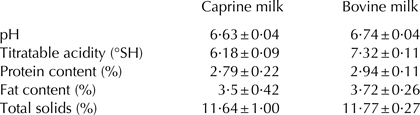Goat milk production is a dynamic and growing industry, it has become an important part of the economy in many countries (Silanikove et al. Reference Silanikove, Leitner, Merin and Prosser2010). The current trend of returning to land and nature, the popularity of organic farming and the rediscovered respect for authentic original products linked to specific geographic areas have all contributed to a significantly increased interest in goat milk products (Morand-Fehr et al. Reference Morand-Fehr, Boutonnet, Devendra, Dubeuf, Haenlein, Holst, Mowlem and Capote2004).
Caprine (goat) milk has been studied far less than bovine (cow) milk (Raynal-Ljutovac et al. Reference Raynal-Ljutovac, Park, Gaucheron and Bouhallab2007) and, often, the similarities in gross composition of caprine and bovine milks (Park, Reference Park, Park and Haenlein2006) have led to flawed assumptions: the extrapolation of results obtained with the bovine milk to the caprine milk.
Caprine milk differs from bovine milk in several physico-chemical characteristics, resulting in a major difference in the technological properties of these two types of milk. Because of the different mineral composition and the size and hydration of the casein micelle, caprine milk has a lower colloidal stability (Park, Reference Park2007). Furthermore, caprine milk is more sensitive to ultra high heat treatments (Anema & Stanley, Reference Anema and Stanley1998; Morgan et al. Reference Morgan, Micault and Fauquant2001; Raynal-Ljutovac et al. Reference Raynal-Ljutovac, Park, Gaucheron and Bouhallab2007), probably due to its higher ionic calcium content and lower micelle solvation (Park & Guo, Reference Park, Guo, Park and Haenlein2006). It was reported earlier (Rafael & Calvo, Reference Rafael and Calvo1996) that even sub-ultra high heat treatments (80°C for 20 s) can disturb the colloidal system of caprine milk proteins to great extent, causing a significantly higher precipitate formation on heating surfaces of the equipment when caprine milk is heated, compared with bovine milk. There are only a few studies of the effect of sub-ultra high heat treatments on aggregation of caprine milk proteins (Rafael & Calvo, Reference Rafael and Calvo1996; Henry et al. Reference Henry, Mollé, Morgan, Fauquant and Bouhallab2001; Pesic et al. Reference Pesic, Barac, Stanojevic, Ristic, Macej and Vrvic2012).
Another example of lower colloidal stability is that caprine skim milk exhibits markedly lower ethanol stability than bovine skim milk (Guo et al. Reference Guo, Wang, Li, Qu, Jin and Kindsted1998; Raynal-Ljutovac et al. Reference Raynal-Ljutovac, Park, Gaucheron and Bouhallab2007).
To the best of our knowledge and surprisingly, no paper has been published that considers sensitivity of colloidal casein micelles in caprine milk towards skimming process. In the literature, one comes across a great variety of centrifugal conditions used in laboratories for milk skimming: 8500 g/15 min/4°C (Lindmark-Månsson et al. Reference Lindmark-Månsson, Timgren, Aldén and Paulsson2005), 5000 g/20 min/4°C (Sandra & Dalgleish, Reference Sandra and Dalgleish2007), 4500 g/20 min/20°C (García-Risco et al. Reference García-Risco, Ramos and López-Fandiño2002), 3000 g/30 min/5°C (Calvo & Espinoza, Reference Calvo and Espinoza1999), 3000 g/30 min/15°C (Lieske et al. Reference Lieske, Konrad and Faber1997), 2000 g/30 min/32°C (Devold et al. Reference Devold, Brovold, Langsrud and Vegarud2000), 2000 g/30 min/4°C (Moatsou et al. Reference Moatsou, Samolada, Panagiotou and Anifantakis2004), 1620 g/10 min/20°C (Shuiep et al. Reference Shuiep, Giambra, El Zubeir and Erhardt2013), 1000 g/30 min/4°C (Law & Leaver, Reference Law and Leaver2000), 600 g/15 min/20°C (Raynal & Remeuf, Reference Raynal and Remeuf1998). It is not clear why such a great variety exists, nor what is the criterion in choosing a particular skimming condition.
In our preliminary tests, when we were defatting the milk in order to prepare samples for electrophoresis, we noticed the formation of precipitate during centrifugation.
We decided to measure and compare the amount of precipitate formed during skimming with three different centrifugal forces and also to analyse the quantity and the composition of proteins lost by precipitation. In addition, we wanted to find out whether the heating of milk prior to centrifugation has any effect; and, we wanted to study, in all the areas, the differences in colloidal stability between caprine and bovine milks.
