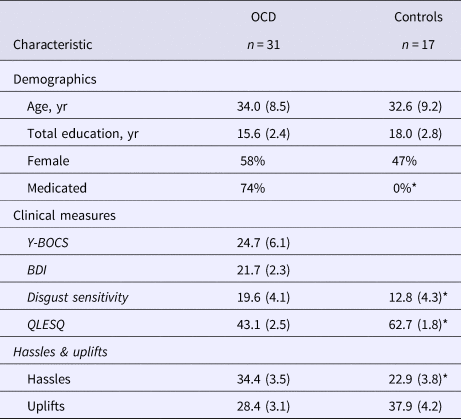
Note. Values displayed are means with standard deviations in parentheses.
Significant group differences at the p < 0.05 level are indicated with an asterisk (*). Comparisons of washing and checking subtypes revealed no differences in any of the measures listed above
Behavioral data
Within-scanner image ratings were subjected to separate multilevel models for anxiety and disgust ratings. Group (OCD v. Control) was included as a group level factor, and image type (Neutral v. Aversive) was included as a within-participant factor. Valenced images evoked reliably greater anxiety [t(1150) = 15.83, p < 0.001] and disgust [t(1150) = 12.14, p < 0.001] than neutral images, an effect qualified by group × valence interactions for both anxiety [t(1150) = 5.59, p < 0.001] and disgust [t(1150) = 2.78, p < 0.01] ratings, an effect driven by universally higher ratings in the OCD group (online Supplementary Table S1). The OCD subgroups did not differ in their ratings except for anxiety ratings for contamination/washing images, for which the washing subtype showed greater anxiety than the checking subtype [t(29) = 2.55, p < 0.05] (online Supplementary Table S2).
Imaging data
Across all participants, emotion provocation was associated with widespread activation of visual cortices, bilateral anterior insula, dorsomedial PFC, and dorsolateral PFC, and reduced activity in bilateral parahippocampal gyrus, superior temporal gyrus, and caudate nucleus (Fig. 1A). Contrasting emotion provocation effects between the OCD and Control groups revealed greater OCD-related neural reactivity in the posterior cingulate gyrus (PCg), precuneus, and calcarine sulcus (Fig. 1B), a region which was deactivated in the Control group in response to emotion provocation [t(16) = 2.86, p = 0.01], but was activated in the OCD group [t(30) = 4.40, p = 0.0001]. The peak PCg voxel from this contrast (x = −9; y = −51; z = 12) was subsequently used as a region of interest (ROI) for examining whole brain connectivity. All regions generally demonstrating emotional reactivity, as well as regions demonstrating differences between OCD and Control groups, are listed in the online Supplementary Materials (Table S3).
