Sea otters were once common around the North Pacific, with a population between 100,000 and 150,000 (Kenyon Reference Kenyon1969:198). Indigenous peoples across the region—including Ainu, Kamchadals, Aleut, Sugpiaq, Alutiiq, Tlingit, Haida, Nuu-chah-nulth, Makah, and groups south to Baja California—have hunted sea otters over thousands of years (Corbett et al. Reference Corbett, Causey, Clementz, Koch, Doroff, Lefevre, West, Rick and Erlandson2008; Fedje et al. Reference Fedje, Mackie, Wigen, Mackie, Lake, Fedje and Mathewes2005; McKechnie and Wigen Reference McKechnie, Wigen, Braje and Rick2011; Ravalli Reference Ravalli2018; Rick et al. Reference Rick, Braje, DeLong, Braje and Rick2011; Salomon et al. Reference Salomon, Huntington and Tanape2011, Reference Salomon, Wilson, White, Tanape, Happynook, Larson, Bodkin and VanBlaricom2015). Because sea otters have thick, luxurious fur, they were the primary target of the maritime fur trade that originated in northeast Asia in the fifteenth century and that accelerated with the Russians moving eastward to North America following Bering's 1741 expedition (Jones Reference Jones2014; Ravalli Reference Ravalli2018:4). Commercial exploitation resulted in the serial depletion of sea otters across the North Pacific coast and near extirpation of the species (Bodkin Reference Bodkin, Larson, Bodkin and VanBlaricom2015; Lensink Reference Lensink1962:9–15). In 1911, sea otters were afforded protection under the International Fur Seal Treaty, but by that time, fewer than 2,000 animals remained in 13 remnant colonies. These were located in the Kuril Islands, Kamchatka Peninsula, and Commander Islands in Russia; in the Aleutian Islands, Alaska Peninsula, Kodiak Island, and Prince William Sound in Alaska; in scattered remnants on Haida Gwaii in Canada and in central California; and around the San Benito Islands of Mexico (Bodkin Reference Bodkin, Larson, Bodkin and VanBlaricom2015:47).
Today, sea otters are distributed patchily along the Pacific coast, despite protections offered through the U.S. Marine Mammal Protection Act (MMPA) of 1972 and Endangered Species Act of 1973, Canada's Protection of Species at Risk Act of 2002, conservation status in Russia, and the Law of Hunting Control of Sea Otters and Fur Seal in Japan (Davis et al. Reference Davis, Bodkin, Coletti, Monson, Larson, Carswell and Nichol2019:2). Three regional subspecies of Enhydra lutris have been defined: E. l. lutris in Asia, ranging from Hokkaido to the Kuril Islands, Kamchatka Peninsula, and Commander Islands; E. l. kenyoni, ranging from the Aleutian Islands to Prince William Sound, Alaska, and along the Pacific coast of Canada into Oregon; and E. l. nereis, ranging from central California to Baja (Cronin et al. Reference Cronin, Bodkin, Ballachey, Estes and Patton1996). In some places, sea otter recovery is well underway (e.g., southeast Alaska), while in other places, sea otters remain absent (e.g., Oregon). The southern sea otter (E. l. nereis) in California and the southwest-Alaska stock of E. l. kenyoni are listed as threatened under the Endangered Species Act, whereas E. l. kenyoni stocks in southcentral and southeast Alaska are not listed. In Canada, E. l. kenyoni is listed as a species of “special concern” under the Species at Risk Act. Each population has been subject to different conservation challenges. For example, sea otters in the Aleutians have suffered heavy predation by killer whales (Doroff and Burdin Reference Doroff and Burdin2015:2; Estes et al. Reference Estes, Bodkin, Ben-David, Perrin, Würsig and Thewissen2008:810), whereas some populations in central California are struggling to expand because of density-dependent resource limitations (Tinker, Tomoleoni et al. Reference Tinker, Tomoleoni, Weitzman, Staedler, Jessup, Murray, Miller, Burgess, Bowen, Miles, Thometz, Tarjan, Golson, Batac, Dodd, Berberich, Kunz, Bentall, Fujii, Nicholson, Newsome, Melli, LaRoche, MacCormick, Johnson, Henkel, Kreuder-Johnson and Conrad2019:220).
Although some coastal communities are considering reintroducing sea otters, others are experiencing negative consequences from growing sea otter populations. In conservation efforts to restore ecosystems across the North Pacific, archaeologists are well positioned to remind agencies, regulators, and the general public that Indigenous peoples have maintained relationships with sea otters over thousands of years. The nature of those relationships, however, varied substantially across space and time, and these specifics are relevant to local and subregional management decisions.
In southeast Alaska, the commercial fur trade forced the extirpation of sea otters by the mid- to late 1830s (Hoyt Reference Hoyt2015:66). Because sea otters are relatively sedentary (with male home ranges of 1–24 km2 and female home ranges of 1–11 km2; Bodkin Reference Bodkin, Larson, Bodkin and VanBlaricom2015:47), spatially concentrated hunting can lead to localized depletions or extirpation. Kashevarov (Reference Kashevarov, Black, Dauenhauer, Dauenhauer and Black2008 [1822]:98) estimated that Russian-sponsored Aleut hunters took 12,900 sea otters from the vicinity of Sitka between 1799 and 1803. Yet between 1844 and 1849, fewer than 500 sea otters were taken from all of Alaska (Bodkin Reference Bodkin, Larson, Bodkin and VanBlaricom2015:46).
Alaska's sea otter population is now divided into three stocks: southwest Alaska (Aleutians), southcentral Alaska, and southeast Alaska. Focusing on Tlingit territory, here we are concerned with the southeast Alaska stock. Between 1965 and 1969, the Alaska Department of Fish and Game (ADFG) translocated 413 sea otters from Prince William Sound and Amchitka Island to Yakobi Island, Chichagof Island, Baranof Island, Prince of Wales Island, and Cape Spencer in southeast Alaska. As Carswell and colleagues (Reference Carswell, Speckman, Gill, Larson, Bodkin and VanBlaricom2015) explained,
although neither the State of Alaska nor USFWS [U.S. Fish and Wildlife Service] documented clear objectives for the reintroductions, both the translocations and the “experimental harvests” that occurred in the 1960s (which yielded 1,000 pelts for public auction in 1968, the first that had occurred since 1911) were apparently viewed in the practical context of game management, with incidental benefits for scientific study [Carswell et al. Reference Carswell, Speckman, Gill, Larson, Bodkin and VanBlaricom2015:350–351].
