Published online by Cambridge University Press: 19 January 2004
Previous studies have shown that the secreted phosphorylcholine-containing glycoprotein of filarial nematodes, ES-62, is only present in the post-infective life-cycle stages, but that the mRNA is transcribed throughout the worm's life-cycle. The aim of this current study was to investigate whether the presence or absence of protein expression simply reflects differences in mRNA abundance. To this end, we investigated the relative abundance of ES-62 using TaqMan real time RT-PCR, in different life-cycle stages of 2 model filarial nematode parasites, Acanthocheilonema viteae and Brugia pahangi. For B. pahangi, microfilariae, infective larvae and adult worms were each found to have approximately similar levels of ES-62 mRNA. However, the corresponding stages of A. viteae differed greatly from each other with a pattern of increased mRNA production with maturation. As a rule A. viteae had higher levels of ES-62 mRNA than B. pahangi, and this was particularly noticeable in the adult stage where the difference was approximately 3500-fold higher. However, this significant difference in mRNA abundance was not reflected in the quantity of ES-62 protein secreted by the adult worms of each species, as A. viteae only secreted ~3 times as much ES-62 as B. pahangi. Thus, overall, the results obtained from this study indicate that ES-62 protein production does not solely reflect mRNA levels, and also suggest that the 2 nematodes may employ different mechanisms for regulating protein production.
Currently, there are approximately 150 million people infected, and a further 1000 million at risk of infection, with one of the three filarial nematodes of major medical importance: Wuchereria bancrofti, Brugia malayi and Onchocerca volvulus. Although these parasites are not a major cause of mortality, they cause significant levels of morbidity. One problem in trying to control these organisms, and hence reduce such morbidity, is the ability of the parasite to survive in an infected individual for up to 15 years (Richards et al. 2001).
Longevity in filarial nematodes has been linked to the ability of the parasite to evade the host's immune response. One evasion mechanism, which has been well characterized in many nematodes, relates to the release of parasite-derived excretory–secretory (ES) molecules into the host environment. Many of these ES molecules contain phosphorylcholine (PC) and are known, to be immunomodulatory via this PC component (reviewed by Harnett & Parkhouse, 1995). The best-characterized PC-containing immunomodulatory molecule is ES-62, the dominant ES product secreted by the rodent filarial nematode Acanthocheilonema viteae. ES-62 has previously been shown to downregulate lymphocyte proliferative responses by modulating the signal transduction pathways associated with ligation of the antigen receptor (reviewed by Harnett & Harnett, 2001). More recent work has demonstrated that ES-62 is also able to induce maturation of dendritic cells capable of inducing Th2 responses (Whelan et al. 2000) and to suppress the IFN-γ/LPS-induced production of IL-12, IL-6 and TNF-α by macrophages (Goodridge et al. 2001). Thus, overall, ES-62 promotes the induction of an anti-inflammatory, Th2-like immune response (reviewed by Harnett, Harnett & Byron, 2003).
Initial work by Harnett et al. (1989) indicated that ES-62 is only present in the L4 and adult worm stages of A. viteae, a finding confirmed by Stepek et al. (2002). However, the latter study also showed that although ES-62 was not present throughout the life-cycle of A. viteae, the mRNA could be detected. An ES-62 homologue is also found in B. pahangi, which is closely related to B. malayi (Stepek et al. 2002). Also, the recent finding of homologous EST sequences to ES-62 in the larval cDNA libraries of this parasite and also O. volvulus (http://nema.cap.ed.ac.uk/fgn/filgen1.html) suggests that ES-62 may be secreted by all filarial nematodes. Conversely, there are no reports of it being found in gastrointestinal nematode parasites and it also appears to be absent from C. elegans.