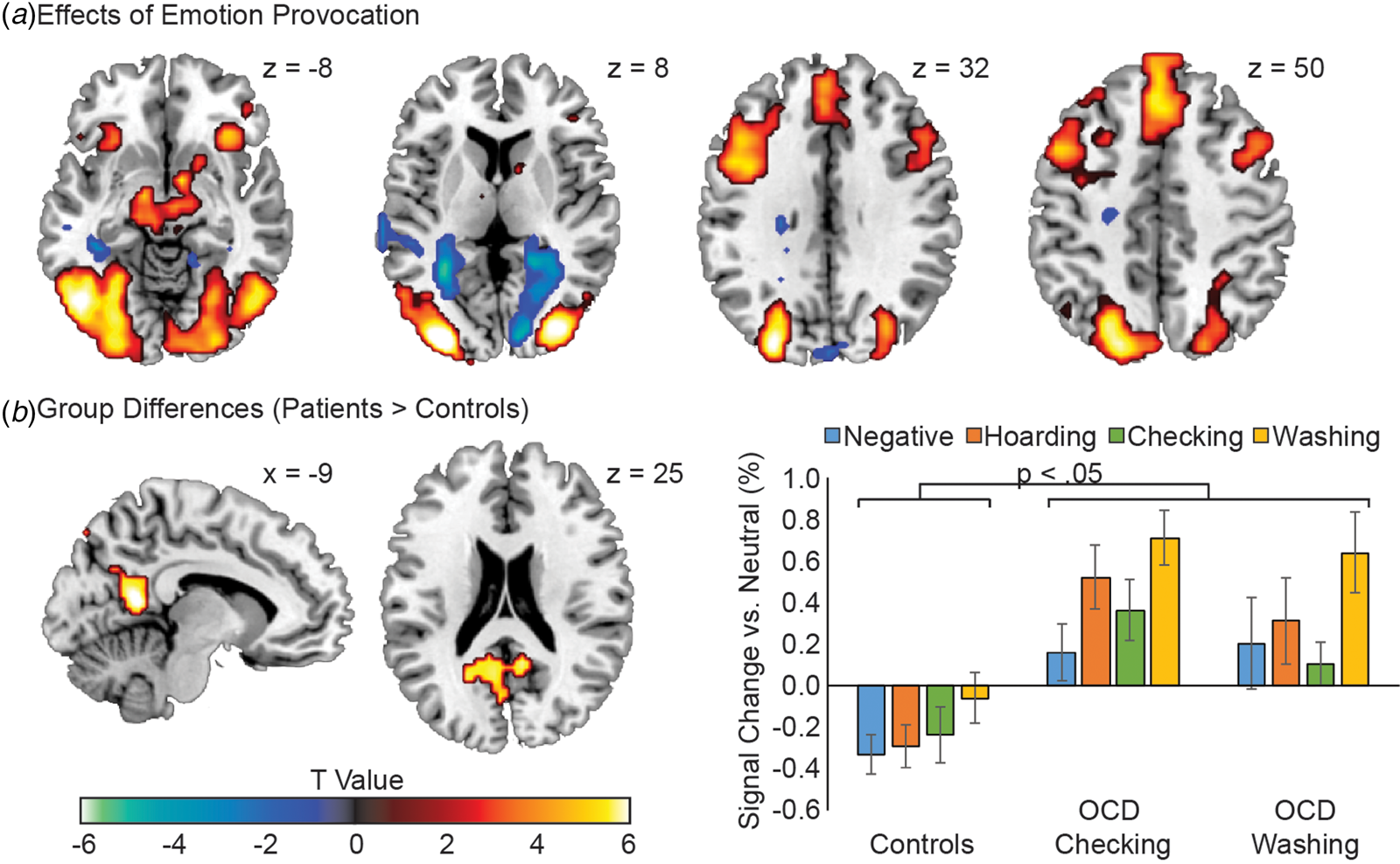
Fig. 1. Neural effects of emotion provocation in OCD and control participants. (A) Main effects of emotion provocation, collapsing across all participants, using condition-based analysis (provocative image v. neutral image viewing). (B) Group differences in the emotion provocation effect between the OCD group and Healthy Control group participants. The mean provocation values (compared to within-participant neutral image neural activity) are displayed for the Healthy Control, OCD Checking, and OCD Washing groups. All OCD v. Control group comparisons within a stimulus category were significant at the p < 0.05 level. Error bars are standard errors. Images are displayed in ‘neurological’ convention, meaning left is left and right is right.
Psychophysiological interaction (PPI) analyses modelled changes in PCg connectivity as a function of emotion provocation (Fig. 2, online Supplementary Table S4). Across all participants, emotion provocation was associated with increased PCg functional connectivity throughout the occipital lobe, including primary visual cortex (Panel A), but reduced functional connectivity with the cingulate cortex, insula, superior temporal lobe and thalamus (Panel B). Relative to the Control group, OCD patients demonstrated elevated emotion-provoked PCg functional connectivity with the visual cortices, cerebellum, and the right anterior insula/OFC (Panel C). Using the regions of group-modulated PCg coupling with as ROIs (online Supplementary Table S4) we examined the association between PCg – ROI coupling and symptom severity scores on the YBOCS. Within the OCD group, YBOCS scores were only correlated with emotion-provoked coupling between the PCg and visual cortices [r(29) = 0.50, p = 0.004] (Panel C).
