INTRODUCTION
Birds are hosts to a number of intracellular blood parasites, including Haemosporidia of the genera Plasmodium, Haemoproteus and Leucocytozoon, Haemogregarinidae of the genus Hepatozoon and piroplasmids of the genus Babesia. These parasites may exert important ecological and evolutionary pressures on life-history traits of avian hosts (e.g. Merino et al. Reference Merino, Moreno, Sanz and Arriero2000; Hõrak et al. Reference Hõrak, Ots, Vellau, Spottiswoode and Møller2001; Marzal et al. Reference Marzal, de Lope, Navarro and Møller2005).
The prevalence of infection varies greatly among different bird taxa (e.g. Bennett et al. Reference Bennett, Bishop and Peirce1993; Valkiūnas Reference Valkiūnas2005). For example, seabirds are often free from blood parasites (e.g. Peirce and Brooke, Reference Peirce and Brooke1993; Merino et al. Reference Merino, Barbosa, Moreno and Potti1997a ; Merino and Minguez, Reference Merino and Minguez1998; Engström et al. Reference Engström, Dufva and Olsson2000). A recent review of blood parasite prevalence in birds suggested that multiple factors are responsible for patterns of association between parasitic infections and ecological and life history traits in seabirds (Quillfeldt et al. Reference Quillfeldt, Arriero, Martínez, Masello and Merino2011). Indeed, life history and ecological parameters that increase the exposure time to arthropod vectors may be important. Furthermore, historical/phylogenetic factors may also influence susceptibility to different parasitic infections. For example, there is a relatively high prevalence of Haemoproteus in gulls and frigatebirds and Plasmodium in penguins, while Hepatozoon are apparently confined to albatross and storm-petrel species (Quillfeldt et al. Reference Quillfeldt, Arriero, Martínez, Masello and Merino2011).
This study used genetic methods to detect parasites in five species belonging to two genera of seabirds that breed on tropical islands off Brazil. There have been no previous studies in this region that focus on protozoa in seabird blood (Quillfeldt et al. Reference Quillfeldt, Arriero, Martínez, Masello and Merino2011). In particular, we were interested in inter- and intraspecific differences in prevalence patterns of blood parasites in these sympatrically breeding birds. Based on published data, we hypothesized that noddies would most likely be infected with Haemoproteus and boobies with Babesia.
METHODS
Study sites and species
Boobies and noddies are circumtropical species that breed yearly. They have mean body masses varying from 100–200 and 800–1500 g in noddies and boobies, respectively. These seabirds were sampled at five breeding colonies on offshore islands of the Atlantic coast of Brazil (for sample sizes, dates and locations see Tables 1 and 2):
-
1. Fernando de Noronha (3·854°S, 32·424°W): This site consists of one large island and 19 small adjacent islets, within a total area of 26 km2. It contains the most diverse seabird community in Brazil, with 11 breeding seabird species and c. 25 000 breeding pairs (Antas, Reference Antas and Croxall1991; Schulz-Neto, Reference Schulz-Neto and Branco2004a ). These islands provide different nesting habitats. For example, black noddies, Anous minutus and red-footed boobies, Sula sula nest in dense (vegetation cover 80%) forest of the Mulungu tree, Erytrina velutina (Schulz-Neto, Reference Schulz-Neto and Branco2004a ), while brown noddies, Anous stolidus and brown boobies, Sula leucogaster nest on the rocky ground. Masked boobies, Sula dactylatra nest in medium (∼0·5 m height) or tall grass (>0·5 m height). Arthropods reported on these islands include ticks (Ixodidae – Rhipicephalus microplus and Astigmatina), flies and mosquitoes (Flechtmann, Reference Flechtmann1987). Black noddies on Fernando de Noronha also nest in small platforms on the ground of vertical, well-protected cliffs.
-
2. Abrolhos archipelago (17·926°S, 38·935°W) is a group of five small islands located ∼65 km off the Brazilian coast. Six seabird species breed at this site, with c. 3000 breeding pairs (Alves et al. Reference Alves, Couto, Efe and Ribeiro2000). The percentage of vegetation cover varies between 0 and 40%. Rock, which is sparsely covered with short grass ∼0·2 m high (Alternanthera maritima, Cyperus imbricatus, Blutaparon portulacoides; Hazin and Macedo, Reference Hazin and Macedo2006) provides nesting habitat for brown noddies, while masked and brown boobies predominantly nest in sites containing tall grass (C. imbricatus, Borreria verticilata; IBAMA/FUNATURA, 1991) as well as short grass. Ticks, mosquitoes and flies (Olfersia spinifera; Graciolli and Carvalho, Reference Graciolli and Carvalho2003) have been reported on these islands.
-
3. Ilha da Trindade (20·517°S, 29·300°W) is located at the far east of the Vitória-Trindade submarine ridge, 1160 km off Brazil. The island has five breeding species, including the brown noddy that nests in areas of large stones, pebbles, bare rock and medium grass (predominantly Cyperus atlanticus and Bulbostylis nesiotis, vegetation cover 40%). Flies, O. spinifera have been recorded (Graciolli and Carvalho, Reference Graciolli and Carvalho2003), however there are no records of ticks or haematophagous mosquitoes.
-
4. Atol das Rocas (3·856°S, 33·817°W) is located 145 km west of Fernando de Noronha (Kikuchi and Leão, Reference Kikuchi and Leão1997) and has the largest seabird colony with c. 150 000 individuals (Schulz-Neto, Reference Schulz-Neto1998), including five seabird species which breed on both islands (Schulz-Neto, Reference Schulz-Neto and Branco2004b ). However, although black noddies and red-footed boobies breed on Fernando de Noronha they also rest and forage on Atol das Rocas (Schulz-Neto, Reference Schulz-Neto and Branco2004b ). These islands are mostly sandy with bushes. Brown noddies, masked and brown boobies nest on the ground, in areas with 80–90% of short grass vegetation cover, Portulaca oleracea, as well as medium grass, Cyperus ligularis (Schulz-Neto, Reference Schulz-Neto and Branco2004b ). Black noddies breed in coconut palm trees (Cocos nucifera), while red-footed boobies rest in coconut palm trees.
-
5. The São Pedro and São Paulo Archipelago (SPSPA) (0·917°N, 29·335°W) is a remote group of 10 small rocky islands located ∼1000 km from the Brazilian coast, and 610 km from Fernando de Noronha. It contains the smallest colony of seabirds, with three breeding species of approximately 1000 individuals (Both and Freitas, Reference Both, Freitas and Branco2004). There is no vegetation on SPSPA, so birds are crowded in a small area of rocky outcrops.