In this study, we have examined the expression of ES-62 by A. viteae and B. pahangi in more detail. Specifically, we have compared stage-specific and species-specific differences in mRNA production by TaqMan RT-PCR and protein production by biosynthetic radio-isotope labelling and semi-quantitative SDS-PAGE. Curiously, A. viteae appears to show stage-specific differences in ES-62 mRNA abundance that are largely absent from B. pahangi. Furthermore, we demonstrate that while the adult worms of both species express very different levels of ES-62 mRNA, this is not accurately reflected in the amount of protein released, demonstrating that ES-62 expression in filarial nematodes is controlled post-transcriptionally.
Adult A. viteae were recovered by direct visualization of the skin and underlying body surfaces of infected jirds (Meriones libycus) according to the method of Worms, Terry & Terry (1961). Adult B. pahangi were harvested from the peritoneal cavities of infected jirds (Meriones unguiculatus) infected 3–4 months previously (Devaney, 1988). Recovered worms were washed 5 times in RPMI ‘complete’ medium (RPMI 1640 medium with added glutamine (2 mM), penicillin/streptomycin (100 units/ml) (all from Invitrogen, Paisley, UK) and glucose (1% w/v) (Sigma, Dorset, UK)) prior to further analysis. A. viteae microfilariae (mf) were recovered from infected jird blood as described by Lucius & Textor (1995). Briefly, mf-infected blood was overlaid with RPMI ‘complete’ medium and incubated in the dark for 2 h whereupon the mf were recovered from the medium (‘blood mf’). Alternatively, A. viteae mf released from adult female worms in culture were retrieved from the medium (‘in vitro mf’). B. pahangi mf were obtained from the peritoneal cavity of an infected jird and purified as described by Osborne & Devaney (1998). L3 of A. viteae and B. pahangi were obtained from infected ticks (Ornithodorus moubata) or mosquitoes (Aedes aegypti), respectively and washed 3 times in RPMI complete medium prior to use.
RNA was extracted from mf (approximately 500000), L3 (500–700) and adult worms (approximately equal numbers of males and females) of A. viteae (12 worms) or B. pahangi (75 worms) by homogenization in RNeasy RLT lysis buffer (Qiagen Ltd, Crawley, UK) following the RNeasy Mini Kit protocol (Qiagen), except that 350 μl of wash buffer RW1 were added before and after the RNA was treated with DNaseI (Qiagen) for 15 min at room temperature. The total RNA was then stored at −70 °C.
First-strand cDNA synthesis was performed by heating 100 ng total RNA and 10 ng oligo(dT)12–18 primer (Invitrogen) and 0·5 mM dNTP to 65 °C for 5 min and then quick-chilling on ice. Samples were treated with the RNaseOUT recombinant ribonuclease inhibitor (Invitrogen) by incubation at 42 °C for 2 min in the presence of 10 μM DTT. Then 200 U Superscript II Reverse Transcriptase (Invitrogen) was added and the reaction was incubated at 42 °C for 50 min before inactivation by heating at 70 °C for 15 min. The cDNA was stored at −20 °C.
TaqMan real-time RT-PCR was performed according to the manufacturer's instructions (Applied Biosystems, Foster City, USA). Primers and fluorogenic probes (Table 1) were designed using the PrimerExpress v1.0 program and purchased from Biosource (Nivelles, Belgium). PCR reactions were performed in the ABI-prism 7700 Sequence Detector, which contains a Gene-AMP PCR system 9600 (Applied Biosystems). PCR amplifications were performed in a total volume of 25 μl of 10 mM Tris–HCl buffer, pH 8·3 containing 0·5 μl of cDNA sample, 50 mM KCl, 10 mM EDTA, 200 μM dATP, dCTP, dGTP and 400 μM dUTP, 5 mM MgCl2, 300 nM each primer, 200 nM probe, 0·625 U AmpliTaqGold and 0·25 U AmpErase Uracil N-Glycolase (Eurogentec, Seraing, Belgium). Each PCR amplification was performed in triplicate using the following conditions: 2 min at 50 °C and 10 min at 94 °C followed by 40 or 45 two-temperature cycles (15 s at 94 °C and 1 min at 60 °C). Data analysis was performed using the Applied Biosystems Sequence Detection Software and samples were normalized against actin mRNA.