Not until the last 25 years has sea otter population expansion been experienced as an economic threat to some fishers. Carswell and colleagues (Reference Carswell, Speckman, Gill, Larson, Bodkin and VanBlaricom2015:352) traced the history of the sea otter–shellfish fishery conflict and the impact of the 2005 McDowell report, which estimated that sea otters caused the loss of $11.2 million in economic activity between 1996 and 2005 because of their predation of invertebrates. As of 2014, there were approximately 26,000 sea otters in southeast Alaska (USFWS 2014:5), and today, sea otters compete with commercial fishers for abalone, geoduck clam, Dungeness crab, red sea urchin, and sea cucumber (Hoyt et al. Reference Hoyt, Eckert, Gill and Rice2014; Larson et al. Reference Larson, Hoyt, Eckert and Gill2013). Subsistence harvesters also pursue abalone, sea cucumbers, and Dungeness crab, in addition to butter clams, cockles, and chitons—which are also eaten by sea otters—and they are likewise affected by the resurgence of sea otters in some locations. Yet some people stress ecosystem benefits; as a top predator, the sea otter has removed grazing invertebrates, permitting kelp forest and seagrass habitats to expand (Estes Reference Estes, Larson, Bodkin and VanBlaricom2015; Hughes et al. Reference Hughes, Eby, Van Dyke, Tim Tinker, Marks, Johnson and Wasson2013).
The federal MMPA allows for any Alaska Native (who resides on the coast) to harvest sea otters for subsistence, provided the harvest is not wasteful. Only Alaska Natives can buy and trade raw pelts or other sea otter parts with each other, and they can make and sell handicrafts out of sea otter fur and other parts. Today, sea otter pelts are used to make clothing, bedding, hats, dance blankets, and other regalia, as well as to trim ceremonial garments. The U.S. Fish and Wildlife Service (USFWS) is responsible for sea otter management, and it has promulgated regulations and guidelines governing the production of sea otter handicrafts. The Southeast Alaska Regional Dive Fisheries Association (representing harvest divers and processors) is one organization lobbying for increasing the scale of the hunt. Some would like to see sea otter hunting reach the potential biological removal (PBR) level. The MMPA defines the PBR as the maximum number of animals (not including natural mortalities) that may be removed from a marine mammal stock while allowing that stock to reach or maintain its optimum sustainable population. For southeast Alaska, the PBR is 2,179 (USFWS 2014:8). Subsistence hunters in southeast Alaska took 850 sea otters in 2012 and 1,500 in 2013 (Carswell et al. Reference Carswell, Speckman, Gill, Larson, Bodkin and VanBlaricom2015:361), well below the PBR. Tinker, Gill, and colleagues (Reference Tinker, Gill, Esslinger, Bodkin, Monk, Mangel, Monson, Raymond and Kissling2019:1073) project that the carrying capacity for sea otters in southeast Alaska is 74,650.
Some conservationists are currently seeking to define “traditional” Tlingit use of sea otters as not only utilizing their pelts but as consuming them as food: in their view, both of these conditions have to be met before Alaska Natives would be entitled to the exemption to the MMPA to harvest sea otters (Charles Smythe, personal communication 2015). Although some Alaska Natives do eat sea otter, some Tlingit say they never did, and clearly, sea otters were not available for taking during their parents’ and grandparents’ time, so there is a gap in transmission of culinary knowledge. Zooarchaeological data can shed light on these issues. Negotiations regarding sea otter hunting should take into consideration the long-term history of the relationship of Tlingit people to sea otters that can be revealed from the archaeological record. As will be shown, Tlingit ancestors have been managing sea otters as part of larger marine ecosystems for thousands of years.
Across the North Pacific, other local communities are interested in how sea otters affect marine ecosystems. Although sea otters consume vast quantities of shellfish, their removal of sea urchins from subtidal rocky reefs benefits the development of kelp forests and the fishes and fisheries they support (Davis et al. Reference Davis, Bodkin, Coletti, Monson, Larson, Carswell and Nichol2019). Although hunting sea otters is prohibited in British Columbia, Washington, Oregon, and California, some First Nations (e.g., Ka:‘yu:‘k't'h’/Che:k'tles7et'h’ First Nation; Pinkerton et al. Reference Pinkerton, Salomon and Dragon2019) would like to resume hunting, and others might be interested, should sea otter populations expand to the extent that they could be hunted sustainably. Whether or not Canadian First Nations consumed sea otters as food has not been explicitly examined archaeologically (e.g., Szpak et al. Reference Szpak, Orchard, McKechnie and Gröcke2012). Although Gibson (Reference Gibson1992:7) suggested that First Nations did eat sea otter, contemporary groups appear to be primarily interested in acquiring pelts and controlling sea otter populations to protect important macroinvertebrates near their settlements (Pinkerton et al. Reference Pinkerton, Salomon and Dragon2019; Salomon et al. Reference Salomon, Wilson, White, Tanape, Happynook, Larson, Bodkin and VanBlaricom2015).
The current project grew out of a conversation with Rosita Worl in her office at Sealaska Heritage Institute (SHI),Footnote 1 where she described challenges that SHI was experiencing as it sponsored skin-sewing workshops in the face of public sentiment against sea otter hunting. This article explores the longer-term history of sea otter use by posing the simple question: did Tlingit people eat sea otters during the precontact period? This question can be addressed through zooarchaeology, and SHI has supported this effort to better understand the relationships between Tlingit ancestors and sea otters over the long period of time in which they have been interacting. SHI supports the rights of Alaska Natives to manufacture handicrafts—specifically, producers who live in villages who have few economic opportunities. SHI also aims to educate the general public, believing that if it and resource managers better understood long-standing cultural practices, the rights that derive from this history could be afforded the respect they deserve. This new information can help Tlingit and Haida groups regain control over their resources and reestablish their relationship with sea otters. It is hoped that this research can illustrate how modern zooarchaeological work can contribute to the decolonization of subsistence regulations (Moss Reference Moss2010) and to the respect of cultural rights and traditional knowledge.
Ethnographic Background
In the Tlingit moral universe, all animals have souls and were once human beings (de Laguna Reference de Laguna1972:823–826). Sea otters, however, are rarely depicted in Tlingit art, and they are not typically used as a crest animal. Many animals are associated with the Raven and/or Wolf-Eagle moieties and are displayed in clan regalia. These include mammals (wolf, sea lion, seal, brown bear, black bear, porpoise, killer whale, whale, dog, land otter,Footnote 2 marten, beaver, mouse, cow), birds (raven, eagle, golden eagle, thunderbird, goose, swan, puffin, murrelet, petrel, owl, flicker), fish (sockeye salmon, coho salmon, dog salmon, shark, halibut, herring, dogfish, needlefish, bullhead, black sea bass), amphibians (frog), and invertebrates (devilfish [octopus], woodworm, snail, starfish, king crab, giant clam; Hope Reference Hope2003; Swanton Reference Swanton1908). The animals most commonly associated with shamanism include land otter, devilfish, raven, frog, mountain goat, oystercatcher, and crane.