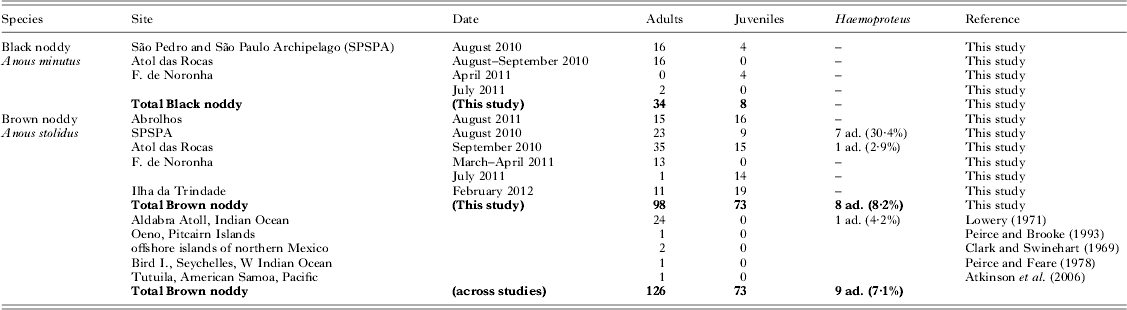
Table 1. Studies of blood parasite prevalence in noddies, including the present results. The results of PCR-based screening for Haemoproteus are given, and published studies of the same species are summarized (ad. = adults). In the present study, all individuals were negative in PCR-based screening for Babesia and Leucocytozoon
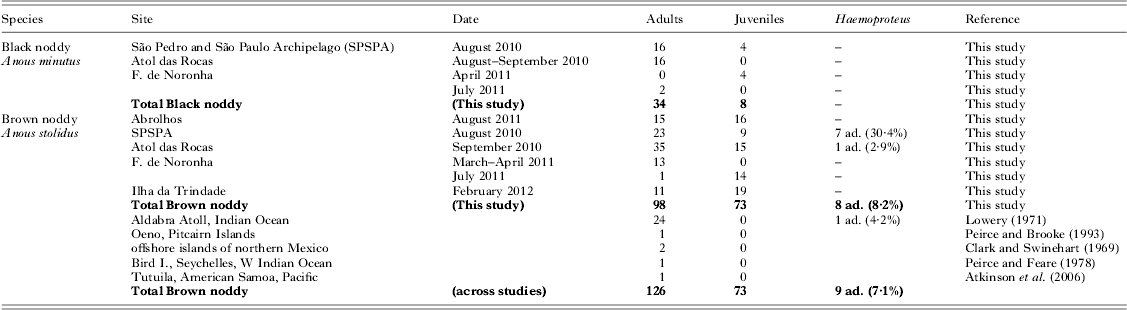
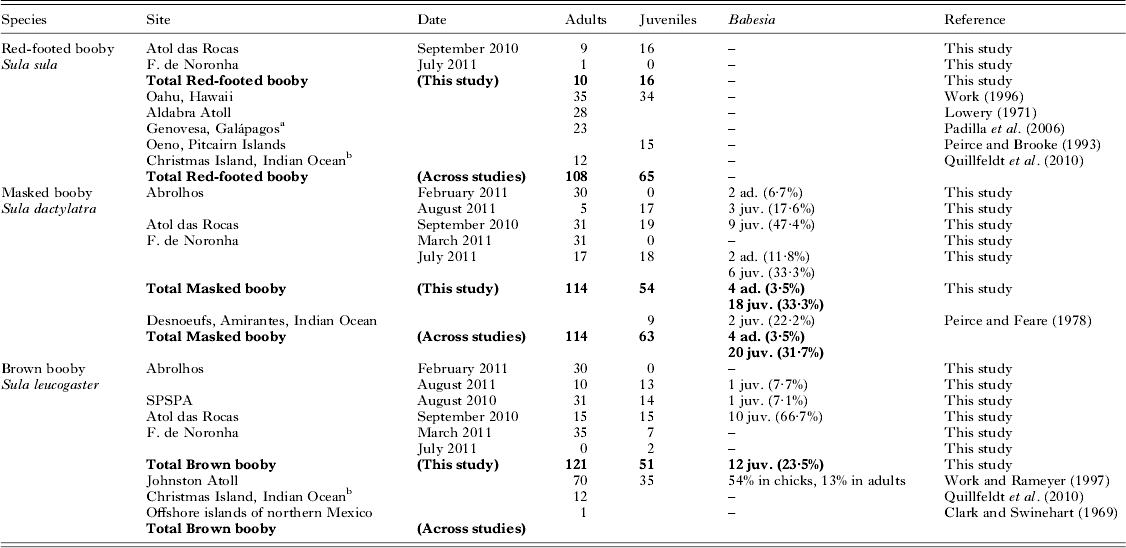
Table 2. Studies of blood parasites in boobies, including the present results. The results of PCR-based screening for Babesia are given, and published studies of the same species are summarized (ad. = adults, juv. = juveniles). In the present study, all individuals were negative in PCR-based screening for Plasmodium/Haemoproteus and Leucocytozoon
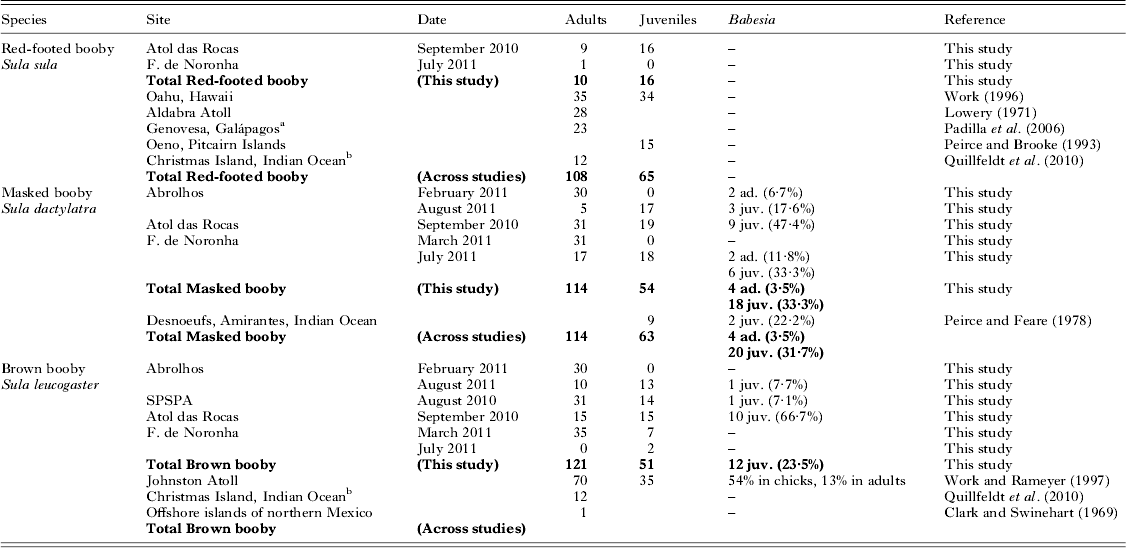
a 9% of the Red-footed boobies on Genovesa were infected with Haemoproteus.
b Samples analysed by Quillfeldt et al. (Reference Quillfeldt, Martinez, Hennicke, Ludynia, Gladbach, Masello, Riou and Merino2010) for Plasmodium/Haemoproteus and Leucocytozoon were subsequently also screened for Babesia using the methods outlined in the present paper (Javier Martínez, unpubl. data).
Sample collection and PCR screening
Chicks and adults were caught either by hand or by using dip nets. Each bird was weighed to the nearest 5 or 10 g using a Pesola® spring balance. Standard structural measurements were recorded as follows: culmen and tarsus lengths to the nearest 0·1 mm using callipers, wing and tail lengths to the nearest mm using stopped wing and feather rulers, respectively.