Groups of 5 adult female A. viteae or 20 adult female B. pahangi were incubated in 20 ml choline-depleted RPMI medium at 37 °C in an atmosphere of 5% CO2/95% air for 24 h. Fresh medium was then added containing 1·5 MBq [methyl-3H] choline chloride (2·22 TBq/mmol) (Amersham Biosciences UK Ltd, Bucks, UK). The worms were then cultured for a further 96 h, replacing the medium and radio-isotope every 24 h.
Groups of 3 adult female A. viteae or 20 adult female B. pahangi were incubated in 20 ml of L-methionine-depleted MEM medium (Sigma, UK) at 37 °C in an atmosphere of 5% CO2/95% air for 1 h. Fresh medium was then added containing 1 MBq [35S]-L-methionine (>37 TBq/mmol, Amersham Biosciences, UK). The worms were then cultured for a further 72 h, replacing the medium and radio-isotope every 24 h.
The spent medium from each worm culture was filtered using 0·22 μm membranes to remove microfilariae and then concentrated to 300 μl and dialysed into PBS, pH 7·4 using Centricon microconcentrators with a 10 kDa membrane (Millipore (UK) Ltd, Watford, UK). Concentrated ES samples were stored at −20 °C. Incorporated radioactivity was measured by precipitation of 10 μl aliquots of radio-isotope labelled ES using 10% (w/v) TCA containing 10 mM choline or 10 mM methionine as described previously (Harnett et al. 1994). Determination of radioactivity was undertaken using a Beckman LS 6500 Multipurpose Scintillation Counter. The bicinchoninic acid (BCA) protein assay was performed using the MicroBCA Protein Assay Reagent Kit (Pierce, Illinois, USA), according to the manufacturer's instructions.
Polyacrylamide gel electrophoresis (PAGE) in the presence of sodium dodecyl sulphate (SDS) was performed on a Bio-Rad electrophoresis cell (Bio-Rad Laboratories Ltd, Hemel Hempstead, UK) according to the manufacturer's instructions. Thus 10% (w/v) acrylamide gels were employed and 2-mercaptoethanol was used for reduction of the samples. [14C]-labelled molecular weight markers (Amersham Bioscience) were also run to allow for molecular weight estimations. Gels were treated with the fluorographic reagent AMPLIFY (Amersham Bioscience) before exposure to pre-flashed Hyperfilm™ MP (Amersham Bioscience) and storage at −70 °C.
Polyacrylamide gel electrophoresis (PAGE) in the presence of sodium dodecyl sulphate (SDS) was performed as previously described. Kaleidoscope pre-stained molecular weight markers (Bio-Rad) were also run to allow for molecular weight estimations. Each lane was immediately sliced into 2 mm fragments and placed in 1·5 ml microcentrifuge tubes containing 200 μl 1×PBS. These gel slices were homogenized and 1 ml of scintillant was added and the amount of radioactivity in each gel fragment was measured using a Beckman LS 6500 Multipurpose Scintillation Counter.
TaqMan real-time RT-PCR was performed to assess ES-62 mRNA levels in microfilariae (mf), L3 and adult worms of A. viteae and B. pahangi. Normalization of ES-62 mRNA against actin mRNA levels revealed that the 2 filarial nematodes exhibit different patterns of ES-62 mRNA expression through their life-cycles (Fig. 1A and B). ES-62 is predominantly expressed by the adult worms of A. viteae, with significant but lower expression by L3 (~5% adult levels) and substantially lower expression by microfilariae (~0·19% and ~0·13% adult levels for in vitro and blood mf respectively; Fig. 1A). In contrast, the abundance of B. pahangi ES-62 mRNA remained relatively constant, with slightly lower levels in L3 (~76% and ~16% adult levels for mf and L3 respectively; Fig. 1B). The Ct (threshold cycle, PCR cycle number at which an increase in fluorescence above a threshold can first be detected) values for A. viteae and B. pahangi actin were similar (<2-fold different; Fig. 1C). This indicates that the expression levels of this housekeeping gene are equivalent, since roughly equal amounts of cDNA were used for each species. The dilution of adult worm cDNA indicated that the amplification efficiencies of both sets of probes and primers were comparable since parallel Ct profiles were obtained and hence the ΔCt (Ct ES-62−Ct actin) for both species remained constant across the dilution series (Fig. 1D). This validates the comparison of mRNA levels.