The most prominent of the shamanic animals is the land otter, a being that occurs frequently in Tlingit literature. The Tlingit view land otter as the “single most powerful supernatural in their universe” (Jonaitis Reference Jonaitis1986:90). One way land otter wields its power is to transform into a person (typically “Land Otter Man,” Kóoshdaa kaa) and back again. Some land otters were transformed persons who had been lost in boating accidents, drowned, and then captured and transformed (de Laguna Reference de Laguna1972:744–745). Whereas land otters were deeply feared and respected by laypeople, they were sought out by Tlingit shamans. Traditionally, the land otter was the shaman's most potent spirit helper, or yéik.
Biologically, the land otter is closely related to the sea otter; both are in the mustelid family, but they are different species. The sea otter is larger, and it has thicker vibrissae, larger flipper-like hind feet, and a short, less tapered tail. The two species have distinctive patterns of abundance, geographic distribution, and different behaviors and life histories. The prominence of land otter in Tlingit literature and its association with shamanism is the most significant difference. A review of the more than 3,500 place names in Thornton's (Reference Thornton2012) atlas, Our Grandparents’ Names of the Land, revealed 20 names associated with land otters and 14 associated with sea otters. This insignificant difference suggests that people were equally aware of and knowledgeable about both species. Although the land otter was hunted after 1840, there were taboos against eating it (Emmons Reference Emmons1991:54, 136). No such taboos precluding the consumption of sea otter meat are known.
Archaeological Background
Even though sea otters were once common in southeast Alaska, patterns of their precontact abundance and distribution are largely unknown. Zooarchaeological remains provide a record of animal distribution and abundance over varying temporal and spatial scales. In the Herring Synthesis Project (Moss et al. Reference Moss, Butler, Elder, Moss and Cannon2011; Thornton, Butler et al. Reference Thornton, Butler, Funk, Moss, Hebert and Elder2010; Thornton, Moss et al. Reference Thornton, Moss, Butler, Hebert and Funk2010), we compiled zooarchaeological data on animal abundances in archaeological assemblages dating from approximately 8000 cal BP to the historic era. At least 16 archaeological sites in southeast Alaska have yielded sea otter bones (Table 1). The oldest evidence of sea otter use by Tlingit ancestors derives from Coffman Cove on Prince of Wales Island, where sea otter bones date to roughly 4000 cal BP (Moss et al. Reference Moss, Hays, Bowers and Reger2016). People have been hunting sea otter even longer based on sea otter bones from Kilgii Gwaay in Haida territory that date to more than 10,600 cal BP (Fedje et al. Reference Fedje, Mackie, Wigen, Mackie, Lake, Fedje and Mathewes2005). Sea otters were hunted over millennia continuing to the historic era. Although the number of bones identified from most sites is small, these data have not been analyzed in a way that can yield new information relevant to some of today's sea otter management challenges.
Table 1. Southeast Alaskan Archaeological Sites with Sea Otter Bones.
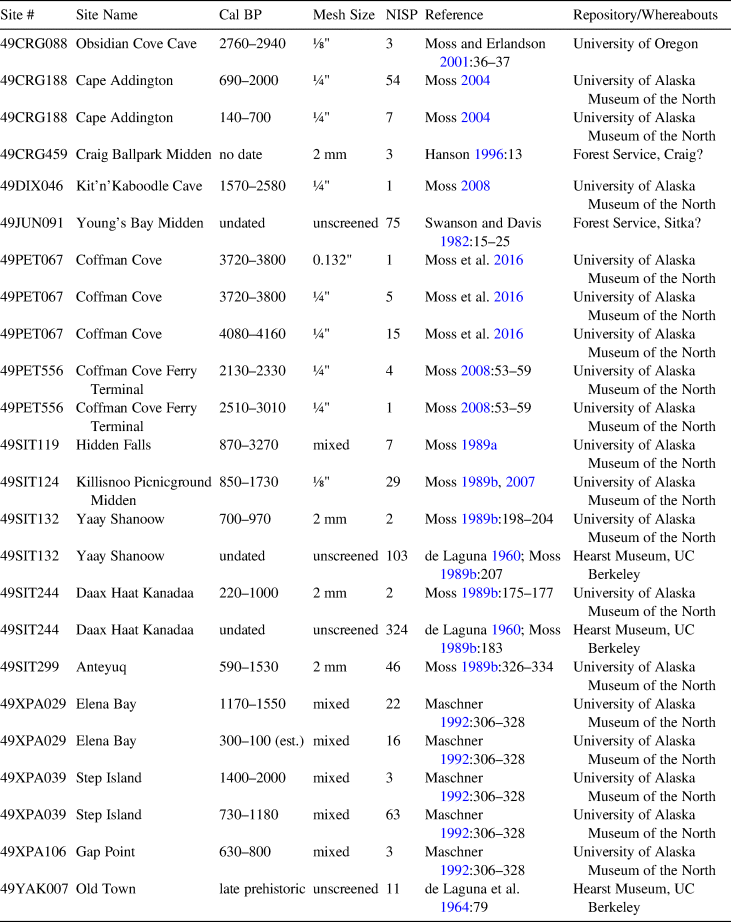
The largest collections of sea otter bones in southeast Alaska derive from two archaeological sites near Angoon: Daax Haat Kanadaa (49SIT244) and Yaay Shanoow (49SIT132). Both sites were excavated by Frederica de Laguna (Bryn Mawr College) and her team in 1950. The artifact collections are held by the University of Pennsylvania Museum of Archaeology and Anthropology, whereas the faunal remains reside at the Phoebe A. Hearst Museum at the University of California, Berkeley. Initially, de Laguna traveled to northern Tlingit territory looking for a place where she could conduct an “integrated program of archaeological, ethnological, and acculturation studies” (de Laguna Reference de Laguna1960:ix). Further, she sought a community “sufficiently integrated to exhibit a coherent social life and sufficiently old-fashioned to have retained some institutions derived from the aboriginal culture” (de Laguna Reference de Laguna1960:ix). In 1949, she selected Angoon and Yakutat for further work. In 1950, she and Catherine McClellan did ethnological work in Angoon, while Francis Riddell (then a graduate student at UC Berkeley) and Lloyd Collins (a graduate student at University of Oregon) started excavations. De Laguna and McClellan worked on the excavations in the last weeks of the summer of 1950. The faunal identifications listed in the appendix of de Laguna (Reference de Laguna1960) and in the original Hearst Museum catalog were made by Francis Riddell, assisted by Sheilagh Thompson, J. Arthur Freed, and Dr. Seth Benson, Curator of Mammals, Museum of Vertebrate Zoology, UC Berkeley (de Laguna Reference de Laguna1960:93).