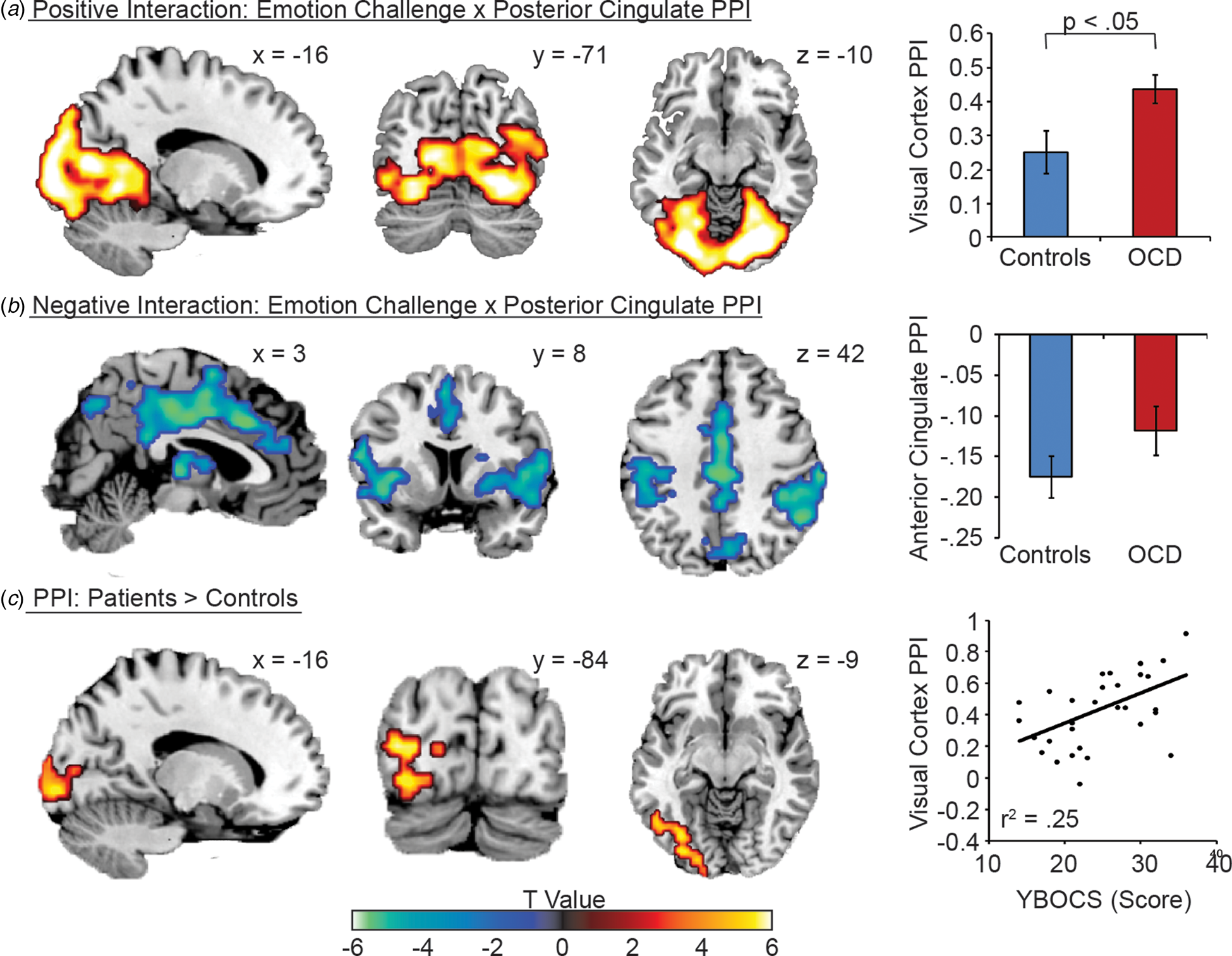
Fig. 2. Psychophysiological interaction (PPI) analysis of PCg activity between emotion v. neutral image viewing conditions. (A) Positive interaction effect, i.e., increased PCg connectivity during emotion relative to neutral image viewing. (B) Positive interaction effect, i.e., decreased PCg connectivity during emotion relative to neutral image viewing. (C) PPI effect differences between the OCD and Control groups. Right panel visualizes the relationship between PCg -visual cortex PPI effects and YBOCS scores. Error bars are standard errors. Asterisk (*) indicates a significant difference at the p < 0.05 level. Images are displayed in ‘neurological’ convention, meaning left is left and right is right.