Fig. 1. TaqMan real-time RT-PCR measurement of Acanthocheilonema viteae and Brugia pahangi ES-62 mRNA. ES-62 mRNA levels in microfilariae (mf), L3 and adult worms of A. viteae (A) and B. pahangi (B) were assessed by TaqMan real-time RT-PCR and expressed relative to actin mRNA. The graphs show 3 experiments using 3 separates preparations of the life-cycle stages of each species. Dilution of separate adult worm cDNA shows equivalent amplification efficiencies of ES-62 and actin probe and primer sets for both species (C and D). (C) Threshold cycle (Ct) values for ES-62 and actin. (D) ΔCt values (Ct ES-62−Ct actin) for each species.
A. viteae ES-62 mRNA levels were much higher than those of B. pahangi. Mean ES-62 mRNA levels relative to actin were ~3500-fold higher in adult worms of A. viteae than those of B. pahangi. A. viteae L3 larvae expressed ~1100-fold more ES-62 mRNA than B. pahangi L3, while ES-62 mRNA was ~9- and ~6-fold more abundant in A. viteae in vitro and blood microfilariae respectively, compared to B. pahangi microfilariae.
In order to determine how the levels of ES-62 mRNA correlated with the levels of ES-62 protein in the adult worms of the 2 species, adult female worms were radio-isotope labelled with [3H]-choline or [35S]-methionine. Since there is a significant difference in the size of A. viteae and B. pahangi, it was necessary to weigh both species of worm and calculate levels of protein as μg of protein/g of worm and [3H]-choline and [35S]-methionine incorporation as (cpm)/g of worm. During a 120 h culture in the presence of [3H]-choline, ES products were prepared from adult A. viteae and B. pahangi at 24 h intervals and subjected to TCA precipitation analysis. Fig. 2 demonstrates that, per gram of worm, A. viteae secretes a higher level of [3H]-choline-containing proteins at each 24 h period than B. pahangi. However, a bicinchoninic acid (BCA) protein assay of the same ES samples revealed that B. pahangi secretes comparable amounts of total protein per gram of worm to A. viteae (Fig. 3). This indicates that although B. pahangi secretes a similar amount of protein to A. viteae, these proteins contain less choline. A. viteae secretes 2 choline-containing proteins as observed by fluorography (Fig. 4A: lane 1). The lower molecular weight protein was initially considered as a degradation product of ES-62 since immunoprecipitation with a rabbit polyclonal antibody specific for ES-62 detected it. However, immunoprecipitation with this antiserum depleted of anti-PC activity resulted in loss of antibody binding, suggesting in fact that this lower molecular weight molecule (approximately 20 kDa) was another PC-containing molecule. This molecule was not detected by earlier studies (for example, Harnett et al. 1993) almost certainly because different membrane sizes were employed when concentrating the ES by ultrafiltration (30 kDa membranes were used in the previous studies, whereas 10 kDa membranes were employed in this current study).

Fig. 2. TCA precipitation of [3H]-choline labelled ES of Acanthocheilonema viteae and Brugia pahangi. Five adult female worms of A. viteae and 20 adult female worms of B. pahangi were cultured in the presence of [3H]-choline for 120 h in total. The ES was prepared for each 24 h culture period and subjected to TCA precipitation analysis. The [3H]-choline incorporation into ES products was calculated to [3H]-choline secreted (cpm) per 24 h per gram weight of worm. The data are the mean of duplicate experiments.

