INTRODUCTION
Ascidians are among the dominant groups of benthic invertebrates in many marine sessile communities, on both natural and artificial substrates (Jackson, Reference Jackson1977). Solitary and colonial ascidians are frequently seen covering large portions of rocky substrates in the intertidal and shallow subtidal zones, especially in shaded areas (Lambert, Reference Lambert2005). Coastal areas are frequently exposed to intense perturbations due to human colonization and consequent urbanization; as a result, ascidians are exposed to diverse anthropogenic disturbances that may reduce their abundance and diversity (Naranjo et al., Reference Naranjo, Carballo and García-Gomes1996). In contrast, local increase in the number of species is apparently the result of modifications of the marine environment, affecting the sessile community organization, and facilitating the invasion of exotic ascidians from around the world (Chapman & Blockley, Reference Chapman and Blockley2009). Human-mediated reductions or increases in ascidian abundances and diversity are both potentially threatening to sessile communities, because they may reshape the trophic structure of marine communities (Byrnes et al., Reference Byrnes, Reynolds and Stachowicz2007; Stachowicz et al., Reference Stachowicz, Bruno and Duffy2007), and also to the human economy, especially associated with impacts on mariculture (McKindsey et al., Reference McKindsey, Landry, O'Beirn and Davies2007).
In Brazil, the human population is mainly in coastal areas or near coastal cities, all along its 8500 km shoreline. São Sebastião (SS) is one such city, located on the south-eastern coast (Figure 1), about 180 km from Brazil's largest city, São Paulo. The coastal area near SS has alternating rocky shores and sandy beaches, with many coastal islands. The climate is subtropical, and water temperatures range from 12°C at the bottom (50 m deep) to 28°C on the surface during the summer months (Silva et al., Reference Silva, Miranda and Castro-Filho2005). The most populated area is confined between the hills of the Serra do Mar and the São Sebastião Channel (SSC), a passage formed between the continent and São Sebastião Island, the largest coastal island in the region. Channel waters have a maximum depth of 47 m and are relatively well-protected, which provided the motive for the construction of an oil terminal, a harbour and a few marinas.

Fig. 1. Map of the São Sebastião channel on the south-eastern Brazil, showing localities from where data were compiled, where: AI, Alcatrazes Island; AR, Araçá Beach; BA, Baleia Beach; BP, Baleeiro Point; BR, Barequeçaba Beach; BI, Búzios Island; CE, São Sebastião Island (Centro Beach); CG, Cabelo Gordo Beach; DS, São Sebastião Island (Dart shipwreck); FI, Figueira Beach; GR, Grande Beach; JP, Jarobá Point; MT, Montão de Trigo Island; PC, Pontal da Cruz Pier; PE, São Sebastião Island (Perequê Beach); PI, Pitangueiras Beach; PP, Petrobras Pier; PR, Preta Beach; SE, Segredo Beach; SF, São Francisco Beach; SG, São Sebastião Island (Saco da Ponta Grossa); SI, Serraria Island; SP, São Sebastião Island (Saco do Poço); SU, Sumítica Island; VS, São Sebastião Island (Velasquez shipwreck); YC, São Sebastião Island (Yatch Club).
Since the 1950s this region has undergone severe changes, for the most part as a result of urbanization. For example, the São Sebastião Harbour was officially inaugurated in 1955 and another terminal for oil and gas began operation in 1969 (http://www.portodesaosebastiao.com.br). The busy traffic of international ships during the past 40 years greatly increased the probability of introduction of alien species in the region. Today, almost 70,000 people live in SS, with more than double that number during summer vacations, while on the other side of the SSC, the population of Ilhabela city increases ten-fold during the summer (CETESB, 2009). The intense degradation of the hills along the coast has also caused an increasing amount of erosion, turning the water almost permanently murky. It is reasonable to expect that the arrival of alien species with the incoming ships and the continuous degradation of coastal habitats might have caused important shifts in the marine fauna and flora.