To understand symptom specific profiles of emotion reactivity, we conducted an explatory sub-analysis of Washing and Checking subtypes of OCD (Fig. 3). Checkers demonstrated greater provocation-related activity in the PCg, slightly dorsal and medial to the PCg region implicated in the overall OCD v. Control contrast (Panel A). Washers demonstrated greater provocation-related activity in the bilateral anterior insula and occipital gyrus (Panel B). With respect to the PPI analyses, Checkers demonstrated stronger emotion-provoked PCg connectivity with motor cortices (Panel C), while washers demonstrated stronger emotion-provoked PCg connectivity with the anterior insula, middle cingulate, and superior temporal gyurs (Panel D).
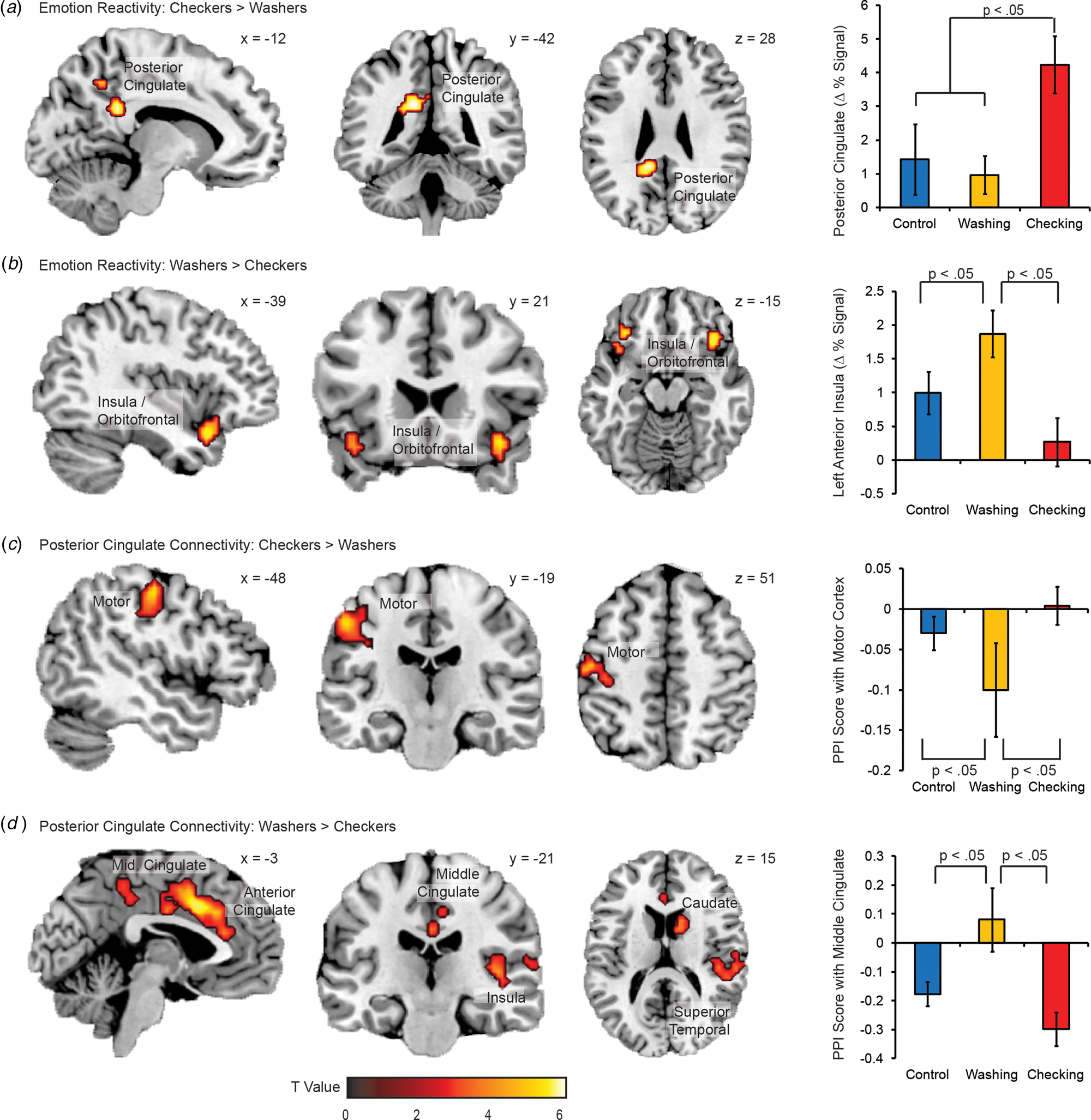
Fig. 3. Activation and connectivity differences between OCD subtypes. Regions of greater reactivity to emotion images (compared to neutral images) for (A) checkers than washers, and (B) washers than checkers. Regions of greater PPI with the PCg as a function of image condition (emotion v. neutral) for (C) checkers than washers, and (D) washers than checkers. Error bars are standard errors. Images are displayed in ‘neurological’ convention, meaning left is left and right is right.