Fig. 3. Protein assay of Acanthocheilonema viteae and Brugia pahangi ES. The ES of A. viteae and B. pahangi were subjected to bicinchoninic acid (BCA) protein assay. The concentrations of the ES for each 24 h culture period were calculated to μg of protein secreted per 24 h per gram weight of worm. The data are the mean of duplicate experiments.

Fig. 4. SDS-PAGE/fluorography of Acanthocheilonema viteae and Brugia pahangi radio-isotope-labelled ES. Day 3 [3H]-choline- (A) and [35S]-methionine-labelled (B) ES samples of adult A. viteae (lane 1) and adult B. pahangi (lane 2) were run on 10% SDS-polyacrylamide gels under reducing and denaturing conditions. All samples contained approximately the same amount of ES. The gels were fixed, placed in the fluorographic reagent AMPLIFY, dried and then exposed to pre-flashed autoradiograph film at −70 °C. Molecular weight markers (kDa) are indicated on the left of the gels and the solid arrows indicate ES-62. The dashed arrows indicate the choline-containing proteins (A) and all proteins (B) in B. pahangi.
In contrast to the 2 [3H]-choline labelled proteins described above, B. pahangi female worms appeared to secrete 4 [3H]-choline labelled proteins, 1 at 62 kDa and minor components at 46, 52 and >100 kDa (Fig. 4A: lane 2). The 62 kDa molecule was previously shown to be the B. pahangi equivalent of A. viteae ES-62 as determined by its reactivity for an antibody raised against the A. viteae product (Stepek et al. 2002). Previous work by Maizels and colleagues also found that Brugia spp. secreted several PC-containing molecules (Maizels, Burke & Denham, 1987). Although these latter molecules were calculated as having slightly different molecular weights – 51, 67 and 90 kDa, it is tempting to speculate that they may correspond to 3 of the molecules detected in the present study. In order to obtain an estimate of the total number of proteins secreted by each species, SDS-PAGE/fluorography was performed on the [35S]-methionine labelled ES from both A. viteae and B. pahangi. Fig. 4B (lane 1) only reveals the existence of what appears to be the 2 proteins labelled with [3H]-choline in the ES of [35S]-methionine labelled A. viteae. In contrast, B. pahangi secretes at least 5 [35S]-methionine-labelled proteins, only 3 of which (52, 62 and >100 kDa) co-migrate with those shown in Fig. 4A (lane 2).
Semi-quantitative SDS-PAGE was performed on the [3H]-choline-labelled ES products from both worms, specifically on the day 5 samples. Fig. 5A confirms that A. viteae secreted 2 choline-containing proteins, with the first peak being ES-62 and the second peak the 20 kDa molecule. Fig. 5B shows that B. pahangi secreted up to 4 choline-containing proteins, with the peak composed of samples 9–11 being ES-62. For estimation of the total number of secreted proteins from each species, semi-quantitative SDS-PAGE was performed on methionine-labelled ES from both parasites. Fig. 5C and D confirm that A. viteae secretes only 2 [35S]-labelled proteins, but B. pahangi secreted at least 6 proteins. Using these latter results, the percentage of the ES from both species which is ES-62 was calculated to be 82·6% for A. viteae and 24·7% for B. pahangi. Taking into account that the level of ES-62 secretion from B. pahangi is clearly a more crude estimate due to the lack of sharpness in the appropriate peak, these results provide an opportunity to estimate the amount of ES-62 in the protein secretions from both A. viteae and B. pahangi. Per gram of body weight, 4262 μg protein/72 h is secreted from A. viteae, with 3521 μg being ES-62 whereas 5144 μg of protein/72 h is secreted from B. pahangi, with 1271 μg being ES-62. Therefore, these results suggest that the adult worms of A. viteae secrete ~3-fold more ES-62 protein, than the adult worms of B. pahangi. This, however, does not reflect the relative abundance of ES-62 mRNA, which was estimated to be ~3500 more abundant in A. viteae than in B. pahangi.