The aim of the study was to select the optimal skimming conditions that would achieve both the high efficiency and the low protein loss. We assume it could be useful in all the electrophoretic techniques where skimming is a necessary step in sample preparation. The aim was also to provide additional information about the differences in colloidal stability of caprine and bovine milks, bearing in mind that the colloidal stability of biological systems such as milk is of great scientific interest (Tuinier & de Kruif, Reference Tuinier and de Kruif2002).
Materials and methods
Sample preparation
Raw milk was collected from local farms: caprine milk from a single flock of Saanen goats and bovine milk from a single herd of Holstein-Friesian cows. All samples were collected during the months of April and May. The compositional analysis and the heat treatment of raw milk were performed immediately after the sample collection.
Heat treatment
Raw bovine and caprine milks were heated to 40°C, stirred and then cooled down to 20°C. Aliquots (10 ml) of all samples were placed in polypropylene sealed tubes and then heated in a water bath. Temperature was measured inside the control tube filled with 10 ml milk. Three different heating temperatures were applied for 5 min: 70, 80 and 90°C. After the heat treatment, milk samples were immediately cooled in tap water until the temperature reached 20°C. All the samples were left overnight in the refrigerator at 7–10°C.
Skimming
Milk samples were re-heated and cooled down to 40 and 20°C, respectively. The milk samples were centrifuged (centrifuge model 5430; Eppendorf AG, Hamburg, Germany) at 20°C for 15 min using three different forces: 600, 2000 and 4500 g. After centrifugation, the liquid phase was carefully pipetted from the centre of the tube. Because of the formation of precipitate, the term ‘supernatant’ will be used in the text for the liquid phase instead of a commonly used term ‘skimmed milk’. Supernatant and precipitate samples were further analysed.
Compositional analysis of raw milk, supernatant and precipitate samples
The composition of raw milk was determined by the following methods: total solids by standard drying method at 102±2°C (International Dairy Federation (IDF), 1987); fat content according to Gerber method (IDF, 1981); protein content by Kjeldahl method (AOAC, 1990). The titratable acidity (°Soxhlet-Henkel) was determined using 0·1 m NaOH and phenolphthalein as an indicator. The pH was measured using digital pH-meter (Consort, Turnhout, Belgium).
Fat content of supernatants was also determined according to Gerber method (IDF, 1981), and protein content of supernatants by Kjeldahl method (AOAC, 1990).
Precipitate weight ratio measurement
At the beginning of the experiment, an empty tube (w 0) and a tube filled with raw milk (w 1) were weighed. After centrifugation, supernatant and fat residues were carefully removed, and the tubes with precipitates were weighed (w 2). The precipitate weight ratios were then calculated as (w 2−w 0)/(w 1−w 0)×100.
Preparation of precipitate and supernatant samples for the SDS-PAGE
Precipitates obtained by centrifugation at 4500 g/15 min/20°C were prepared for SDS-R(reducing)-PAGE. Samples were prepared by resolving precipitates with SDS-R-PAGE resolving buffer pH 8·00, containing 0·125 m Tris-HCl, 4% (w/v) SDS, 5% (v/v) β-mercaptoethanol. Precipitates were mixed in mass ratio 1 : 20 with resolving buffer in polypropylene sealed tubes, which are then placed in the boiling water bath until all the precipitates were resolved. Furthermore, resolved precipitates were mixed in mass ratio 1 : 3 with SDS-R-PAGE sample buffer pH 6·8, containing 0·055 m Tris–HCl, 2% (w/v) SDS, 7% (v/v) glycerol, 5% (v/v) β-mercaptoethanol, and 0·0025% (w/v) bromophenol blue. In this way, the final dilution of all precipitate samples was 1 : 60.
Supernatants were prepared in reducing (SDS-R-PAGE) and non-reducing (SDS-NR-PAGE) conditions, according to the method of Anema & Stanley (Reference Anema and Stanley1998). Supernatants were ten-fold diluted by mixing aliquots of each supernatant with SDS-R-PAGE and SDS-NR-PAGE sample buffers. The sample buffer used for SDS-R-PAGE was the same as for the precipitate samples. SDS-NR-PAGE sample buffer differed only by lacking reducing agent: β-mercaptoethanol.
SDS-PAGE
SDS PAGE was performed according to the method of Laemmli (Reference Laemmli1970). Samples were run with 4% stacking gel of pH 6·80, and 12·5% resolving gel pH 8·85. The running buffer was Tris–glycine buffer containing 0·05 m TRIS, 0·19 m glycine, and 0·1% (w/v) SDS. Before the analysis, all the samples were heated in boiling water for 5 min. Aliquots of 5 μl supernatant and precipitate samples were loaded per well.