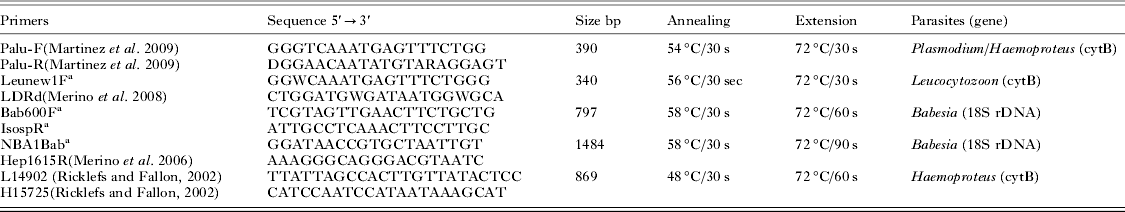
All adults were sampled during the breeding season, while on their nest incubating eggs or attending young, except for black noddies and red-footed boobies, which were sampled at Atol das Rocas, even though they breed on Fernando de Noronha (Schulz-Neto, Reference Schulz-Neto and Branco2004a , Reference Schulz-Neto and Branco b ). Each bird was individually marked with a numbered metal ring to avoid sampling the same bird multiple times. For each bird, a drop of blood was obtained from the tarsal or brachial vein and stored on FTA classic cards (Whatman International Ltd., UK). Small pieces of FTA card were cut with sterilized scissors. Genomic DNA was extracted from FTA cards as indicated by Martínez et al. (Reference Martínez, Martínez de la Puente, Herrero, Del Cerro, Lobato, Rivero de Aguilar, Vasquez and Merino2009). Polymerase chain reactions (PCR) were used to detect haemoparasites. Information about primers can be found in Table 3. PCR were performed in a 10 μL reaction volume, containing 20–100 ng template DNA, 50 mm KCl, 10 mm Tris–HCl, 1·5 MgCl2, 0·2 mm dNTPs, 0·25 μ m primer, and 1·25 U of AmpliTaq Gold 360 (Applied Biosystems, Foster City, CA, USA). PCR using the Veriti thermal cycler (Applied Biosystems, Foster City, CA, USA) conditions are as follows: 95 °C for 10 min (polymerase activation), 40 cycles at 95 °C for 30 s, annealing temperature (see Table 2), extension temperature (see Table 3), and a final extension at 72 °C for 10 min. PCR assays were checked using agarose gel electrophoresis. Amplicons were recovered from agarose gels (UltraClean GelSpin DNA Purification kit, MO BIO) and subjected to direct sequencing using an ABI 3130 (Applied Biosystems) automated sequencer. Positive and negative controls were routinely used.
Table 3. PCR settings and primer pairs used to perform the molecular screening in the present study (PaluF/PaluR, Leunew1F/LDRd, and Bab600F/IsospR). All amplicons achieved were sequenced. The primers Palu F/R can detect both Plasmodium and Haemoproteus. In order to achieve a larger amplicon to perform a reliable phylogenetic inference, one Babesia and one Haemoproteus isolate were amplified by using the sets of primers NBA1Bab/Hep1615R and L14902/H15725
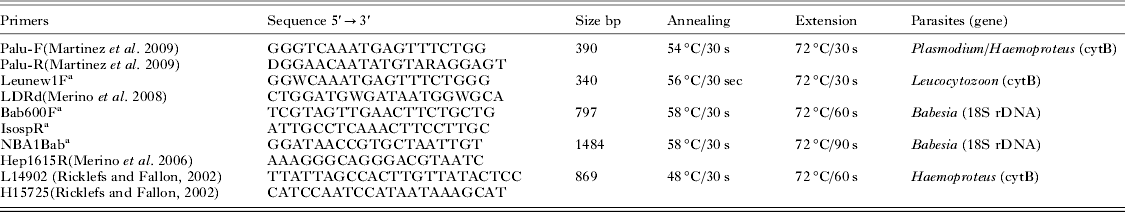
a Primers designed in this study.
Phylogenetic analysis
DNA sequences for Haemoproteus (cytochrome B) obtained from noddies were aligned with 77 other sequences belonging to Haemoproteus or Parahaemoproteus species that can be found on GenBank. The alignment was performed using the CLUSTALW algorithm implemented in BIOEDIT (Hall, Reference Hall1999). The final alignment contained 558 positions and 80 sequences, including two lineages of Leucocytozoon as the outgroup. The alignments were analysed using Bayesian inference, implemented in MrBayes 3.2 (Ronquist and Huelsenbeck, Reference Ronquist and Huelsenbeck2003), setting the substitution model GTR+G. The model was previously selected using corrected AIC (Akaike Information Criterion) implemented in JMODELTEST 0.0.1 (Posada, Reference Posada2008). This analysis consisted of 2 runs of 4 chains each, with 3 000 000 generations per run and a burn-in of 300 000 generations (54 000 trees for consensus tree). The final standard deviation of the split frequencies was lower than 0·01. Convergence was checked using TRACER v1.5 software (a program for analysing the trace files generated by Bayesian MCMC runs; Rambaut and Drummond, Reference Rambaut and Drummond2007). All model parameters were higher than 100 indicating convergence.
The Babesia DNA sequence (18S rDNA) obtained from boobies was aligned together with 20 other sequences belonging to Babesia species that were listed in GenBank. The alignment was performed using PROBCONS (http://toolkit.tuebingen.mpg.de/probcons). Poorly aligned positions and divergent regions of the alignment were suppressed using GBlocks (Talavera and Castresana, Reference Talavera and Castresana2007) selecting the following options: ‘Minimum Number of Sequences for a Conserved Position’ to 11, ‘Minimum Number of Sequences for a Flank Position’ to 17, ‘Maximum Number of Contiguous Nonconserved Positions’ to 3, ‘Minimum Length of a Block’ to 10, and ‘Allowed Gap Positions’ to ‘With Half’. The final alignment contained 1404 positions. In this case, the substitution model GTR+I+G was selected to perform the Bayesian analysis. Only 1 000 000 generations were necessary to obtain convergence. The tree was rooted on the midpoint.
In addition, the maximum-likelihood inference was also performed using PhyML (Guindon et al. Reference Guindon, Dufayard, Lefort, Anisimova, Hordijk and Gascuel2010). This analysis was performed with the two alignments. The substitution models were those indicated above, the subtree pruning and regrafting and the nearest-neighbour interchange tree-rearrangements were selected, and the approximate likelihood-ratio test was used to obtain the clade support.
Microscopic analyses
Studies using blood smears may not detect parasites if the intensity of infection is low (e.g. Valkiūnas, Reference Valkiūnas2005). Genetic methods using PCR can detect infections missed by blood smears (e.g. Feldman and Freed Reference Feldman and Freed1995; Bensch et al. Reference Bensch, Stjernman, Hasselquist, Ostman, Hansson, Westerdahl and Pinheiro2000; Perkins and Schall, Reference Perkins and Schall2002; Ricklefs et al. Reference Ricklefs, Swanson, Fallon, Martinez-Abrain, Scheuerlein, Gray and Latta2005; Parker et al. Reference Parker, Whiteman and Miller2006; Merino et al. Reference Merino, Moreno, Vásquez, Martínez, Sánchez-Monsálvez, Estades, Ippi, Sabat, Rozzi and McGehee2008). PCR has also been shown to be more sensitive than microscopic-based diagnosis of Babesia spp. (Almeria et al. Reference Almeria, Castella, Ferrer, Ortuno, Estrada-Pena and Gutierrez2001; Ano et al. Reference Ano, Makimura and Harasawa2001). However, for identification of the parasite species, molecular methods should be combined with light microscopy (Valkiūnas et al. Reference Valkiūnas, Iezhova, Krizanauskiene, Palinauskas, Seghal and Bensch2008).
A drop of blood from each bird was immediately smeared and air-dried, fixed with methanol and later stained with Giemsa stain (1/10 v/v) for 30 min. Blood smears from birds that were positive for blood parasite infections by PCR were scanned using an optic microscope following methods described by Merino et al. (Reference Merino, Potti and Fargallo1997b ). In brief, one-half of every blood smear was scanned at ×200 to look for extracellular parasites. Intracellular stages of haematozoa were sought at ×400 in the other half of the sample. The oil immersion objective was used when a possible parasite was sought at ×400. All samples were scanned using a microscope Olympus B061.
Statistical data analysis
Statistical analyses were carried out in SigmaStat 3.5. and SPSS 11.0. Prevalence was given with 95% confidence intervals (95% CI). In order to compare the body condition of birds infected or free from parasites, and among birds of archipelagos with different rates of infection, we calculated mass residuals, i.e. the difference between observed mass and predicted mass. Predicted masses were calculated using a linear regression of body mass on four measures of structural size (e.g. Dehnhard et al. Reference Dehnhard, Quillfeldt and Hennicke2011). To account for temporal changes in body mass, only birds sampled in August–September were included in these analyses. Predicted masses were calculated for brown noddy adults according to the regression equation: M mean = −330·9+1·4* Culmen+3·2*Tarsus+1·2*Wing+0·3*Tail (R = 0·597, F = 9·3, P<0·001). The calculated mass residuals were compared among birds of the different archipelagos using analysis of variance (ANOVA). Predicted masses for juvenile masked boobies were calculated according to the regression equation: M mean = −2278·2+17·1* Culmen+27·8*Tarsus−0·9*Wing+5·3*Tail (R = 0·612, F = 7·3, P<0·001). The calculated mass residuals were compared among individuals with and without parasites using t-tests.