In the mid-1980s, I excavated column samples at these two sites to quantify fish and shellfish remains that had not been adequately sampled in 1950, in pursuit of understanding broader patterns in the subsistence economy (Moss Reference Moss1989b). I radiocarbon-dated the sites for the first time and demonstrated that they were occupied between 900 and 300 cal BP (Moss Reference Moss1989b). The two sites were probably occupied contemporaneously, as these two fort sitesFootnote 3 are associated with two intermarried clans, and they are located only 250 m away from each other (de Laguna Reference de Laguna1960; Moss Reference Moss1989b). After having tested these sites, I went to UC Berkeley in 1985 and obtained the handwritten catalog of identifications because all that was published were animal bone counts in the appendix of the 1960 publication. I summarized the information from the catalog in my 1989 dissertation but did not examine the faunal remains firsthand.
Methods—Collections Research and Ethnoarchaeology
Until mid-July 2014, the 1949–1950 faunal materials had been in their original bags in the basement of the museum's facilities in the Hearst Memorial Gymnasium on the UC Berkeley campus. Before my visit, Hearst Museum personnel rebagged and relabeled them in preparation for the move out of the Hearst Gymnasium to new facilities at the Berkeley Global Campus in Richmond Bay. I was able to study these materials before they went into long-term, inaccessible storage during the museum's move. With financial support from IPinCH (the Intellectual Property Issues in Cultural Heritage Project, Simon Fraser University), I studied the collections during the summer of 2014.
I examined the bones from the Angoon sites to identify cutmarks indicative of butchery for food and/or skinning for furs, and to distinguish between these uses. This information is interpreted with the help of ethnoarchaeology and traditional ecological and ethnographic knowledge. Sea otters and seals (Phoca vitulina) are the two most common mammals found at the Angoon sites, making up 49% and 21% of the assemblage, respectively, by NISP.Footnote 4 As will become clear, people were bringing whole sea otters and seals to these sites, where they were processed. Based on the prevalence of these two species and the proximity of the two sites, I lumped the assemblages together. Hunters appear to have been taking seals and sea otters from the waters adjacent to the sites.
Since people were undoubtedly consuming seal represented in these two assemblages, I compared seal and sea otter remains, looking at skeletal representation, cutmarks, and dog damage (gnawing). My initial assumption was that if patterns of skeletal representation and cutmarks on seals and sea otters were similar, then one could infer that people were eating sea otters.
In the collection of 940 bones from the two sites, I examined 461 sea otter and 195 seal specimens. I used a hand-held double lens magnifier (8x) under strong light to examine each specimen. I recorded modifications on illustrations (templates) of sea otter and seal elements (Post Reference Post2004, Reference Post2006), described these on excel spreadsheets, and documented them in 516 photographs. The modifications included burning, calcination, and the number, orientation and anatomical placement of cutmarks. I recorded pre-depositional damage due to dog chewing and gnawing. I noted the number of carnivore tooth punctures and what element portions had been chewed or chewed off.
Nearly all of the faunal remains from these two sites have weathered substantially since their initial collection by de Laguna's team. It is understandable that many of these specimens (which have been out of the ground for ~70 years) have dried out, weathered, split and cracked longitudinally, exfoliated, and crumbled. Because these specimens were stored in their original bags in a facility that also hosts a swimming pool, it seems possible that some quantity or concentration of airborne gases affected the condition of faunal materials that were contained in permeable paper bags until mid-2014.
Binford (Reference Binford1981) developed a functional typology of cutmarks based on 108 anatomical locations and their orientations, primarily on caribou. He generalized that cutmarks are left in sequential order: skinning, dismemberment, filleting, and marrow removal. Skinning is indicated by cutmarks left on “points of entry,” such as the head and the lower limbs, metapodials, and phalanges. Dismemberment or disarticulation marks usually occur at primary joints. Filleting marks include longitudinally oriented cuts and short oblique cuts, made to free the meat from the bone or sever muscle insertions. These cutmark types were identified ethnoarchaeologically on caribou, but also on moose, bear, and mountain sheep. Binford's (Reference Binford1981) illustrations are mostly of caribou, and before I traveled to Berkeley, I took his numbering system and descriptions of cutmarks and adapted them to templates of sea otter and seal bones. I intended to use this system to diagnose the function of each cutmark. Yet because the placement and orientation of cutmarks on the sea otter and seal bones did not easily match those documented by Binford, I abandoned this approach. I nonetheless recorded every cutmark on a template and photographed every cutmarked bone. I recorded gnawing, but I did not photograph every gnawed bone.
After I collected the primary data and considered skeletal element representation, it became clear that I needed to learn more about skinning sea otters for pelts and butchering for meat. Working with Sealaska Heritage Institute, I pursued these through ethnoarchaeology. In 2016, I observed Tlingit hunter Kyle Barry skin a sea otter, a process documented by SHI videographer Kathy Dye. Mr. Barry did a skillful job, removing the sea otter pelt without making any holes in it. Mr. Barry's mother, Louise Kadinger, is the person who taught her son how to skin a sea otter, and she will use this sea otter pelt in sewing. I received authorization from the USFWS to obtain the sea otter carcass. At the University of Oregon, the carcass was fed to dermestid beetles, and it has been further macerated and degreased. I examined all of the bones and described and photographed each cutmark made during the skinning process. After repeated viewing of the video, I was able to isolate one or a few times during skinning when each cutmark was made by Mr. Barry. I also researched seal skinning and butchery to better understand how cultural practices incorporate knowledge of seal anatomy. Tlingit ancestors had deep experiential knowledge of the biology of all the animals they used, although here we focus on sea otters and seals.
Results—Skeletal Representation, Cutmarks, and Dog Damage
Of 940 cataloged vertebrate remains from these sites, 461 are sea otter. Femora provided an MNI of 25, with 15 adults, 1 juvenile, and 9 pups represented. Among the 195 seal bones, scapulae provided an MNI of 14, with 6 adults and 8 pups represented. The presence of seal pups indicates occupation of the sites during the summer, probably July or August. Skeletal element survivorship was scaled to 25 for sea otters and 14 for seals. Sea otters are shown in the top of Figure 1, with elements ordered by MNE. The same element order is used for the seals in the bottom of Figure 1 to show the differences in element survivorship. Sea otter limb bones (femur, tibia, humerus, ulna) survive at higher rates than seal limb bones. The scapula was the element with the highest survival among seals.

Figure 1. Element survivorship (MNE) of sea otter and seal bones from Angoon sites scaled to 25 individual sea otters and 14 individual seals.