Electrophoresis was performed using 20·5×10 cm TV200YK twin-plate electrophoresis unit (Consort) together with Electrophoresis Power Supply EV202 (Consort). The power supply was set at constant current of 80 mA and the maximum voltage of 300 V was applied for 1·5 h.
Following electrophoresis, the gels were fixed and stained for 1 h at the temperature of 45°C, in 250 ml Coomassie blue dye solution containing 0·23% (w/v) Coomassie brilliant blue R250, 3·9% (w/v), trichloroacetic acid, 6% (v/v) acetic acid and 17% (v/v) methanol. The staining was followed by three destaining steps, with a destaining solution containing 8% (v/v) acetic acid and 18% (v/v) methanol. The gels were scanned using HP Scanjet G2710 (Hewlett Packard, California, USA).
Protein quantification and nomenclature
The densitometric analysis of scanned gels was done using LabImage 1D L340 software (Kapelan Bio-Imaging GmbH, Leipzig, Germany).
Proteins obtained by SDS-NR-PAGE are those dispersible to monomers in SDS solution and are called ‘SDS-monomeric’ proteins, while those obtained by SDS-R-PAGE, where disulphide bonds were reduced by β-mercaptoethanol to free thiols are called ‘total-reducible’ proteins (Havea, Reference Havea1998).
The level of irreversible denaturation of each protein fraction was calculated as a percentage of ‘SDS-monomeric’/‘total-reducible’ of the corresponding protein peaks. Thus, all the samples prepared in non-reducing conditions were taken as control samples for their non-reduced counterparts.
Statistical analysis
A split-plot design was used to determine the effects of the heating temperature, the centrifugal force during skimming and their interaction on the precipitate weight ratio. Both factors were defined as random. The main plot was defined with centrifugal force and block, while the subplot was defined with heat treatment and interaction between centrifugal force and heat treatment. Three caprine and three bovine milk batches were taken on separate days, and considered as blocks. The precipitate weight ratio of each block was a mean of three replicated trials. The analysis of variance for the split-plot design was carried out using a general linear model (GLM) procedure by Statistica 10.0 software (Stat Sof. Inc., Tulsa, USA). The same software was used for mean comparisons performed by t-test, with the level of significance of 0·05.
Results
Composition of raw caprine and bovine milk samples
The gross composition of caprine and bovine milk samples are presented in Table 1.
Table 1. Composition of raw milk samples

Values presented are the means of three replicates ±sd.
Precipitate weight ratio
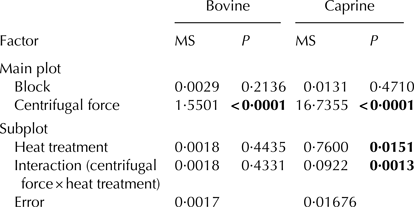
The split-plot statistical analysis (Table 2) showed that the applied centrifugal (g-force) had significant effect on precipitate formation of both caprine and bovine milks.
Table 2. Statistical analysis of precipitate weight ratios

In the case of caprine milk, the heat treatment performed prior to skimming had a significant effect on the precipitate weight ratio formed during skimming: an increase of temperature caused an increase of precipitate weight ratio.
There was no influence of block on precipitate weight ratio, in both caprine and bovine milks.
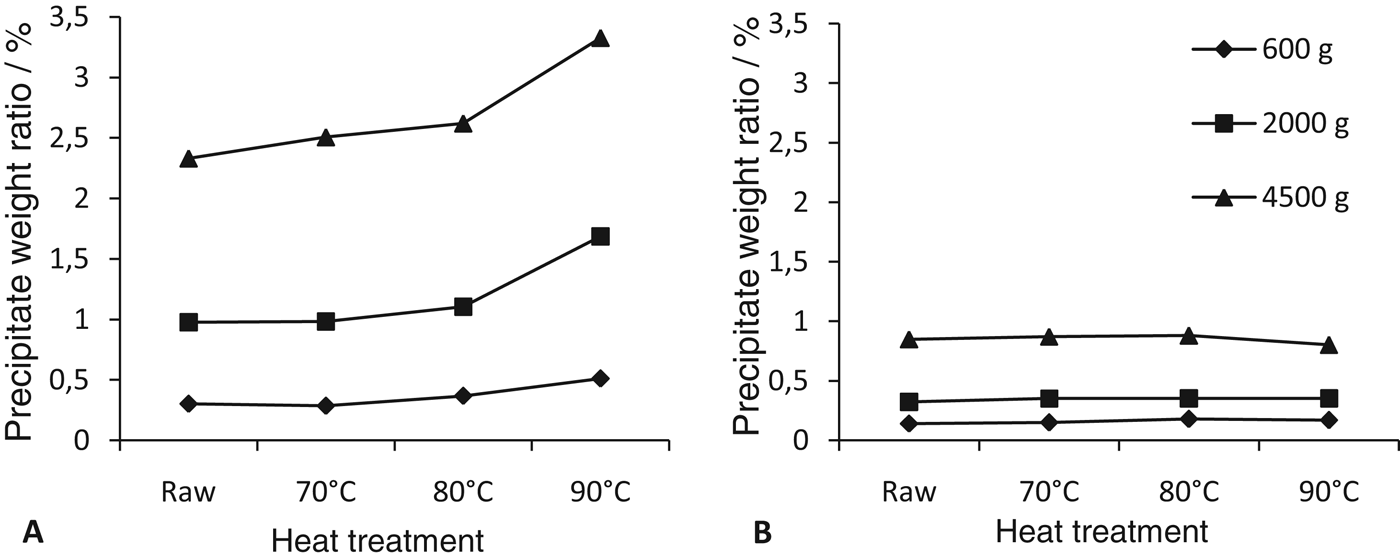
The effect of the skimming regime and of the heat treatment on the weight ratio of precipitate formed after centrifugation, for both the caprine and the bovine milks is shown in Fig. 1. There was a noticeable difference between amount of formed precipitate in the two milks: the weight ratio of caprine milk precipitates is two to four times higher than the ratio of the bovine milk precipitate, depending on the choice of the g-force and the applied heat treatment.