Results
Noddies
We did not detect any parasites in black noddies (N = 34 adults and 8 juveniles, Table 1). In brown noddies, 8 of 98 adult birds (8·2±5·4%) were infected with Haemoproteus (GenBank accession number KC754967), while none of the juveniles (N = 73) were infected. Haemoproteus DNA was detected in adult brown noddies from two of five sampling sites (Table 1), namely a high prevalence on SPSPA (30·4±18·8%, N = 23) and a single bird at Atol das Rocas (2·9±5·5%, N = 35). The proportions of infected adult brown noddies varied among colonies (Chi-square test: χ 2 = 20·1, d.f. = 4, P<0·001). The mass residuals of adult brown noddies did not differ among archipelagos with different occurrence of Haemoproteus (ANOVA: F 2,69 = 1·4, P = 0·248). We did not detect parasites from blood smears, indicating a low intensity of infection.
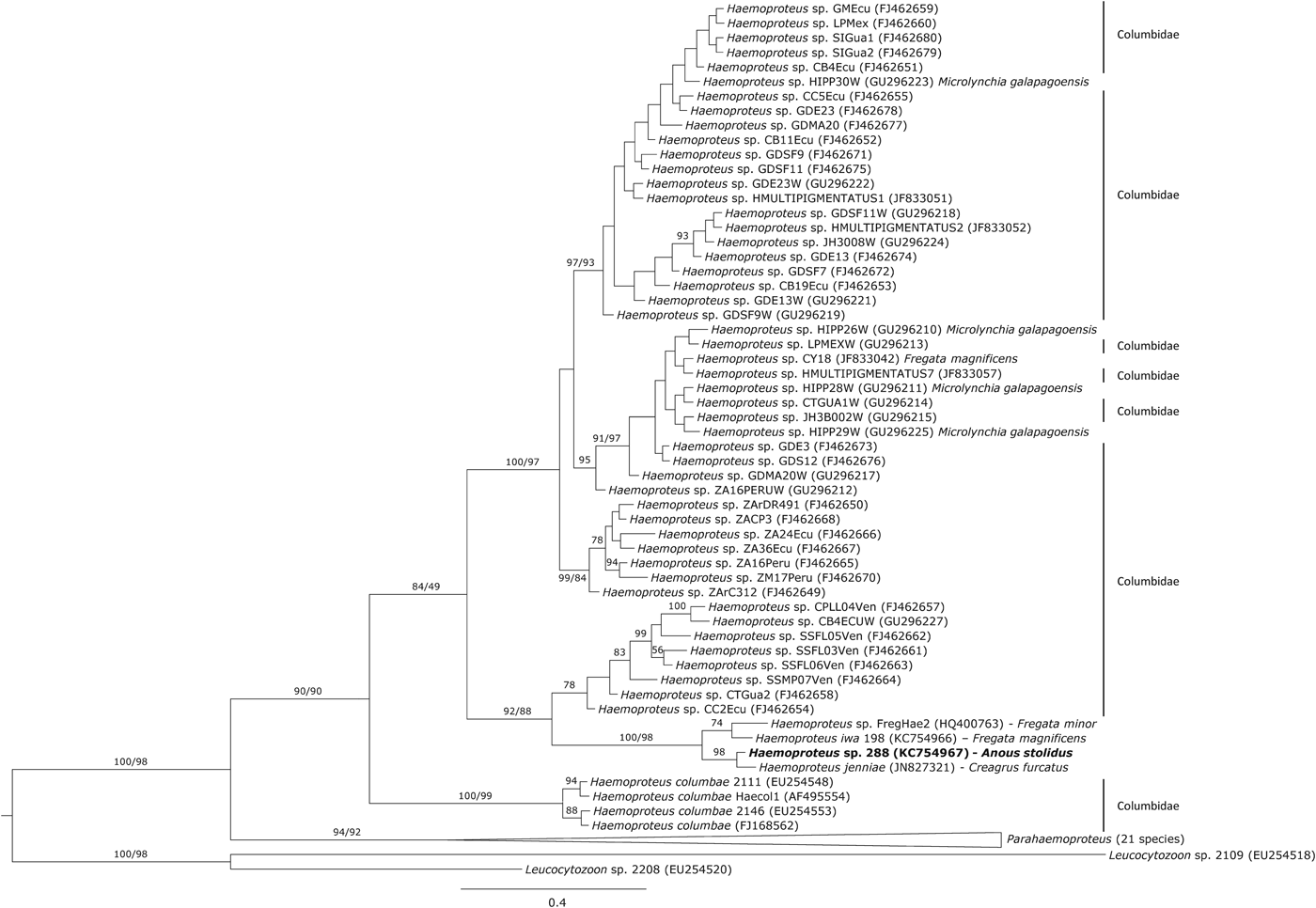
The phylogenetic analysis showed that the haplotype 288 isolated from noddies clustered with three Haemoproteus haplotypes isolated from other seabirds (Fig. 1), of which Haemoproteus jenniae isolated from swallow-tailed gulls (Creagrus furcatus) was the closest species (99·5%). The genetic distance between haplotype 288 and Haemoproteus iwa isolated from magnificent frigatebird Fregata magnificens was 1·3%.