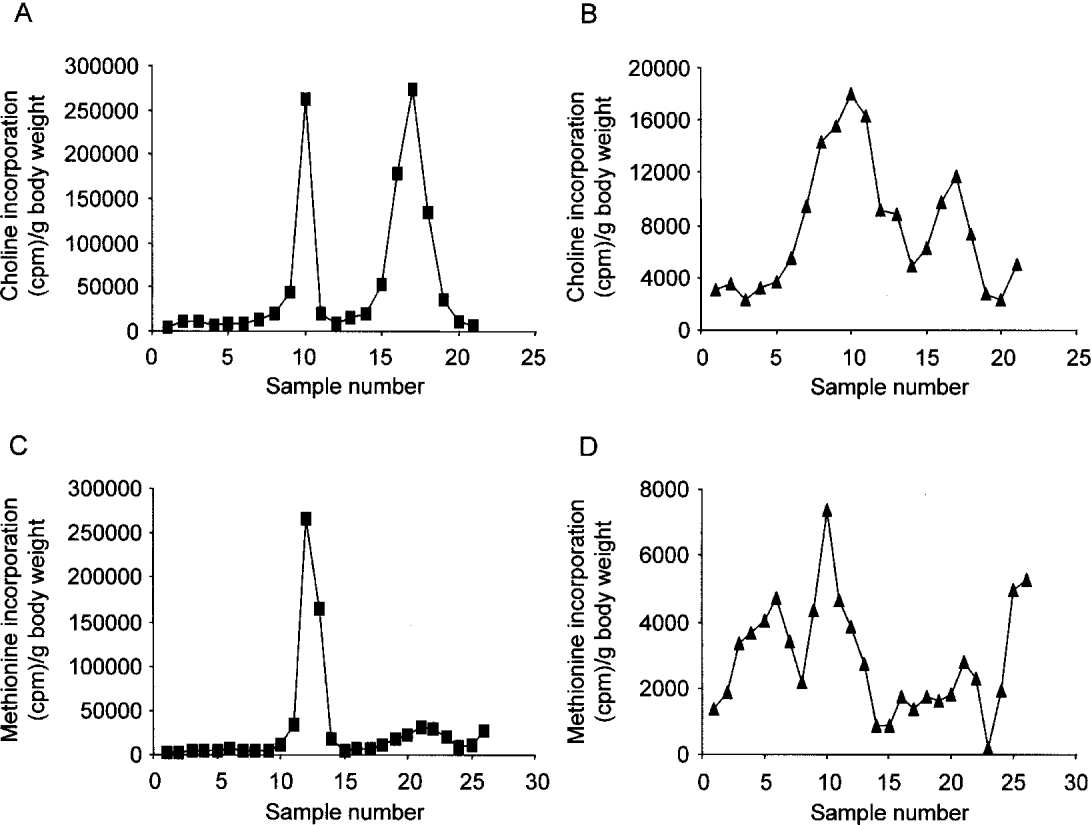
Fig. 5. Semi-quantitative SDS-PAGE of Acanthocheilonema viteae and Brugia pahangi radio-isotope labelled [3H]-choline- (A and B) and [35S]-methionine-labelled (C and D) ES samples of A. viteae (A and C) and B. pahangi (B and D) were run on 10% SDS-polyacrylamide gels under reducing and denaturing conditions, sliced into 2 mm fragments and then the incorporation of [3H]-choline and [35S]-methionine was measured. Sample 1 is derived from the top of the gel.
Although ES-62 is secreted only in the post-infective life-cycle stages of Acanthocheilonema viteae (Harnett et al. 1989; Stepek et al. 2002), a recent study has shown that ES-62 mRNA is expressed in all the life-cycle stages of A. viteae and, in addition, B. pahangi (Stepek et al. 2002). This current study began by comparing the levels of ES-62 transcribed in the different stages of these two nematodes.