Fig. 1. Weight ratio of precipitate formed during centrifugation of raw and heat-treated caprine (a) and bovine (b) milks. Values presented are the means from three blocks.
Figures 1a and b show that with the increase of applied centrifugal force (g-force), the heat treatment had a significantly more pronounced effect on precipitate weight ratio in the case of caprine milk. Furthermore, as the centrifugal force (g-force) increases, the difference between precipitate weight ratios of samples heated at 80°C and at 90°C became more pronounced.
Fat and protein content of raw milk supernatants
Raw milk supernatants were analysed in terms of fat content and the protein content. For the fat content, the same results were obtained for both caprine and bovine milks. After skimming with the g-force of 600 g, 0·1% milk fat remained in supernatant; with a force of 2000 and 4500 g the skimming was complete.
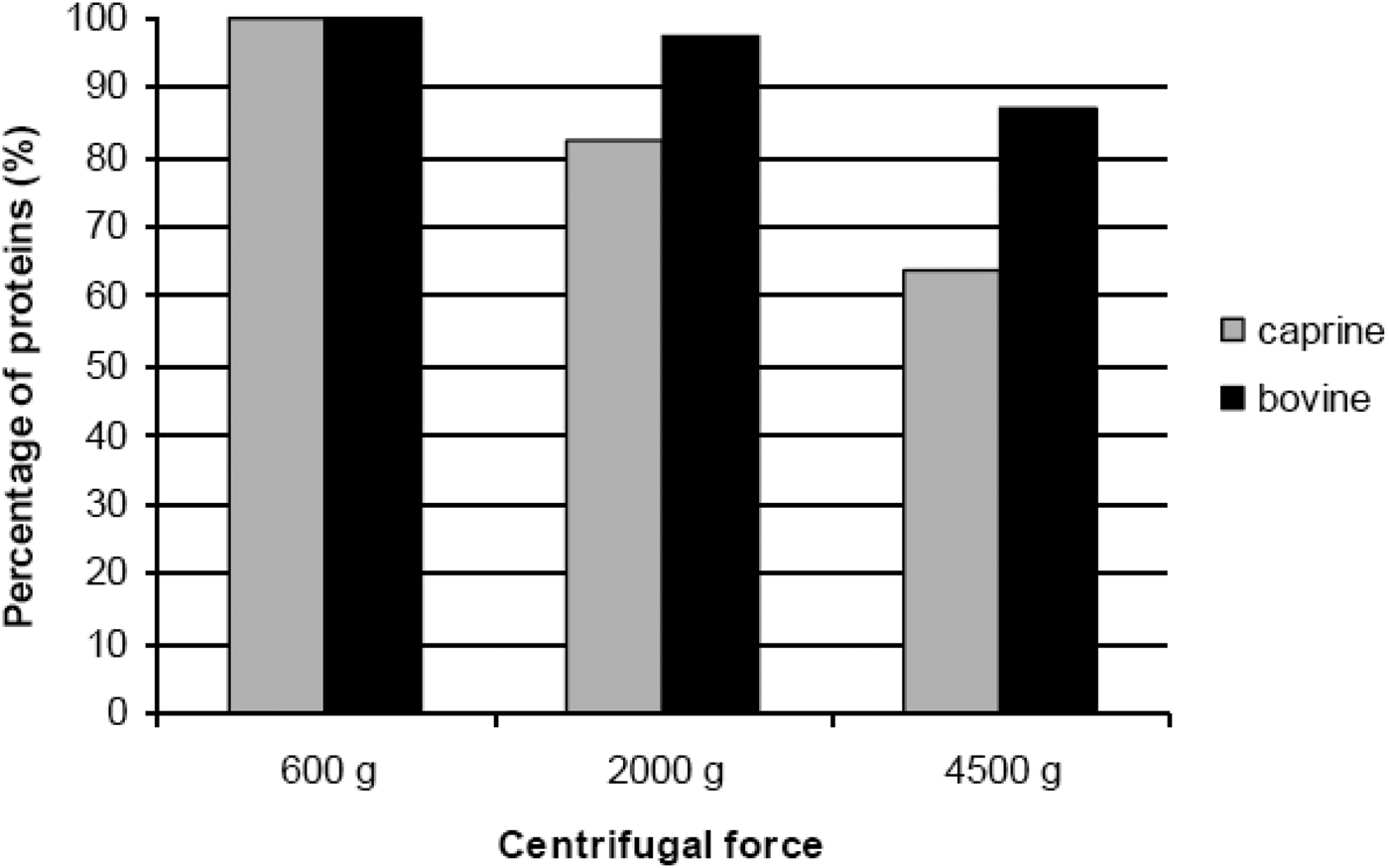
The protein content of supernatants was expressed as a percentage of protein content of supernatants obtained at the mildest centrifugal force (600 g). Hence, protein content of supernatants obtained at the mildest centrifugal force (600 g) was presented as 100%. The protein content of supernatants depended on the g-force used during centrifugation (Fig. 2): the content was decreased with the increase of g-force and the decrease is significantly more pronounced in the case of the caprine milk.

Fig. 2. Protein content of raw milk supernatants of caprine and bovine milks, expressed as percentages of protein contents of milk skimmed at 600 g.