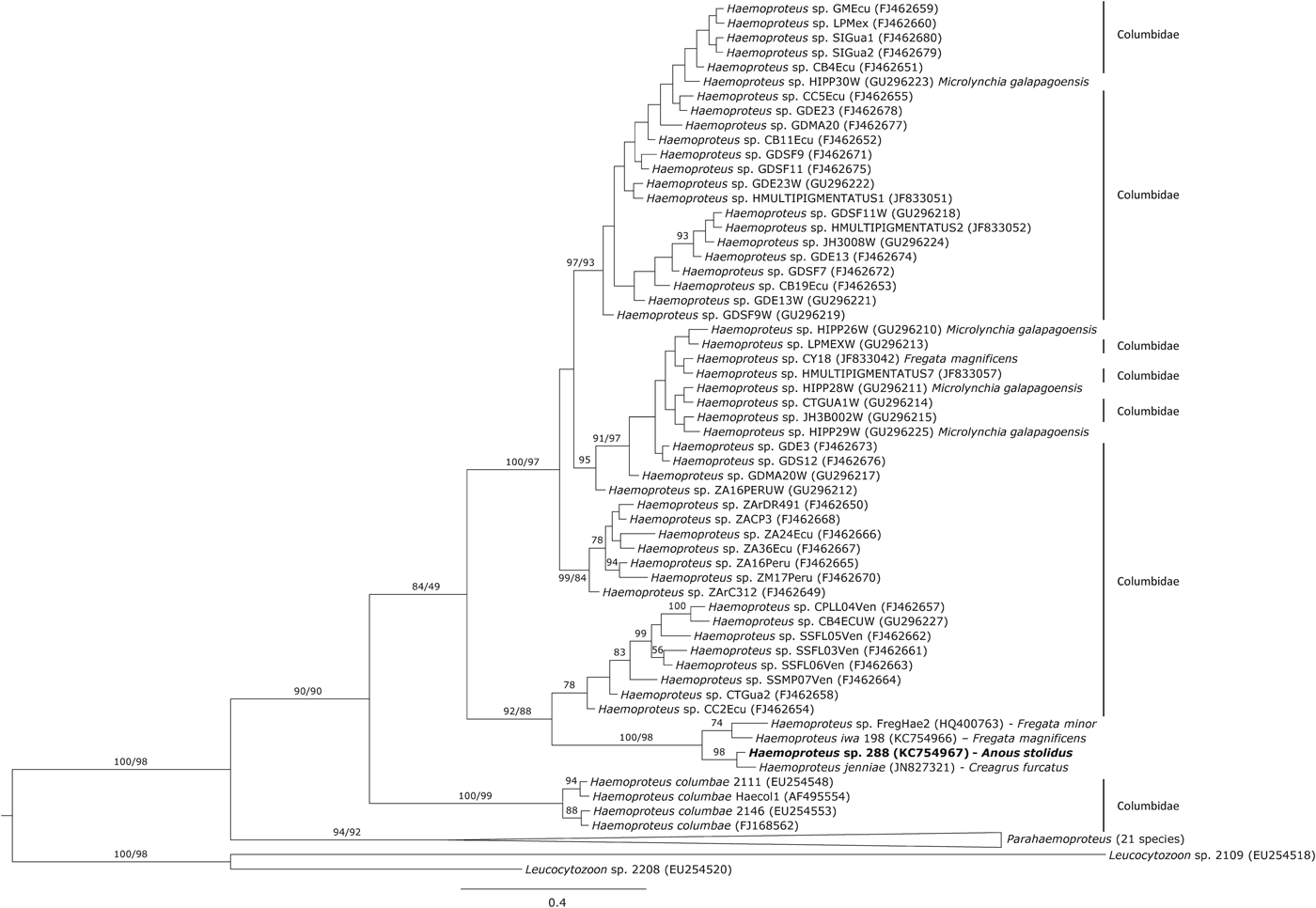
Fig. 1. Phylogenetic inference of the Haemoproteus haplotype found in brown noddies Anous stolidus. Phylogenetic tree was obtained with the program MrBayes v3.2 using the substitution model GTR+G. When clades present two support values, the first one corresponds with the Bayesian support and the second one with that achieved by maximum likelihood inference. The Haemoproteus haplotype isolated in the present study is marked in bold.
Boobies
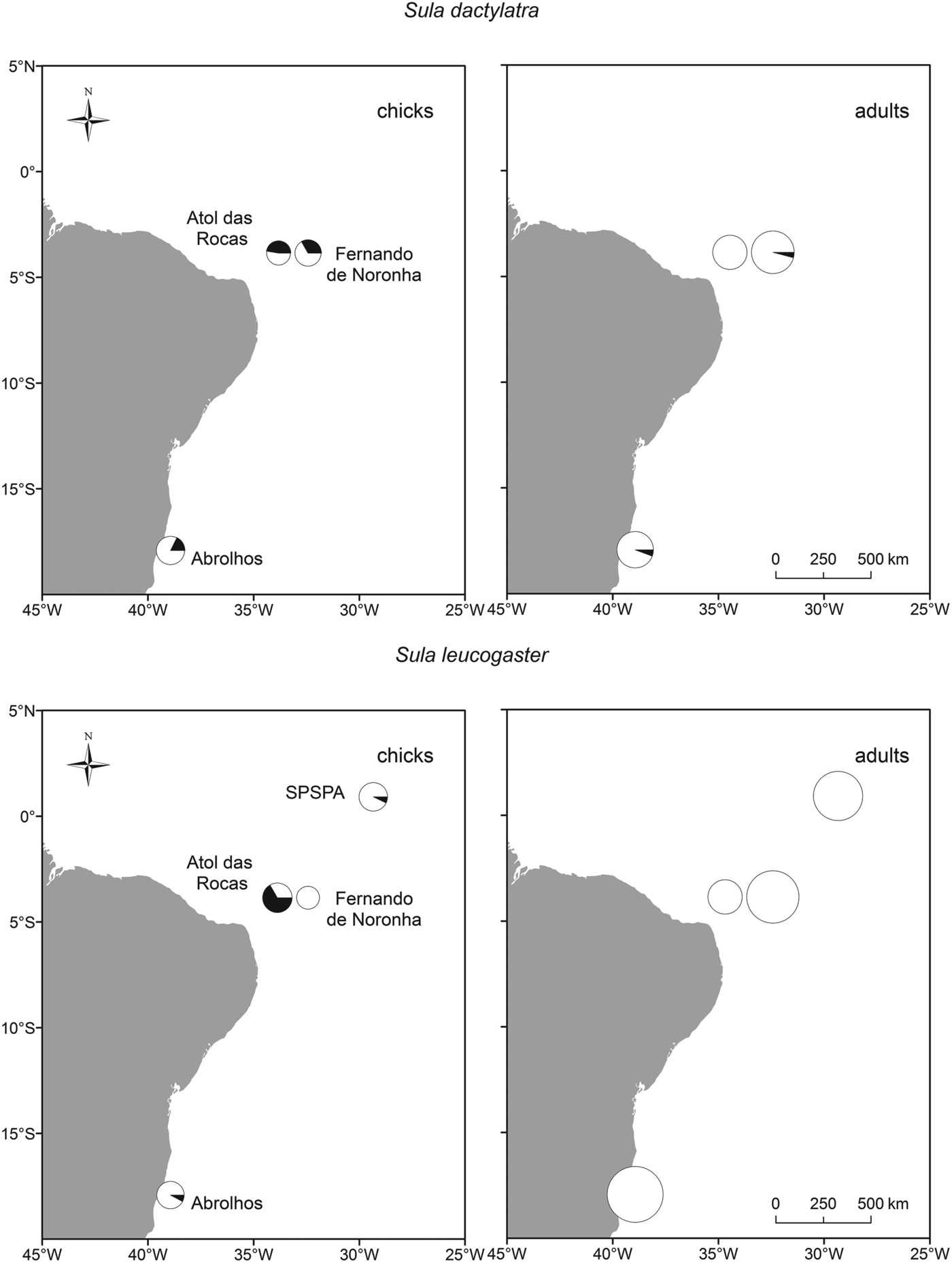
We did not detect any parasites in red-footed boobies (N = 10 adults and 16 juveniles, Fig. 2, Table 2). Brown boobies and masked boobies were infected with Babesia (GenBank accession number KC754965).