Figure 2 depicts the percentage NISP of cutmarked bones. Here, elements are listed in order of the seal meat utility index (Lyman et al. Reference Lyman, Savelle and Whitridge1992) to demonstrate the differences between seals and sea otters.Footnote 5 Of the seal bones, 23 of 179 are cutmarked; 55 of 431 sea otter bones are cutmarked. About 13% of both seal and sea otter bones are cutmarked. The intensity of butchery is roughly the same, with 4.3 cutmarks per specimen. Which elements are cut differs, however: (1) higher proportions of seal pelvises and femora are cutmarked (50–70%) than of sea otters, (2) twice the percentage of sea otter humeri are cut compared to seals, and (3) higher proportions of sea otter ribs, metatarsals, radii, and mandibles are cutmarked (5–14%) than of seals. Overall, 13 different sea otter elements (including the baculum, not shown here) are cutmarked as opposed to eight seal elements.

Figure 2. Percent NISP of cutmarked skeletal elements of sea otter and seal from Angoon sites.
In assessing carnivore damage, a bone portion cannot be expected to exhibit cutmarks if it is absent—that is, if it has been chewed away. The percentage of gnawed elements indicates the intensity of carnivore damage. Figure 3 illustrates the frequency of dog-damaged bones. The sea otter element with the highest frequency of gnawing was the scapula, but more than half of the following were chewed: radii, femora, vertebrae, fibulae, humeri, ulnae, and pelvises. In this figure, the elements are ordered by frequency with which sea otter bones were damaged by dogs. Seal bones, however, were also heavily chewed by dogs. More than 90% of seal femora, vertebrae, humeri, and pelvises were chewed—and ribs, metatarsals, and mandibles were chewed more intensively compared to those of sea otters. Lyman and colleagues (Reference Lyman, Savelle and Whitridge1992:549) observed that in Oregon Coast sites, very few marine mammal bones exhibit dog damage, whereas in Arctic sites, dog damage is common. Ethnographically, Inuit and Iñupiat routinely fed dogs seal meat (Boas Reference Boas1964:109; Henshaw Reference Henshaw2000:64–65). Yet Kopperl (Reference Kopperl2003:198) found little evidence of carnivore gnawing on Alutiiq marine mammal bones on Kodiak. Many factors may explain this variability, but it appears that the residents of these Angoon sites fed bones to dogs or allowed dogs to feed on seal and sea otter bones.

Figure 3. Percent of sea otter and seal elements gnawed by dogs.
The different patterns of skeletal element representation, cutmarks, and dog damage suggest that Angoon Tlingit ancestors processed sea otters and seals differently. This analysis, however, did not provide a straightforward answer to the question of whether or not residents of the Angoon sites consumed sea otter meat.
Results—Ethnoarchaeology of Skinning and Butchering
Sea Otters
Sea otters are substantially smaller than harbor seals. An adult sea otter weighs 30 kg, while an adult seal weighs 82 kg. A sea otter pup weighs less than 2 kg, while a seal pup weighs 11 kg. Seal anatomy differs from that of the sea otter, which is the only North American mustelid considered a marine mammal. Virtually all the fat in a seal is in the blubber, which occurs in a distinct layer just under the skin but above the muscles and viscera. Unlike seals, sea otters have little body fat, with their insulation coming from their thick layer of fur. SHI consultants explained to me that a seal's shoulder and rump are much meatier than a sea otter's shoulder and rump, whereas a sea otter's backstrap (muscle from the lower back that runs along the spine) and ribs may be preferred for consumption. The differences in anatomy in the skeleton and musculature affect how the animals are skinned and how (and if) meat is (or was) butchered.
In 2016, I observed hunter Kyle Barry skin a sea otter using an open skinning method. In open skinning, the animal is opened up with cuts across the belly so the skin can be removed like unzipping and taking off a jacket.Footnote 6 Mr. Barry made cuts encircling the hind foot and then worked up to the vent to the other foot, which was again encircled. He then split the tail with a hook and knife and worked the tail and legs free of the pelt. Mr. Barry then cut through the skin of the torso from vent to chin, removing the pelt from the fascia toward both right and left sides. He then made cuts encircling each fore foot and up along the forelimbs to the chin, working the pelt away from the fascia. Mr. Barry turned the carcass on its belly to work the pelt away from the back of the sea otter. Then, he worked from the chin up on each side of the face and across the back of the skull to remove the pelt. There was not a single nick in the removed pelt. Finally, Mr. Barry gutted the animal at my request, although this would not have been done under normal circumstances. Today, most sea otters are skinned aboard a boat or onshore near where they are killed. Their carcasses are usually left behind to feed other marine animals, and only rarely are they brought back to town to process.
Consultants in Juneau had told me that if a sea otter is skinned properly, there should not be any cutmarks on the bones. Mr. Barry was skillful and careful, removing the pelt from the sea otter without making any holes in it. After I was able to study the cleaned bones, I identified cutmarks on various elements as shown in Figure 4. Even though Mr. Barry used a sharpened steel skinning knife and most of his short strokes were oriented away from the bones, cutmarks were made, but not just at points of entry. There were no cuts between the skull and the axis vertebra (where predicted following Binford [Reference Binford1981] and Lyman [Reference Lyman1987]). Cuts on or near the long bone ends were not for disarticulating the carcass; Mr. Barry kept the carcass intact. The video shows that cuts near the joints resulted from applying leverage to remove the pelt. Using standard typologies, such marks would be misdiagnosed as dismemberment or disarticulation, not skinning. Binford acknowledged that “skinning for skins differs from skinning as a stage of butchering” (Reference Binford1981:107).

Figure 4. Cutmark locations on sea otter skinned by Mr. Kyle Barry in July 2016.
Supplemental Table 1 presents information on the cutmarks made in 2016. In some cases, it is possible to isolate exactly when a cut was made. In other cases, this can be narrowed down to two or three times during the video. As an example, the sternal cuts and that on the second rib were made at about 20 minutes into the skinning process. Supplementary Figure 1 illustrates how the cuts differ in morphology even though they were made with the same steel knife within minutes of each other. The cut on the sternum is a single sharp line, whereas the cut on the rib is coarse and deep because it was made through porous bone at the sternal end. The cut on the lowest sternebra represents multiple knife strokes.
Cutting down through sea otter fur dulls the knife. The knife stays sharper if, after the initial cut, the person skinning the animal cuts up through the skin from the inside of the animal. Mr. Barry cut down through the fur occasionally, and this sometimes resulted in cutmarks on bone. Although Mr. Barry usually cut away from the bone, he did not do so (1) around the ankles, (2) working atop chest, (3) around the wrists, and (4) around the skull and mandible. This resulted in cuts around the paws, the sternum (top and bottom), and the rib but not the head. Mr. Barry used the knife as leverage to remove the pelt around sections of the carcass, which resulted in cuts around the paws, the femur, and perhaps the pelvis. The forelimbs were hard to work around because they were stiff and drawn in toward the chest. Because sea otter skin is very tight around the forelimbs, a processor has to cut entirely around the fascia surrounding the limb bones to loosen and slip the limbs free of the pelt. Somewhat surprisingly, there were no cuts on the back of the skull, which Mr. Barry had to follow very closely in order to remove the pelt from the head. The carcass retained the hide on its paws. Even though Mr. Barry carefully avoided cutting into muscle tissue, there were cutmarks on bones.