TaqMan real-time RT-PCR analysis on cDNA prepared from the microfilariae, L3 and adult worms from both species identified a very significant variation between A. viteae and B. pahangi in the amount of ES-62 mRNA present. B. pahangi worms contained roughly similar amounts of mRNA regardless of stage, whereas A. viteae stages were highly variable. Consequently, A. viteae adult worms expressed on average ~3500 times more ES-62 mRNA than their Brugian counterparts, while for the L3 and mf of A. viteae the difference was ~1100-fold and ~6 to 9-fold respectively.
L4 and adult stage filarial nematodes secrete ES-62. Although the former only produce trace amounts, the latter secrete sufficient amounts to allow quantitative analysis. Such analysis demonstrated that ES-62 protein is secreted in a different abundance from adult worms of the two filarial nematodes. Based on measurement of total protein released allied to the percentage of this protein that is ES-62 as determined by [35S]-methioine labelling it was calculated that A. viteae secretes ~3 times as much ES-62 as B. pahangi. However, the 3-fold higher secretion by A. viteae compared to B. pahangi is not compatible with the difference in mRNA production observed. As we also cannot explain this difference by increased synthesis and storage of ES-62 protein by A. viteae (Stepek et al. 2002) the reason for such incompatibility needs to be explored.
One possibility is that there are differences in ES-62 mRNA stability in the two species. mRNA stability is an important factor in the control of gene expression in eukaryotes (reviewed by McCarthy, 1998) and this has recently been highlighted in trypanosomes (D'Orso, et al. 2003). Studies in yeast indicate that individual mRNAs vary widely with respect to stability and this is usually determined by rates of both deadenylation and decapping (Herrick, Parker & Jacobson, 1990). Control of mRNA turnover is not fully understood but a role is indicated for adenylate, uridylate-rich (AU-rich) instability elements (AREs) in 3′ untranslated regions (reviewed by Chen & Shyu, 1995). AREs typically range from 50 to 150 nucleotides in length and increase deadenylation rates and subsequent mRNA degradation (Ford et al. 1999). Each ARE represents a combination of functionally and structurally distinct sequence motifs or domains, such as AUUUA motifs, UUAUUUA(U/A)(U/A) nonamers, U stretches and/or a U-rich domain. AREs in mRNA molecules are the binding sites for proteins that can stabilize or destabilize transcripts.
We have previously described the presence of AREs in 2 out of 3 cDNA clones of ES-62 obtained from a female adult A. viteae cDNA library (Harnett et al. 1999). Specifically, these clones were 77·1% A/U rich in the 3′ end of the cDNAs and contained the nonamer referred to above. Thus there is evidence to indicate rapid ES-62 mRNA turnover in A. viteae but, at present, we do not have ES-62 cDNAs from B. pahangi to make a comparison. However, we have recently isolated an ES-62 cDNA from each of the microfilaria, male and female stages of the close relative B. malayi. Each of these ES-62 cDNAs also possesses an ARE. These are identical but distinct from the ARE of the A. viteae ES-62 cDNA. Of particular note, the AUUUA motif is present but not as part of the nonamer. It has been suggested that the nonamer is a better indicator than the AUUUA motif in predicting the potential destabilizing function of an ARE (Zubiaga, Belasco & Greenberg, 1995). However, the B. malayi AUUUA motif has U stretches nearby and this combination has previously been found to provide a similar destabilizing function to the nonamer (Chen & Shyu, 1994). With present knowledge we therefore cannot state which of these AREs is likely to be more effective but if B. malayi mirrors the situation in B. pahangi ES-62 mRNA instability certainly appears to be present in the latter organism. Perhaps postulated differences in ES-62 mRNA stability between filarial nematode species may also involve differences in interaction with ARE binding proteins that contribute to regulation of mRNA turnover.