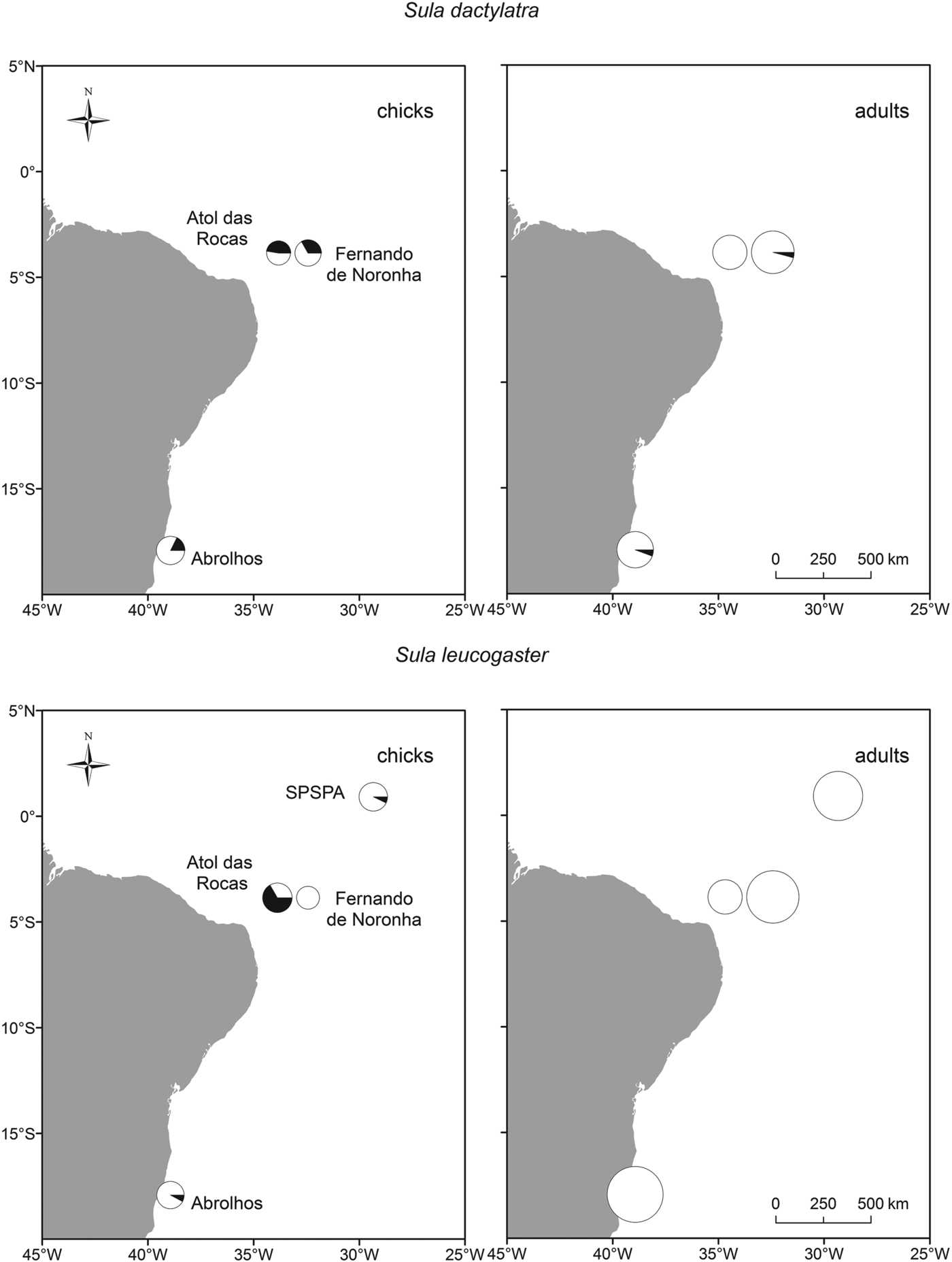
Fig. 2. Infection of brown and masked boobies with Babesia poelea on Brazilian Atlantic islands. The size of the symbols represents the sample size, and the portion of black in the circle represents the percentage of infected animals.
In brown boobies, only juveniles were infected (23·5±11·6%, N = 51), while all adults (N = 121) were free from blood parasites. Babesia DNA was detected in brown booby juveniles from three of four breeding sites (Table 2), with low prevalence on Abrolhos (7·7±14·5%, N = 13) and SPSPA (7·1±13·5%. N = 14), but high prevalence in the largest colony, on Atol das Rocas (66·7±23·9%, N = 15). The proportions of infected brown booby juveniles varied among colonies (χ 2 = 22·2, d.f. = 3, P<0·001). However, brown booby juveniles from archipelagos with different occurrence of Babesia did not differ in their mass residuals (ANOVA: F 2,36 = 0·2, P = 0·830). Likewise, the mass residuals were similar between brown booby juveniles whether infected or free of Babesia (t-test: t = 0·2, d.f. = 37, P = 0·824).
In masked boobies, the prevalence was nearly ten times higher in juveniles (33·3±12·6%, N = 54) than in adults (3·5±3·4%, N = 114). The difference in prevalence between adults and juveniles was statistically significant (χ 2 = 26·1, d.f. = 1, P<0·001). Adults were infected in two of three colonies, with low prevalences of 5·7±7·7% at Abrolhos (N = 35) and 4·2±5·7% at Fernando de Noronha (N = 48). Juveniles were infected at all three breeding sites, with intermediate to high prevalence of 17·6±18·1% (Abrolhos, N = 17), 33·3±21·8% (Fernando de Noronha, N = 18) and 47·4±22·5% (Atol das Rocas, N = 19). The proportions of infected masked boobies did not vary significantly among colonies for either adults (χ 2 = 1·7, d.f. = 2, P = 0·429, power 0·18) or juveniles (χ 2 = 3·6, d.f. = 2, P = 0·168, power 0·36).
We did not detect parasites from blood smears, indicating the low intensity of infection. However, masked booby juveniles infected with Babesia were on average 74·1±44·6 g lighter than the population mean, while uninfected masked booby juveniles were on average 37·0±28·6 g heavier than the population mean. This difference of c. 7% of body mass was statistically significant (t-test: t = 2·2, d.f. = 52, P = 0·034).
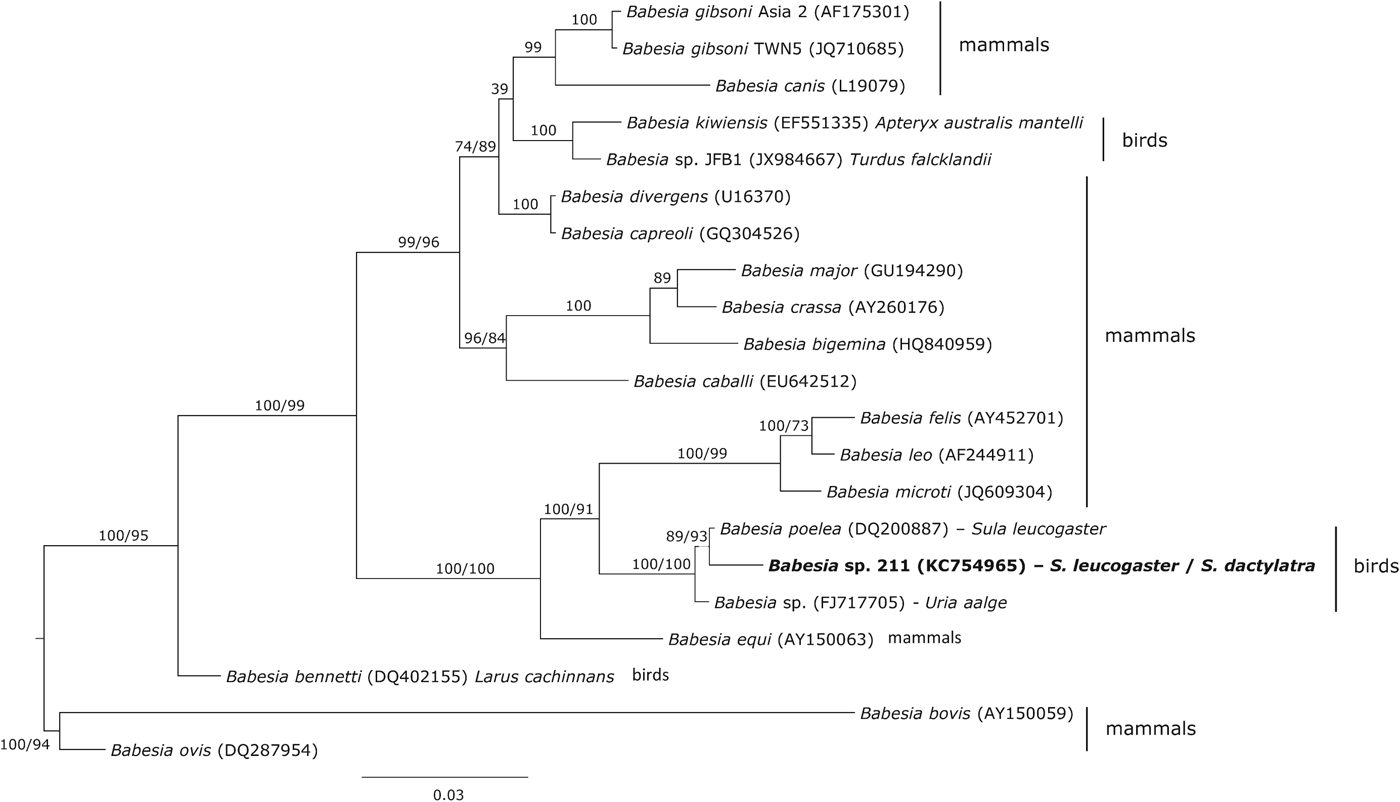
Phylogenetic analysis showed that the haplotype 211 isolated from boobies clustered with two Babesia haplotypes isolates from seabirds, Babesia poelea from brown booby and Babesia sp. from common murre Uria aalge (Fig. 3). The genetic distance between these Babesia species and the haplotype 211 was 0·3 and 0·8%, respectively. However, other Babesia species (Babesia kiwiensis and Babesia bennetti) isolated from birds were phylogenetically distant from this clade.