I recognize that this was a single skinning event and that Mr. Barry used a steel knife, not a sharpened stone or shell tool. As such, I do not claim that all sea otters were skinned in this way during precontact times. Furthermore, I did not observe a person butchering a sea otter for the purpose of obtaining meat. Ideally, one would be able to observe many such skinning and butchery events, but because of wildlife regulations and animal welfare sensitivities, this was not feasible. Yet, this single skinning event opened up the possibility that cutmarks observed archaeologically might be interpreted in new ways. In recognizing the limitations of this study, I do not claim that the inferences described below rule out other possible explanations.
Returning to the archaeological sea otter specimens from the Angoon sites, 51 cutmarks on the mandible, lower limbs (radius, ulna, tibia, and fibula) and paws (metapodials, carpals, and tarsals) all could be made from skinning. Most of the cutmarks on femora and humeri (74 of 100) occur on or near the proximal or distal ends, suggesting use of the cutting tool as leverage to remove the pelt. Three femora exhibit 26 cutmarks on their shafts, and these cuts may represent muscle-stripping, but whether this was for human consumption or to feed to dogs is unclear. Cutmarks on caudal (8) and atlas (11) vertebrae likely result from skinning.
Seventy-four cuts were made on ribs, vertebrae, and pelves. Because many of these cuts were near the head or neck of the ribs, and vertebral cuts occurred all over the centra and various processes, the evidence indicates that the residents of the Angoon sites were butchering these portions of the axial skeleton of some sea otters to provide the backstrap to feed either themselves or their dogs.
In my exchanges with Peter Kawagaelg Williams, a skilled Yup'ik sea otter hunter who lives in Sitka, he described sea otter backstrap as delicious, and he cooks it by panfrying as one would a steak. In contrast, Lee Kadinger of Juneau described sea otter meat as very tough, stating that it is edible only after it has been in a slow cooker for a long time with onions, celery, wine, and other aromatics. De Laguna (Reference de Laguna1972) wrote of the Yakutat Tlingit:
people formerly ate sea otter meat, which is said to taste different from seal meat. If fresh, it spoils in three days’ time, but is good when a day old. It can be preserved for a long time, “two years,” by boiling it, smoking it, and putting it in a five-gallon can covered with seal oil. It must be covered by about 2 inches of oil. If a piece sticks out above the oil, then it spoils quickly [de Laguna Reference de Laguna1972:398; emphasis added].
De Laguna did not specify if people preferred one cut of sea otter meat to another, and boiling was considerably easier in the twentieth century than it would have been in precontact times in the absence of metal or ceramic cooking and storage vessels. Stone boiling in baskets or boxes for a long time would be labor intensive. Pit roasting, baking, or steaming are other possible cooking methods that could have been used in the ancient past.
Seals
Although there are many ways to skin a seal, it can be done more quickly than skinning a sea otter. Although it took Mr. Barry nearly an hour of careful work to skin a sea otter, Eskimo contestants in seal-skinning competitions can skin a seal in under four minutes (World Eskimo-Indian Olympics 2018). A seal is more “sausage-shaped” than a sea otter, with a much shorter tail. It is easier to skin a seal than a sea otter because its limbs do not protrude as far from the body. The layer of seal blubber between the skin and the flesh means that the skin will pull away more easily. The fastest method of skinning a seal is the open skinning method as used in seal-skinning contests. It is harder and takes longer to case skin a seal, which involves hanging the seal upside down by its feet, making a cut in one foot, and continuing up the leg, around the anus and down the other leg. Then, the skin is pulled down the animal as though removing a sweater. A seal can also be case skinned by hanging it head-side up, with a circular cut made around the head. These methods are still employed by Iñupiat and Yupiit people to make sealskin floats (used in marine hunting) or seal pokes (bags) for food storage (Fienup-Riordan Reference Fienup-Riordan2007:145; Foote Reference Foote1992; Frink and Giordano Reference Frink and Giordano2015).
Although skinning a seal may be easier than skinning a sea otter, butchering a seal for meat takes significant amounts of skill, time, and labor (Moss Reference Moss2016). De Laguna (Reference de Laguna1972:395–398) observed Minnie Johnson butcher a female seal and two pups in the summer of 1952 or 1954. After the skin and blubber were removed, the flippers could be disarticulated and the entrails removed. Mrs. Johnson saved only the liver, kidneys, and heart. She explained that the stomach, lungs, and small intestine also would have been saved in the past. She disarticulated the head and discarded it, although parts of this would also have been eaten in the past. The shoulders and thighs were disarticulated, with the latter given to de Laguna to feed to her dog. With a hatchet, Mrs. Johnson chopped one side of the ribs from the backbone. De Laguna wrote that “this is done only with a young seal, for the backbone of an old seal is cut free of the ribs. The old people are said to like the backbone” (Reference de Laguna1972:396). Although she did not save the flippers in this case, Mrs. Johnson roasted the flippers of other seals in the oven in their skins. Mrs. Johnson explained, “You peel them after you cook them. Taste like pig's feet” (de Laguna Reference de Laguna1972:397). Flippers could be singed to remove the hair, cooked, and stored in oil. About this, de Laguna cited Grinnell who wrote in 1899, “Flippers are regarded as especially choice” (Reference de Laguna1972:397).
The Canadian company SeaDNA is marketing the meat of harp seals (Pagophilus groenlandicus) and grey seals (Halichoerus grypus; SeaDNA 2019). Both of these Atlantic species are larger than the harbor seals taken by Angoon Tlingit, with adult harp seals weighing 130 kg and adult grey seals ranging from 100 to 310 kg. The seal-meat cuts marketed include adult loins, “veal” loins (presumably juveniles), flippers (foreflipper only), and “trims.” Burger meat, sausages, salami, terrines, and other processed meat products (e.g., merguez, péperettes, rillettes) are also sold. Although the butchery process is not described on the company website, the marketed cuts surely represent portions of seals that are preferred for their meat value. Interestingly, the foreflippers are cut at the humeral head, but the website photo appears to indicate that only the humerus and radius-ulna are included, not the bones and meat below the wrist (SeaDNA 2019). No adult seal humeri from the Angoon sites show cutmarks indicating they were disarticulated in this way. Only one humerus is cut, and that pup bone suggests stripping for meat along the shaft, not disarticulation. Why SeaDNA does not sell the hindflipper is not explained, but recall that Mrs. Johnson shared seal thighs with de Laguna to feed to her dog, so perhaps this meat is less preferred. The SeaDNA method of butchery may represent “high-grading” seal carcasses to market certain cuts, whereas the rest of the carcass is used in making phoconailles (seal charcuteries). SeaDNA marketing does demonstrate the high meat value of the seal forelimb.