Discussion
In this study, we present the first functional connectivity analysis of OCD and its subtypes in response to emotion-provocation. OCD was broadly characterized by altered functional connectivity relative to healthy controls, with specific distinctions between washing and checking subtypes.
Across subtypes, elevated PCg reactivity to emotion provocation was apparent in OCD. An anatomical bridge between the occipital/parietal and frontal lobes (Morecraft et al., Reference Morecraft, Cipolloni, Stilwell-Morecraft, Gedney and Pandya2004), the PCg is thought to play an important role in directed attention, particularly in the selection of relevant attentional targets (Yamasaki et al., Reference Yamasaki, LaBar and McCarthy2002). Thus a failure to deactivate the PCg in response to emotion provocation may signify a failure to effectively filter out potentially distressing cognitive elaboration.
Connectivity analyses supported this ‘leaky filter’ account of OCD. Across both groups, emotion provocation was linked to stronger functional connectivity between the PCg and both the visual regions in the occipital cortex (Clarke and Miklossy, Reference Clarke and Miklossy1990) and the brain's salience network (SLN) comprised of the ACC and insula (Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss and Greicius2007). However, the direction of connectivity enhancement differed between visual and SLN regions. Stress provoked positive PCg-visual connectivity but also inhibitory connectivity between the PCg and the SLN. Thus, when controls deactivated the PCg in response to emotion provocation, such deactivation would also indicate down-regulated visual processing and up-regulated SLN processing. One interpretation for such dynamics would be the establishment of a functional network which leverages SLN recruitment to down-regulate sensory processing in response to emotionally provocative stimulation. Establishing the causal relationship between these regions is an important question for future research.
While similar PPI profiles were observed in the OCD group, PCg deactivation was absent in response to emotion provocation, suggesting impaired ability to regulate visual processing using the SLN. This suggestion is further supported by OCD-specific positive connectivity between visual cortices, PCg, and OFC in response to emotion provocation. Such enhanced connectivity, combined with a failure to deactivate the PCg during emotion-provocation, may contribute to stimulus triggering of reactive cognition and behavior. Indeed, elevated stimulus-evoked PCg-Visual connectivity was directly related to symptom severity.
With respect to OCD subtypes, patients with predominantly checking symptoms showed greater dorsal PCg activation and greater emotion-enhanced functional connectivity within the dorsal PCg projecting to the motor cortices. By contrast, the washing subtype showed greater insula reactivity and emotion-enhanced connectivity between the PCg and the anterior insula. These findings extend prior work describing similar activation differences between OCD subtypes that inform subtype-related differences in information processing (Mataix-Cols et al., Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004). Checking may be characterized by a lack of inhibitory control over the motor cortex in response to emotion provocation, and is consistent with previous findings of heightened motor activity in OCD that may reflect a compensatory response to safety obsessions (Ciesielski et al., Reference Ciesielski, Rauch, Ahlfors, Vangel, Wilhelm, Rosen and Hämäläinen2012; Cocchi et al., Reference Cocchi, Harrison, Pujol, Harding, Fornito, Pantelis and Yücel2012). The washing subtype, by contrast, showed a lack of inhibitory control between the PCg and the anterior insula, a sensory hub of the SLN (Seeley et al., Reference Seeley, Menon, Schatzberg, Keller, Glover, Kenna, Reiss and Greicius2007). This is consistent with a failure to regulate the motivational importance afforded to pictures depicting violations of hygiene and cleanliness. This distinction between checking and washing as disorders of motor and salience reactivity respectively may be a fruitful avenue for future investigation.