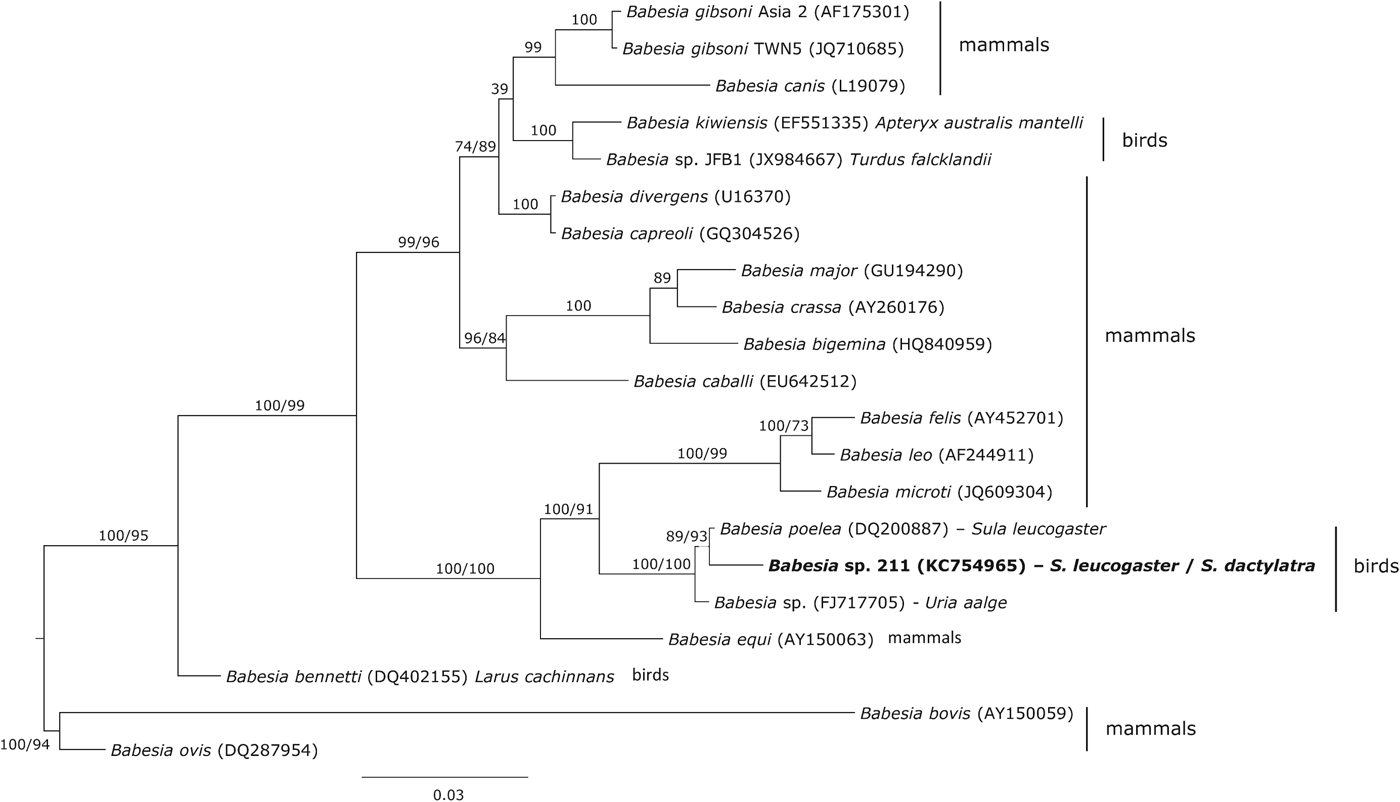
Fig. 3. Phylogenetic inference of the Babesia haplotype found in brown and masked boobies. Phylogenetic tree was obtained with the program MrBayes v3.2 using the substitution model GTR+I+G. When clades present two support values, the first one corresponds with the Bayesian support and the second one with that achieved by maximum likelihood inference. The Babesia haplotype isolated in the present study is marked in bold.
Discussion
In accordance with other seabird studies (reviewed by Quillfeldt et al. Reference Quillfeldt, Arriero, Martínez, Masello and Merino2011), parasite prevalences were low in five species of tropical Brazilian seabirds from Atlantic Ocean islands. Two species were free from parasites, and the other three species had low blood parasite prevalences with parasites detected being the typically found parasite taxa for each genus, with no multiple infections. The absence of infections in black noddies and red-footed boobies might be explained by their different nesting habitats. In contrast to ground-nesting species, these two species nest on trees or bushes, where louse flies (Hippoboscidae), common vectors for Haemoproteus, and ticks (Ixodidae), common vectors for Babesia (e.g. Smith, Reference Smith1996), may be less likely to occur.
Noddies as hosts of Haemoproteus
Haemoproteus sp. are globally distributed in birds with about 100 named species (Peirce, Reference Peirce2005). Within seabirds, Haemoproteus parasites are especially common in frigatebirds, Fregatidae (Quillfeldt et al. Reference Quillfeldt, Arriero, Martínez, Masello and Merino2011; Merino et al. Reference Merino, Hennicke, Martínez, Ludynia, Masello and Quillfeldt2012) and gulls and terns, Laridae (Quillfeldt et al. Reference Quillfeldt, Arriero, Martínez, Masello and Merino2011).
Thus, our present finding of a Haemoproteus infection in a noddy, belonging to, or with close affinity to the Laridae, supports this pattern. The only previous record of a Haemoproteus infection in a noddy was detected on Aldabra Atoll in the Indian Ocean, where one of 24 adult brown noddies was infected by an unknown species of Haemoproteus (Lowery, Reference Lowery1971). The phylogenetic analysis showed that the Haemoproteus haplotype isolated from noddies on Brazilian islands are close to others isolated from seabirds including gulls. The lack of haematic stages in the blood smears indicated that the infection was chronic and not acute (e.g. Madsen et al. Reference Madsen, Valkiūnas, Iezhova, Mercade, Sanchez and Osorno2007). The fact that only adult individuals were found parasitized further suggested that risk of infection is related to their permanence in areas inhabited by suitable vectors.
In general, Haemoproteus parasites are considered relatively benign in birds. Two seabird species affected by Haemoproteus have been studied in detail, through correlative analyses. In magnificent frigatebirds, 16% of males were infected with H. iwa, but all infections were light (<1% of erythrocytes) and were thus classified as chronic (Madsen et al. Reference Madsen, Valkiūnas, Iezhova, Mercade, Sanchez and Osorno2007). In yellow-legged gulls, Larus cachinnans, Martínez-Abraín et al. (Reference Martínez-Abraín, Merino, Oro and Esparza2002) found significant differences in Haemoproteus lari prevalence between two breeding colonies, which were explained with differences in the vector abundance. The birds of both colonies were in equally good body condition and had similar clutch sizes. Further, the intensity of H. lari infection was not correlated with body condition or egg volume (Martínez-Abraín et al. Reference Martínez-Abraín, Merino, Oro and Esparza2002), suggesting that H. lari parasites had little effect on the health of the gulls under normal conditions.