Comparison of protein contents by t-test had shown that there was no statistical difference between supernatants obtained at 600 g and that obtained at 2000 g from bovine milk. However, statistically significant difference (P<0·05) between protein contents of supernatants obtained at 600 and 4500 g showed that skimming at 4500 g might be inappropriate even for bovine milk. For caprine milk there was a statistically significant difference (P<0·05) in protein content of supernatants obtained at 2000 and 4500 g, compared with protein content of supernatants obtained at 600 g.
SDS-PAGE analysis of supernatants
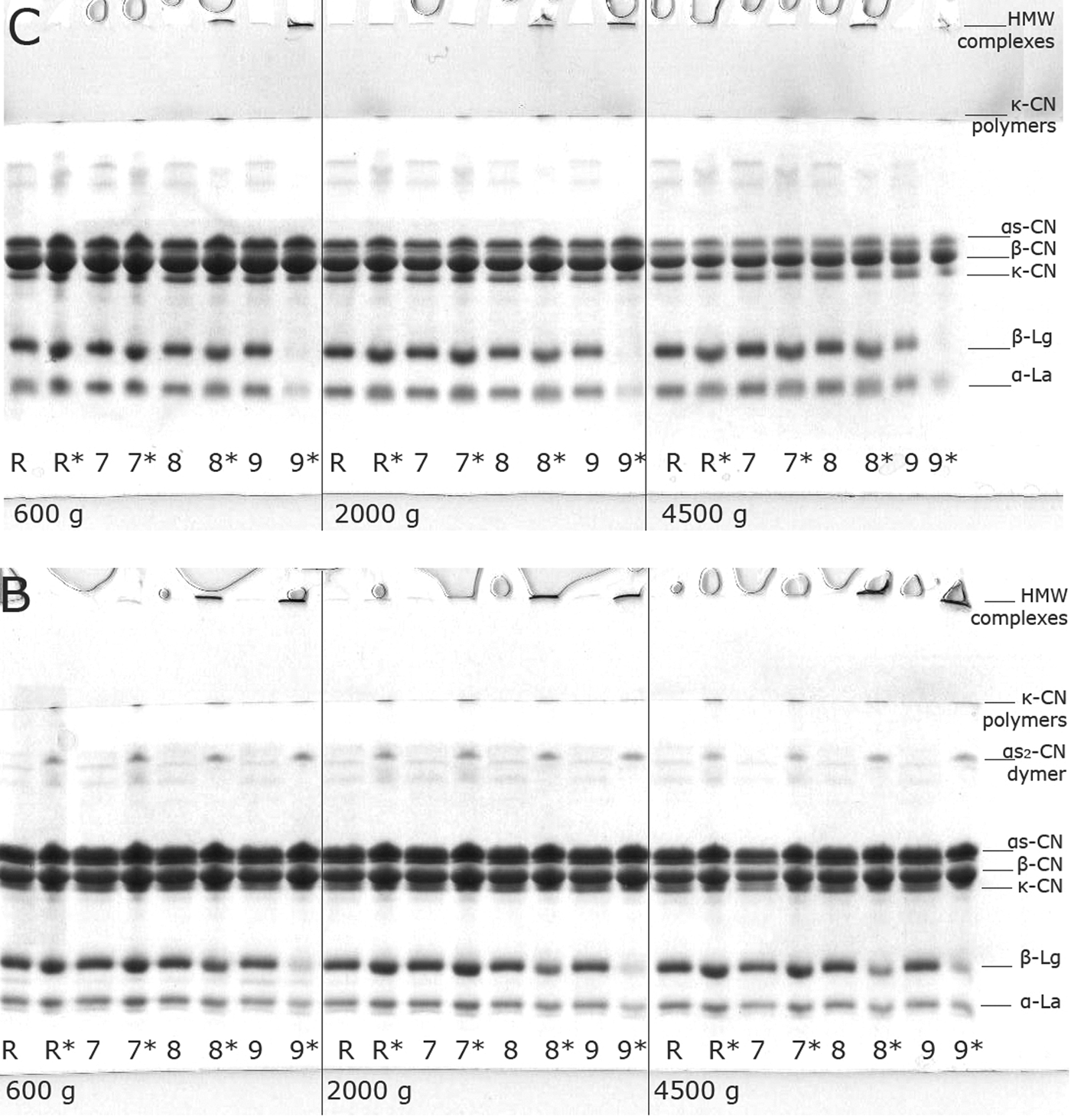
Electrophoretograms of caprine and bovine supernatant samples are shown in Fig. 3. Similar patterns were found for samples from the other two trials (data not shown).

Fig. 3. SDS PAGE patterns of caprine (C) and bovine (B) supernatants prepared in reducing (lanes R, 7, 8, 9) and non-reducing conditions (lanes R*, 7*, 8*, 9*), obtained at 600, 2000 and 4500 g.
There was no difference between the SDS-R-PAGE profiles of unheated and heated caprine and bovine milk samples skimmed at 600 g.
On the other hand, when caprine milk was centrifuged at 4500 g (Fig. 3(C)), the difference is noticeable between the SDS-R-PAGE unheated samples (Fig. 3(C), 4500 g, lane R) and the ones heated at 80 and 90°C (Fig. 3(C), 4500 g, lanes 8 and 9); and particularly so among the bands corresponded to β-lactoglobulin. A statistical comparison of peak volumes, obtained by densitometry, showed that the peak corresponding to β-lg in samples heated to 90°C for 5 min and centrifuged