Over half the seals represented in the Angoon sites are pups, so I reexamined the skeletal representation and distribution of cutmarks. Although seal-pup scapulae, femora, and ulnae are well represented, no pup vertebrae, carpals, metacarpals, tarsals, metatarsals, or phalanges are present in the assemblage. These small elements were probably not recovered in 1950. Apparently, de Laguna's team did not screen the deposits, and small bones were probably not collected. With respect to cutmarks, pups account for most of the cut scapulae (three of the four cut are pups of 17 total) and femora (five of the six cut are pups of nine total). Adult seal pelves are cut more than pups (three or four cut are adult, and one is juvenile of eight total). This pattern is also affected by the soft porous nature of seal pup bones; only two seal pup pelvises occur, and at least one was “chewed to a nub” (according to my notes). The softest seal pup elements are the pelvis and cranium, neither of which is fused when a pup is born. This bone density difference means that dog damage was likely severe on certain pup bones. With so many elements missing (from lack of screening or gnawing), it is not possible to reconstruct how seal pups were butchered at these sites. It is likely, however, that seal pups were butchered differently from larger seals. Baby seals are highly valued for their soft skins, delicate meat, and buttery fat (Louise Kadinger, personal communication 2016).
Figure 5 groups elements functionally. With respect to sea otters, the top group consists of elements that get cutmarked in skinning a sea otter. To skin a sea otter, the processor may make cutmarks on mandibles, tibiae, fibulae, metatarsals, tarsals, radii, and ulnae. All but the mandible were cutmarked by Kyle Barry. The bottom group consists of elements expected to be cutmarked if people removed sea otter backstraps for meat. Femur and humerus make up a group to be discussed below.

Figure 5. Percent of cutmarked elements grouped by function (seal pups removed). The top group represents cutmarks from skinning sea otters and processing seal flippers. The middle group (femur, humerus) represents cutmarks from pulling sea otter limbs away from the pelt and from disarticulating and filleting seal flippers. The bottom group are cutmarks from obtaining sea otter backstraps and seal flanks, loins, hams, and other parts.
To skin a seal, processors do not need to make the same cuts as they would skinning a sea otter. Skinning a seal can be done in a few minutes, whereas Mr. Barry spent about 50 minutes skinning a sea otter. Although the top group consists of elements cut when skinning a sea otter, these elements mean something else in relation to a seal. The cuts on the seal's lower limbs were probably made when consuming the limbs and flippers. To remove a sealskin, processors do not have to get anywhere near the articulations or the surface of the forelimb or hindlimb bones because the blubber provides a thick cushion between the skin and meaty limbs. These limb bones likely will only be cut during disarticulation or when cutting meat away from the bone. Seventy percent of the cuts on seal limb bones occurred on the shafts, presumably from muscle stripping. This is substantially higher than the 50% of the cuts on the shafts of sea otter long bones.
Returning to the middle group in Figure 5, on a sea otter, these elements could be cut if a processor is using the knife as leverage to pull a limb through the hide away from the pelt. On a seal, these elements might be marked by disarticulating the fore and hindflipper to get at the hefty meat packages. To summarize Figure 5, in the top group are the elements cutmarked as a result of skinning sea otters and processing seal flippers for meat. The cutmarked elements in the middle group result from pulling sea otter limbs away from the pelt and from disarticulating and filleting seal flippers. The elements in the bottom group are cutmarked from obtaining sea otter backstraps (for human or canine consumption) and from accessing seal flanks, loins, hams, and other cuts of seal meat.
Discussion and Conclusions
One of the larger methodological results of this study is that conventional cutmark typologies used by archaeologists did not work well in understanding sea otter skinning and butchery. Skinning a sea otter is more labor intensive than skinning a seal. Cutmarks on the same element of different taxa can result from different tasks. This study indicates clearly that Angoon Tlingit ancestors were skinning sea otters for their pelts. They also, at least occasionally, appear to have removed backstraps from some sea otters; whether they consumed these cuts as meat or fed them to dogs cannot be determined. The Angoon Tlingit were skinning seals to process their skins and use their blubber to make seal oil. They butchered and ate seal meat, but they also fed seal bones to their dogs.
Understanding a zooarchaeological assemblage requires knowledge of skeletal anatomy of specific animals and how people used them. Ethnographic and ethnoarchaeological information and traditional ecological knowledge are invaluable in understanding such archaeological patterning. This study supports the right of Tlingit to hunt sea otters for the purpose of using their pelts without eating them because even though Tlingit ancestors probably did eat sea otters at times, these animals were not dietary staples. Both sea otter and seal bones were chewed by dogs at the Angoon sites.
How the meat is cooked is another factor to consider in determining the likelihood of sea otter meat consumption. Ethnographically, across the Northwest Coast, sea otters were cooked by roasting, grilling, stone boiling, smoking, or preserving in seal oil (Kuhnlein and Humphries Reference Kuhnlein and Humphries2017; de Laguna Reference de Laguna1972:398). From a modern perspective, grilling and roasting work best for meats that are tender or that have been tenderized by soaking in a marinade or brine or via mechanical methods. Peter Williams prefers to pan fry sea otter backstraps. Boiling or slow cooking can tenderize tough meats by physically altering the proteins on a microscopic scale. When meat reaches the optimum temperature of 140–153°F, myosin and collagen will have denatured, but actin will retain its form (Potter Reference Potter2010:177). The length of time needed to cook sea otter until it is palatable is unknown, and what is judged “palatable” varies cross-culturally. Although some Tlingit have told me they would never eat sea otter, others have done so. Still others say that even their dogs will not eat it. Sea otter has very little fat, unlike meats typically cooked for a long time in a slow cooker, such as beef brisket, pot roast, or pork shoulder. Today, we take it for granted that we can boil food in metal or ceramic vessels for long periods of time. Traditionally, Northwest Coast groups boiled food using watertight wooden boxes or baskets in which they placed the food, water, and stones that had been heated in a fire. As the heat from the rocks was transferred to the food, cooled rocks would be removed and replaced by hot rocks. One advantage of stone boiling is that the basketry or wood vessel is not charred or damaged through direct exposure to fire. A disadvantage is that boiling food for hours would require a lot of time, effort, and fuelwood. Pit baking or steaming also requires time and fuelwood. In a society where people had access to a range of high-quality sources of protein (such as salmon, seal, deer, halibut, herring, duck, other fish, and shellfish), it is unclear if people would invest time and effort into slow cooking the tough meat of a sea otter.