Likewise, Haemoproteus infection in the present study was not correlated to the body condition of noddies. Studies in other birds also suggest that Haemoproteus numbers are normally kept low by natural immunity and only tend to multiply under stress and other diseases. It has therefore been suggested that increasing Haemoproteus parasitaemia (percentage of red cells infected) can serve as a valuable indicator of an underlying disease (e.g. Remple, Reference Remple2004) or of stress (Valkiūnas, Reference Valkiūnas2005). However, experimental studies suggest important detrimental effects of Haemoproteus blood parasites on bird fitness (Merino et al. Reference Merino, Moreno, Sanz and Arriero2000; Marzal et al. Reference Marzal, de Lope, Navarro and Møller2005). In magnificent frigatebirds, males infected with H. iwa had a less intensely coloured red inflatable gular pouch (Madsen et al. Reference Madsen, Valkiūnas, Iezhova, Mercade, Sanchez and Osorno2007), which is an important ornament used in mate choice (Dearborn et al. Reference Dearborn, Anders and Parker2001). This suggests that even light infections can influence the reproductive success of individuals and thus, be subject to intense selection.
Boobies as hosts of B. poelea
Babesia spp. are tick-transmitted protozoan haemoparasites that infect mammals and birds. Currently, over 100 Babesia species are known, and together with Theileria spp. they are referred to as piroplasmids or piroplasms (Piroplasmida). Of 14–18 Babesia species recognized in birds (Jefferies et al. Reference Jefferies, Down, McInnes, Ryan, Robertson, Jakob-Hoff and Irwin2008; Votýpka, Reference Votýpka2011; Peirce and Parsons, 2012), five infect different seabird groups: B. poelea (boobies), Babesia peircei (2 penguin species), B. bennetti (1 gull species), Babesia uriae (1 auk species) and Babesia ugwidiensis (5 cormorant species). While the majority of species infecting domestic mammals cause disease, only two avian species, Babesia shortti and B. uriae, are known to be pathogenic (Samour and Peirce, Reference Samour and Peirce1996; Yabsley et al. Reference Yabsley, Greiner, Tseng, Garner, Nordhausen, Ziccardi, Borjesson and Zabolotzky2009; Votýpka, Reference Votýpka2011).
Babesia had been found previously in two studies in boobies (Table 2). The first record applied to two of nine nestling masked boobies (22%) that were infected at Desnoeufs, Amirantes, Western Indian Ocean (Peirce and Feare, Reference Peirce and Feare1978), and B. poelea that was then described in Brown Boobies at Johnston Atoll (Work and Rameyer, Reference Work and Rameyer1997). There, B. poelea was found in blood smears from 54% of the chicks, and 13% of the adults. While the prevalence was high, mean parasitaemia in adults and chicks was less than 1% (Work and Rameyer, Reference Work and Rameyer1997), similar to our findings. Babesia infection in the present study was not correlated to the body condition of brown booby juveniles, but masked booby juveniles with a Babesia infection were lighter than those not infected, indicating a slight effect on the health of these birds.
It has been established that once birds become infected with haemosporidian parasites, they remain infected either for life or many years (Garnham, Reference Garnham1966; Valkiūnas, Reference Valkiūnas2005), with infections tending to be dynamic, with relapses occurring. In contrast, juveniles in our study were more susceptible to Babesia infections than adults, and infections apparently decrease in intensity or disappear with increasing age and acquisition of immunity. A higher prevalence of infection in chicks also had been noted in previous studies in boobies (Work and Rameyer, Reference Work and Rameyer1997). Similarly, 20% of juvenile prairie falcons Falco mexicanus were infected with Babesia moshkovskii (Croft and Kingston, Reference Croft and Kingston1975) and Babesia infections in birds are commonly reported from undernourished young individuals (see Merino, Reference Merino1998; Peirce, Reference Peirce2000; Merino et al. Reference Merino, Peirce, Fernández and Lanzarot2002). Boobies and other birds would therefore make interesting models to study mechanisms of condition- or age-related acquired immunity.
Babesia poelea was originally described as an endemic avian haemoparasite in seabirds from the central Pacific (Work and Rameyer, Reference Work and Rameyer1997). The Babesia haplotype isolated in the present study was closely related with B. poelea, the genetic distance between them being 0·3%. Furthermore, the lack of haematic stages in the smears does not make it possible to identify the species. Peirce (Reference Peirce2000) suggested that B. peircei could be a synonym of B. poelea and a molecular study is currently being conducted to solve this problem (see Peirce and Parsons, 2012). The result of the latter study could also help to identify the Babesia species reported here. However, the most relevant issue achieved from the phylogenetic analysis was the lack of monophyly for avian Babesia, as previously reported by Yabsley et al. (Reference Yabsley, Greiner, Tseng, Garner, Nordhausen, Ziccardi, Borjesson and Zabolotzky2009).
The Babesia life cycle typically consists of a sexual phase that takes place in Ixodid ticks, and asexual reproduction inside erythrocytes of vertebrate hosts (Schnittger et al. Reference Schnittger, Rodriguez, Florin-Christensen and Morrison2012 and references therein). Given the abundance of Ixodid ticks in many seabird colonies, the scarcity of piroplasms is surprising. However, the avian piroplasms remain an understudied group of protozoans (Jefferies et al. Reference Jefferies, Down, McInnes, Ryan, Robertson, Jakob-Hoff and Irwin2008), and targeted studies are expected to uncover more avian piroplasms in the future and also to resolve the current taxonomic confusion. There is also debate whether Babesia species are highly adapted to specific vertebrate hosts. Most species of Babesia infecting mammals or birds are host-specific at least to the family level (Votýpka, Reference Votýpka2011). However, some recent studies suggest relatively low host specificity and host switching, such as occasional human infections by Babesia microti, B. divergens, B. duncani (see also Criado et al. Reference Criado, Martínez, Buling, Barba, Merino, Jefferies and Irwin2006; Yabsley and Shock, Reference Yabsley and Shock2013). In the present study, sympatrically breeding seabirds such as noddies were not infected with Babesia, while boobies were infected with B. poelea in far separated sites in the Pacific as well as the Atlantic, thus suggesting high host specificity of this piroplasm.
ACKNOWLEDGEMENTS
We would like to thank Felipe M. Neves, Fernanda P. Marques, Guilherme T. Nunes and Luciana R. Camillo for assistance in the field and Carlos San Juan Martín for assistance in the laboratory.
Fieldwork was supported by Zelinha Brito Silva (Reserva Biológica do Atol das Rocas, ICMBio/RN, Brazil), Ricardo Jerozolimski (Parque Nacional Marinho de Abrolhos ICMBio/BA, Brazil), Ricardo Araújo (Parque Nacional Marinho de Fernando de Noronha ICMBio/PE, Brazil) and Corpo de Bombeiros de Pernambuco from Fernando de Noronha archipelago for permits and logistical support. Brazilian Navy and Comissão Interministerial para os Recursos do Mar (CIRM/SECIRM) provided logistic support for the Trindade and SPSPA expeditions. Also we thank ICMBio for sampling permit No. 22697-1 and CEMAVE-ICMBio for providing us banding permit and leg bands.
FINANCIAL SUPPORT
During the preparation of this work, JM and SM were supported by project CGL2009-09439 from the Spanish Ministry of Science and Technology, and PQ by a grant from DFG, Germany (Qu 148/1-ff). The Brazilian Research Council (CNPq–Grant No. 557152/2009-7) funded the project. PLM was supported by CAPES Foundation (Grant No. 9733/2011-6). LB is a research fellow from the Brazilian CNPq (Proc. No. 308697/2012-0). We thank two anonymous referees for useful comments and Tess Cole for revising the English during the review stage.