at 4500 g, differed significantly (P<0·05) from the rest of samples centrifuged using the same centrifugal force. The peak volume was decreased with an increase of temperature applied during heat treatment. No such effect has been detected in the case of bovine milk (Fig. 3(B)).
For both milk types, the extent of irreversible denaturation of β-lg varied with a choice of skimming conditions. Statistical comparison of percentages of SDS-monomeric/total reducible β-lg in samples heated at 90°C for 5 min showed that for both caprine and bovine milks, there was a significant difference (P<0·05) between samples skimmed at 600 and 4500 g.
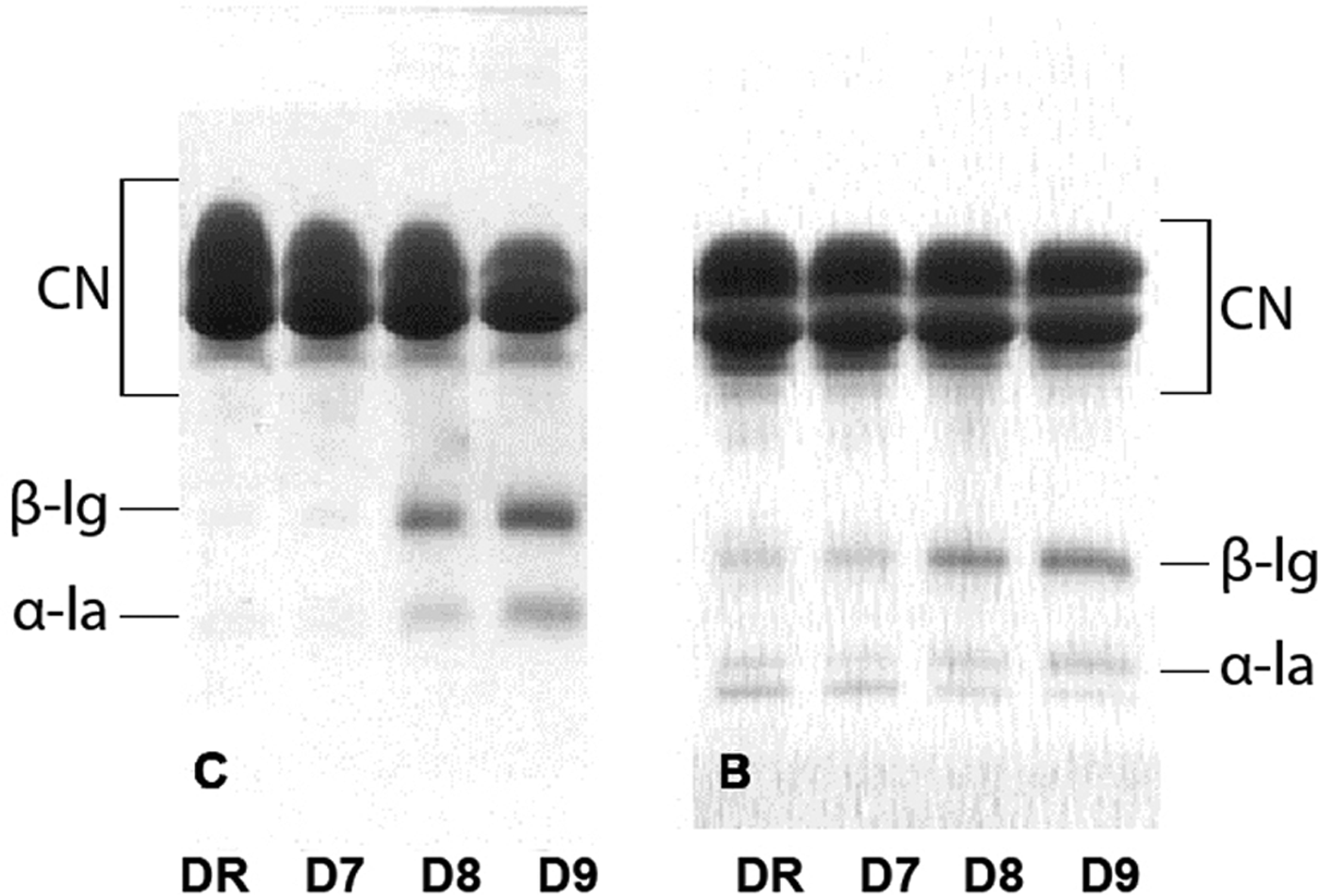
SDS-R-PAGE analysis of precipitates
Precipitates formed by skimming at 4500 g were analysed by SDS-R-PAGE and the patterns are shown in Fig. 4. There was a higher amount of both β-lg and α-lactalbumin in the caprine milk precipitate samples D8 and D9 then in the samples DR and D7. For the bovine milk, a similar pattern was observed only for β-lg.

Fig. 4. SDS-R-PAGE patterns of caprine (C) and bovine (B) precipitates obtained from raw milk (DR), milk heated at 70°C/5 min (D7), 80°C/5 min (D8), 90°C/5 min (D9).
Discussion
Composition of raw caprine and bovine milk samples
Results of the basic composition of caprine and bovine milk were comparable with those reported earlier (Raynal-Ljutovac et al. Reference Raynal-Ljutovac, Park, Gaucheron and Bouhallab2007). It could be observed from the Table 1 that there was a great similarity between basic physico-chemical parameters of bovine and caprine milk.
Precipitate weight ratio