The oldest evidence of sea otter use by the Tlingit and their ancestors is from Coffman Cove, where sea otter bones date to approximately 4000 cal BP (Moss et al. Reference Moss, Hays, Bowers and Reger2016). Based on older evidence from Haida Gwaii (Fedje et al. Reference Fedje, Mackie, Wigen, Mackie, Lake, Fedje and Mathewes2005), First Nations have been hunting sea otters on the Northwest Coast for over 10,000 years. The cumulative weight of the evidence indicates that Tlingit ancestors who resided at Daax Haat Kanadaa (49SIT244) and Yaay Shanoow (49SIT132) occasionally ate portions of some sea otters but that sea otters were not a dietary staple. Sea otters were primarily valued for their pelts, obtained with great skill and effort in the absence of steel knives.
Sea otter bones with meat were fed to dogs, who were also residents of these sites. In addition to guarding villages, houses, and individuals, dogs cleaned residential sites of waste, and they served as pets and hunting partners (Crockford et al. Reference Crockford, Moss and Baichtal2011:50). The number of dogs supported in precontact Tlingit communities is unknown, but dogs would have required considerable food to maintain. This study has revealed differences in how seals and sea otters were processed, but it has also shown that bones of each were fed to dogs, illustrating the broader interrelationships between Tlingit and some of the marine mammals on which they and their dependents (dogs) relied for sustenance.
Sealskins and sea otter pelts were highly valued and used to make clothing and regalia. Seal oil was rendered from blubber and was an important staple food, medicine, and lubricant. Another finding is that standard cutmark typologies employed by archaeologists do not always pertain to the species under consideration. On sea otter bones, marks typically identified as evidence of dismemberment or disarticulation are consistent with using a cutting tool as an anchor against which to pull the pelt away from the fascia—that is, using the cutting tool as leverage to remove the hide. Clearly, a tremendous amount of technical knowledge is involved in the hunting and processing of sea otters and seals, and these are part of the intellectual property and cultural heritage of Tlingit people. If the general public and resource managers had a better understanding of these long-term cultural practices, the rights that derive from this long history could be afforded the respect they deserve, and managers could better incorporate Alaska Native issues and concerns into their planning.
I close with words from Peter Kawagaelg Williams, who eloquently describes the circumstances faced by Alaska Natives who use sea otters today:
My ancestors were first colonized as a result of the Russian fur trade. Colonial powers particularly prized the sea otter for their fur, and their lust for fur spurred on genocide, land theft, and exploitation of nature and indigenous groups. To this day, this oppressive power dynamic continues through American occupation and regulation. Although Alaska Natives theoretically have exclusive rights to hunt and work with marine mammals, non-native people are still creating the definitions and regulations around who qualifies as Alaska Native and what qualifies as Native art, along with numerous other regulations surrounding our inherent rights. The result has been the removal, control, and the constriction of markets for fur artwork and cultural practice. Since the dawn of the colonial fur trade, we as indigenous people have been prevented from controlling our ways of life and from being equals in the world [Peter Kawagaelg Williams, personal communication 2019].
Understanding this larger historical context and the long-term history provided by the archaeological record should help everyone realize how Alaska Natives’ relationships with sea otters connect with issues of self-determination, sovereignty, and heritage. If and when sea otters are able to recolonize parts of their historic range, they will have significant effects on the mosaic of kelp forest and invertebrate-dominated nearshore ecosystems. These impacts will affect both invertebrate and other fisheries and the coastal communities that rely on them. The ancestors of Indigenous groups have been networked within North Pacific ecosystems for more than 12,000 years, and they likely interacted with sea otters in variable ways: hunting them for pelts, as a food source, and/or for the purpose of protecting local shellfish. As communities consider issues of sea otter management, understanding the ecological roles played by people in the past can illuminate alternative solutions to conflicts today.
Acknowledgments
This project grew out of a conversation with Rosita Worl at Sealaska Heritage Institute (SHI). SHI has partnered in this work by providing funding through IPinCH (the Intellectual Property Issues in Cultural Heritage Project, Simon Fraser University) and other sources, and by connecting me with knowledgeable elders, hunters, and skin-sewers. At or through SHI, I am grateful to Rosita Worl, Chuck Smythe, Lee Kadinger, Kathy Dye, Kyle Barry, Louise Kadinger, Mariah Kadinger, Mike Miller, Kenny Grant, and Don Gregory. Thanks are due to George Nicholas and Brian Egan of IPinCH. For their hospitality in Juneau, Sitka, and Berkeley, I thank my dear friends Sue and Tom Koester, Sally Bibb, Brenda Campen, Steve Paustian and Mary Beth Nelson, Steve Papai and Cameron Chardoul, and Christine Hastorf. I am grateful to Natasha Johnson, Michael Black, and Kent Lightfoot at the UC Berkeley Hearst Museum who facilitated the collections work. Robert Lynn, J. Garlich-Miller, and Ed DeCleva at the U.S. Fish and Wildlife Service helped me obtain the sea otter carcass. At the University of Oregon, Hannah Wellman, Patrick O'Grady, and Anna Sloan all helped process and document the carcass. I am grateful to Sofia Vicente-Vidal who provided the Spanish abstract. I thank colleagues Seth Newsome and Emma Elliot Smith (University of New Mexico), Virginia Butler (Portland State University), Thomas Thornton (University of Alaska Southeast), Joshua Reuther and Scott Shirar (University of Alaska Museum of the North), and Lucy Lewis Johnson (Vassar) for their contributions. I thank all the colleagues and friends who attended talks at UC Berkeley and Sealaska Heritage Institute and who helped me think through issues raised here, including Meg Conkey and Les Rountree. I thank Peter Kawagaelg Williams for his pioneering work, inspired teaching, and for permission to use his quote. Finally, I am grateful to the three thoughtful reviewers of this manuscript and Lynn Gamble, editor of American Antiquity.
Data Availability Statement
Microsoft Excel spreadsheets containing primary data are available from Madonna L. Moss, University of Oregon.
Supplemental Materials
For supplementary material accompanying this paper, visit https://doi.org/10.1017/aaq.2019.101.
Supplemental Table 1. Cutmarks Made on Sea Otter Processed by Mr. Kyle Barry in July 2016.
Supplemental Figure 1. Cutmarks on sea otter skinned in July 2016. From top left, close-up and cut on sternum; close-up and cut on second rib; close-up and cut on lowest sternebra. The cuts differ in morphology even though they were made with the same steel knife within minutes of each other. The top cut is a single sharp line. The cut on the rib is coarse because it is going through porous bone at the sternal end. The bottom cut is coarse and probably represents multiple knife strokes.