Our findings add a cortical dimension to previous findings that suggest OCD symptoms originate due to inefficient thalamic gating, leading to hyperactivity in the OFC and ACC (Rauch et al., Reference Rauch, Wedig, Wright, Martis, McMullin, Shin, Cannistraro and Wilhelm2007). Conflation of visual and SLN processes in response to emotion provocation may explain such hyperactivity in OCD, which has been attributed to hyper-vigilance against error that persists in OCD patients even following treatment response (Del Casale et al., Reference Del Casale, Kotzalidis, Rapinesi, Serata, Ambrosi, Simonetti, Pompili, Ferracuti, Tatarelli and Girardi2011). While the CTSC model emphasizes the subcortical interactions leading to the OFC, here we extend this account to implicate cortical regions in regulating the flow of emotionally provocative visual stimulation, and the dysregulation of this network in OCD.
The PCg was central to our findings and worthy of further consideration. The PCg is a highly anatomically connected hub of the brain's default mode network (DMN), which has been used as a biological marker for abnormalities in brain connectivity in a variety of psychiatric disorders such as depression, schizophrenia, autism, ADHD and Alzheimer's disease (Fransson and Marrelec, Reference Fransson and Marrelec2008). The DMN shows increased functional connectivity during ‘rest’ and decreased activity during most tasks where attention is directed externally. However, DMN activity increases when a task requires an internal focus of attention, such as during episodic memory retrieval, daydreaming or introspective thought (Leech and Sharp, Reference Leech and Sharp2014). The lack of emotion-evoked PCg suppression in OCD may therefore imply dysfunction in separating processing of external information from seemingly obligatory cognitive elaboration and behavioral response. Impaired DMN inhibition is theorized to represent a functional reorganization of the brain resulting in overall decreased executive control and may serve as a potential biomarker for a variety of neurological and psychiatric disorders (Chen et al., Reference Chen, Chen, Xie and Li2011). This account is consistent with behavioral research in OCD, where studies suggest that OCD patients have difficulty disengaging attention from threat (Mataix-Cols et al., Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004; Sizino da Victoria et al., Reference Sizino da Victoria, Nascimento and Fontenelle2012), evidenced by the widespread functional networks evoked in OCD relative to controls.
Another potential role of the PCg is in source memory, as OCD patients often report doubt and poor memory (Olson et al., Reference Olson, Hale, Hamilton, Powell, Martin and Savage2016). OCD subtypes such as checking may contribute further to doubt by creating several similar episodes through the repeated checking, thus further reducing confidence in source memory for a particular episode. Olson et al. (Reference Olson, Hale, Hamilton, Powell, Martin and Savage2016) found no behavioral differences between OCD patients and healthy controls, but greater OCD-related activation in the bilateral PCg during source memory retrieval, activation which correlated with symptom severity. Given equivalent performance between the OCD and healthy control group, elevated PCg activity may be compensatory in OCD. However, the ironic consequence of PCg recruitment in memory retrieval may be the hyperactivation of prefrontal regions, increasing obsessions and compulsions rather than alleviating distress.
While the majority of OCD neuroimaging studies do differentiate between symptom subtypes, initial reports from structural and functional neuroimaging studies suggest a distinct neurobiological presentation for each subtype. Albeit at exploratory cluster correction levels, Washers relative to Checkers demonstrated greater provocation-related activity in the bilateral insula and occipital gyrus and had stronger connectivity with the anterior insula for the emotional stimuli. These results are consistent with previous emotion provocation studies of the washing subtype (Mataix-Cols et al., Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004; Jhung et al., Reference Jhung, Ku, Kim, Lee, Kim, An, Kim, Yoon and Lee2014; Okada et al., Reference Okada, Nakao, Sanematsu, Murayama, Honda, Tomita, Togao, Yoshiura and Kanba2015), but differ from a resting state study of OCD subyptes (Harrison et al., Reference Harrison, Pujol, Cardoner, Deus, Alonso, López-Solà, Contreras-Rodríguez, Real, Segalàs, Blanco-Hinojo, Menchon and Soriano-Mas2013), which found no association between contamination subtypes and connectivity, underscoring the importance of the methodological technique used in each investigation. The insula has been associated with perception and expression of disgust (Chapman and Anderson, Reference Chapman and Anderson2012), as well as attention processes, particularly detection of emotionally salient stimuli and affective modulation in response to these stimuli. Indeed, OCD patients may have difficulty switching their attention from an internal focus to an external focus when presented with potentially harmful stimuli (Stern et al., Reference Stern, Fitzgerald, Welsh, Abelson and Taylor2012). Increased stimulus-provoked function connectivity between PCg and the anterior insula in the washer subtype is consistent with the centrality of contamination concerns in the disorder.