For both caprine and bovine milks, centrifugal force (g-force) had a significant effect on the amount of precipitate formed during skimming (Table 2). It led to the conclusion that, for both milks, it is important to choose the appropriate skimming conditions.
It is claimed in a review paper considering thermal processing of milk (Livney et al. Reference Livney, Corredig and Dalgleish2003) that the pasteurization or the pre-heat treatment at temperatures below 100°C do not destabilize milk. Our study showed that, in the case of caprine milk, the stability of colloidal casein micelles has been reduced when such milk was subjected to sub-ultra high temperatures. No such effect has been detected in the case of bovine milk under the same experimental conditions.
Fat and protein content of raw milk supernatants
It could be observed (Fig. 3) that 0·1% fat that remained in supernatants after centrifugation at 600 g, did not affect the visibility of protein bands in the electrophoretograms.
As for the protein content of raw milk supernatants, significantly higher protein loss caused by centrifugation of raw caprine milk, indicated that even greater attention must be paid to the choice of centrifugal force (g-force) during skimming of this type of milk. In this case, in order to avoid protein loss, only 600 g appeared to be an appropriate choice. There is a possibility to perform complete skimming of bovine milk using 2000 g during centrifugation, without any significant protein loss. We suggest that for both types of milks 4500 g and higher centrifugal forces should be avoided.