Thus, at present, we do not know why the adult stages of the two species of filarial nematode should display such huge discrepancy between ES-62 mRNA and protein production. Another related question we need to address is why ES-62 mRNA is translated in a stage-specific manner. With respect to A. viteae one could postulate that ES-62 protein expression simply reflects mRNA abundance. However, we have never detected any ES-62 protein in L1-L3 stages by a variety of procedures and the results of the present study, comparing A. viteae and B. pahangi discussed above do not support this concept. Furthermore, with respect to B. pahangi alone, it cannot possibly be true as all stages examined have roughly similar amounts of ES-62 mRNA. Also, if B. malayi mirrors B. pahangi, stage-specific protein expression cannot reflect mRNA stability as all stages have identical 3′ untranslated regions. Clearly therefore, some other mechanism of translational control must be operating and further analysis will be required to resolve this issue.
W. H. would like to thank the Wellcome Trust for funding.

Table 1. Primers and probes for TaqMan real-time RT-PCR

Fig. 1. TaqMan real-time RT-PCR measurement of Acanthocheilonema viteae and Brugia pahangi ES-62 mRNA. ES-62 mRNA levels in microfilariae (mf), L3 and adult worms of A. viteae (A) and B. pahangi (B) were assessed by TaqMan real-time RT-PCR and expressed relative to actin mRNA. The graphs show 3 experiments using 3 separates preparations of the life-cycle stages of each species. Dilution of separate adult worm cDNA shows equivalent amplification efficiencies of ES-62 and actin probe and primer sets for both species (C and D). (C) Threshold cycle (Ct) values for ES-62 and actin. (D) ΔCt values (Ct ES-62−Ct actin) for each species.

Fig. 2. TCA precipitation of [3H]-choline labelled ES of Acanthocheilonema viteae and Brugia pahangi. Five adult female worms of A. viteae and 20 adult female worms of B. pahangi were cultured in the presence of [3H]-choline for 120 h in total. The ES was prepared for each 24 h culture period and subjected to TCA precipitation analysis. The [3H]-choline incorporation into ES products was calculated to [3H]-choline secreted (cpm) per 24 h per gram weight of worm. The data are the mean of duplicate experiments.

Fig. 3. Protein assay of Acanthocheilonema viteae and Brugia pahangi ES. The ES of A. viteae and B. pahangi were subjected to bicinchoninic acid (BCA) protein assay. The concentrations of the ES for each 24 h culture period were calculated to μg of protein secreted per 24 h per gram weight of worm. The data are the mean of duplicate experiments.
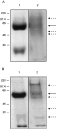
Fig. 4. SDS-PAGE/fluorography of Acanthocheilonema viteae and Brugia pahangi radio-isotope-labelled ES. Day 3 [3H]-choline- (A) and [35S]-methionine-labelled (B) ES samples of adult A. viteae (lane 1) and adult B. pahangi (lane 2) were run on 10% SDS-polyacrylamide gels under reducing and denaturing conditions. All samples contained approximately the same amount of ES. The gels were fixed, placed in the fluorographic reagent AMPLIFY, dried and then exposed to pre-flashed autoradiograph film at −70 °C. Molecular weight markers (kDa) are indicated on the left of the gels and the solid arrows indicate ES-62. The dashed arrows indicate the choline-containing proteins (A) and all proteins (B) in B. pahangi.

Fig. 5. Semi-quantitative SDS-PAGE of Acanthocheilonema viteae and Brugia pahangi radio-isotope labelled [3H]-choline- (A and B) and [35S]-methionine-labelled (C and D) ES samples of A. viteae (A and C) and B. pahangi (B and D) were run on 10% SDS-polyacrylamide gels under reducing and denaturing conditions, sliced into 2 mm fragments and then the incorporation of [3H]-choline and [35S]-methionine was measured. Sample 1 is derived from the top of the gel.