At exploratory cluster thresholding levels, Checkers relative to Washers demonstrated greater provocation-related activity in the posterior cingulate and paracentral gyrus and showed stronger emotion-provoked connectivity with motor cortices. Checking-related anxiety has been associated with greater activation in regions important for attention and motor functions (Mataix-Cols et al., Reference Mataix-Cols, Wooderson, Lawrence, Brammer, Speckens and Phillips2004), and is believed to reflect dysfunction of a circuit that plays a role in attention and motor processes, as well as inhibition of unwanted compulsions, rather than emotion processing. A recent study (Richter et al., Reference Richter, de Jesus, Hoppenbrouwers, Daigle, Deluce, Ravindran, Fitzgerald and Daskalakis2012) suggests a more primary role for the motor cortex in OCD, with impairments in the inhibitory neurotransmitter γ–aminobutiric acid (GABA) leading to reduced ability to inhibit intrusive thoughts and impulses, as well as repetitive motor responses. Here, increased functional connectivity to the motor cortex in the checking subtype supports this notion.
Limitations
This investigation has several limitations. Firstly, most of the OCD participants were taking medication at the time of the study and the confounding effects of these medications cannot be completely excluded, although controlling for medication status did not alter present findings. However, since the OCD patients demonstrated hyper-connectivity compared to controls and previous studies have found that medication reduces brain connectivity even in healthy volunteers (McCabe and Mishor, Reference McCabe and Mishor2011) the potential confounding effects of the medication in our study may not have been significant. Secondly, while all patients identified their predominant symptoms as either safety/checking or contamination/washing subtype, some endorsed more than one symptom dimension, which, while clinically realistic, may have reduced the power of the washing v. checking comparisons. Thirdly, the sample size was modest, limiting power to detect more subtle effects, and precluding strong inferences about OCD subtype differences based upon the current sample. Finally, the study employed a general emotion provocation paradigm, which may provoke only mild OCD symptoms in some patients. Use of individualized provocation paradigms for each symptom subtype may have enhanced the findings (Pergamin-Hight et al., Reference Pergamin-Hight, Naim, Bakermans-Kranenburg, van IJzendoorn and Bar-Haim2015), though at the cost of providing equivalent stimulus presentation across the participant groups.
Conclusion
This study is the first to explore functional connectivity in two of the most prevalent OCD symptom dimensions – aggression/checking and contamination/washing. The findings support the existence of distinct neurocognitive phenotypes for OCD symptom dimensions. They also support the suggestion that PCg plays a key role in moderating the impact of visual cortex activation on frontolimbic activity, and thus, the cognitive/behavioral response to emotional stimuli, a function which may be compromised in OCD. Finally, our exploratory analyses described some of the first functional connectivity differences between OCD subtypes, relating checking-related anxiety linked to motor activation and washing-related anxiety to disgust and emotional salience. Taken together, our results suggest that restoring the PCg-mediated filter between sensory representation and cognitive elaboration may be a critical cortical index of OCD pathophysiology, extending existing CSTC models by implicating the PCg in sensory regulation.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719001090
Acknowledgements
The authors gratefully acknowledge the Grant family, whose generous donation to the CAMH Foundation for research on OCD treatments provided funding for this study.
Funding disclosure
This study was funded by a private donation by the Grant family to the CAMH Foundation, for research on OCD treatments.
Conflict of interest
The authors have no conflicts of interest to disclose.