SDS-PAGE analysis of supernatants
Significant decrease of β-lg peak volume (reduced samples), that appeared when caprine milk was heated at 90°C for 5 min and centrifuged at 4500 g is in agreement with the previously shown data about the precipitate weight ratio and the protein content of supernatants (Figs. 1 & 2). Peak volume of bovine milk β-lg (reduced samples) did not decrease significantly after the same treatments applied, probably because the precipitate weight ratio was more than two times lower than it was for caprine milk.
From the fact that, for both milk types, the extent of irreversible denaturation of β-lg varied with a choice of skimming conditions, it seems that an inappropriate choice of skimming conditions is likely to lead to a wrong conclusion that the extent of β-lg denaturation was higher than it actually was.
SDS-R-PAGE analysis of precipitates
If electrophoretograms of caprine and bovine precipitates (Fig. 4) are compared with their analogue supernatant electrophoretograms (Fig. 3), it could be noticed that the whey proteins were beginning to appear in precipitates of both milk types heated at 80°C, while significant irreversible covalent aggregation of whey proteins was present in supernatants obtained only after treatment at 90°C.
It has been shown in this study that the colloidal system of caprine milk was more susceptible to destabilization during centrifugation than bovine milk. The largest or the most unstable particles are the ones subjected to precipitation. It explains why in samples DR and D7 the majority of proteins were caseins. However, when milk samples were heated to 80 and 90°C for 5 min prior to centrifugation, a noticeable amount of major whey proteins appeared in precipitates. It is possible that by becoming a part of the casein micelles, they also became a part of precipitate. The nature of bonds between whey proteins and casein in precipitates were not determined in this study, thus, all possible bonds could be included: hydrophobic, electrostatic and covalent.
Kinetics of denaturation and aggregation of β-lg is different at 80 and 90°C. At 80°C, activation energy for denaturation of β-lg is significantly higher than at 90°C and the consequence is a slower denaturation at 80°C, while at 90°C the denaturation is almost instantaneous (Donato & Guyomarc'h, Reference Donato and Guyomarc'h2009). Obviously, rapid denaturation of β-lg after 90°C/5 min treatment enables formation of a noticeably larger amount of covalent aggregates. Appearance of β-lg in precipitate samples of milk treated at 80°C, on the other hand, could mean that it has been hydrophobically or electrostatically bonded to casein micelles. This is in agreement with the proposition made by some authors (Donato & Guyomarc'h, Reference Donato and Guyomarc'h2009) that hydrophobic interactions come prior to thiol/disulphide exchange. Also, with mild heating the changes in conformation of whey proteins may be reversible, but with more severe heating, the unfolded whey proteins become associated with other whey proteins or with the casein micelles. The association may initially be through hydrophobic interaction, and later by formation of disulphide bonds with other whey proteins or κ-casein (Law & Leaver, Reference Law and Leaver2000).
Some authors show that denaturation of α-la and β-lg is more than 50 and 75%, respectively, when samples of caprine and bovine milks are heated at 80°C for 5 min (Montilla et al. Reference Montilla, Balcones, Olano and Calvo1995). Others detected even higher level of denaturation, and reported that less than 5% of undenatured β-lg is found when caprine milk is heated at 80°C for 5 min, whereas more than 20% of β-lg remains undenatured in bovine milk (Calvo et al. Reference Calvo, Amigo, Olano, Martin and Ramos1989). It is obvious, from the literature, that significant denaturation of whey proteins occurs after a heat treatments lower than 90°C, but according to our study, significant covalent irreversible aggregation occurred only when milk was heated at 90°C/5 min.
Conclusions
The fact that the amount of precipitate formed after skimming of caprine milk is significantly higher than in case of bovine milk, and the fact that heat treatment prior to skimming affects only the precipitate weight ratio of the caprine milk, represent manifestations of lower colloidal stability of the caprine milk in comparison with the bovine milk. The differences in the stability of colloidal casein micelles between these two milk types is known for decades, but the results of our study could serve as the basis for further investigations on adjusting the skimming conditions for caprine milk in industrial dairy processing environments. We assume it would lead to higher quality of caprine milk and derived dairy products.
Furthermore, since it is determined that small amount of fat remained after skimming at 600 g does not reduce the visibility of protein bands obtained on electrophoretograms, in terms of avoiding protein losses and wrong interpretations of results, we suggest that the centrifugal conditions used in laboratories for milk skimming should be 600 g/15 min/20°C.
This study emphasises the importance of considering caprine milk as a different raw material from bovine milk; and the need for extreme caution when laboratory and/or industrial processes established for bovine milk are being adapted to caprine milk and its specific characteristics.
We are grateful to the Serbian Ministry of Education and Science for providing Grant III 46009 for this study. We are also grateful to dairy plants ‘Beocapra’, and ‘PIK’ from Serbia for providing caprine and bovine milk.