São Sebastião city is also home to the Center of Marine Biology (CEBIMar) of the University of São Paulo. Since 1962 when it was incorporated within the university, this research institute resulted in very important contributions to the knowledge about the local marine biota. Prior to the work of Rodrigues (Reference Rodrigues1962, Reference Rodrigues1966, Reference Rodrigues1977) on Brazilian ascidians, only a few records for the region were noted by Van Name (Reference Van Name1945), based on material collected by Lüderwaldt (Reference Lüderwaldt1929). Later, Millar (Reference Millar1958) also published on Brazilian ascidians, including material from SS. During the 1990s, a series of papers on the biodiversity and taxonomic issues of ascidians was published (Rocha, Reference Rocha1988, Reference Rocha1991; Rocha & Monniot, Reference Rocha and Monniot1993, Reference Rocha and Monniot1995; Rodrigues & Rocha, Reference Rodrigues and Rocha1993), culminating with the production of the first identification guide for Brazilian ascidians (Rodrigues et al., Reference Rodrigues, Rocha and Lotufo1998). By the end of that decade, an important research programme was created, with the ambitious goal of describing the biodiversity of the State of São Paulo. The BIOTA programme compiled a first series of reports of up-to-date data on known diversity for many taxa, including ascidians (Rodrigues et al., Reference Rodrigues, Lotufo, Rocha, Migotto and Tiago1999), and a new series of papers provided additional information (Dias & Rodrigues, Reference Dias and Rodrigues2004; Dias et al., Reference Dias, Duarte and Solferini2006, Reference Dias, Delboni and Duarte2008, Reference Dias, Abreu, Silva and Solferini2009; Lotufo & Dias, Reference Lotufo and Dias2007; Bonnet & Rocha, Reference Bonnet and Rocha2011; Rocha et al., Reference Rocha, Dias and Lotufo2011).
As the SSC is threatened by the future expansion of the São Sebastião Harbour, and because an important body of information on diversity of ascidians from Brazil is not accessible to English-speaking researchers, the purpose of this paper is to summarize the knowledge about the diversity of ascidians from south-eastern Brazil, describing the main shifts in species compositions and dominance of ascidians over the past 50 years, in order to establish a baseline for future monitoring. We also discuss the main taxonomic issues regarding ascidians from south-eastern Brazilian waters and identify profitable areas for future research. Additionally, this study provides one of the most detailed datasets about ascidian diversity from the south-western Atlantic Ocean.
MATERIALS AND METHODS
The list of species and localities was compiled from published data and from material collected by the authors, including unpublished data. Ascidian diversity was studied in 26 localities between 23o45′S 45o50′W and 24o05′S 45o10′W (Figure 1). Vouchers specimens are available at the Museu de Zoologia–USP and the Ascidiacea collection of the Zoology Department, Universidade Federal do Paraná (http://splink.cria.org.br/manager/detail?resource=DZUP-Ascidiacea&setlang=pt&system=-Specieslink).
Abundance was estimated from 1980 to 1985 by reviewing survey studies and description of species published for the studied area. After 1985, abundance was estimated based on observational information by the authors while in the field. In this review, we standardized the relative abundance of the species, considering it rare if not seen in all field trips and when found was represented by no more than one or two colonies/individuals, uncommon if seen in all field trips but in very low abundance, common if seen in all field trips and abundant enough to be found without searching for it, and very common if it was a conspicuous species dominating the encrusting community either on natural or artificial substrate.
Studies reviewed here included surveys at natural substrata in the intertidal zone (Van Name, Reference Van Name1945; Millar, Reference Millar1958; Rodrigues Reference Rodrigues1962, Reference Rodrigues1966, Reference Rodrigues1977; Dias et al., Reference Dias, Abreu, Silva and Solferini2009), the subtidal zone (Rocha et al., Reference Rocha, Lotufo and Rodrigues1999, 1996, 2002, 2008, Reference Rocha, Kremer, Baptista and Metri2009 unpublished data), experimental studies using artificial panels (Rocha, Reference Rocha1988; Dias et al., Reference Dias, Delboni and Duarte2008) and experimental studies on intertidal boulder communities (Rocha, Reference Rocha1991). Most of the observations before 1990 were in the intertidal zone or from submerged experimental panels, supplemented within the past 20 years by SCUBA diving surveys. The surveys were more frequent in the years 1960–1970, 1985–1992, 1994–1996 (SCUBA diving included), 1999–2002 (SCUBA diving included) and 2008–2009 (SCUBA diving included) when different research projects involving ascidians were taking place.
RESULTS
As a result of the almost continuous record of the ascidian fauna for the past 50 years, with only six species recorded for SS in 1945 (Van Name, Reference Van Name1945), a total of 62 species are now known (Figure 2; Table 1). The type locality of eight of these species is the SS region. Among the 12 different families represented by these 62 species, Didemnidae (20 species) and Styelidae (15 species) were the most speciose in the SSC, while the families Clavelinidae, Euherdmaniidae and Cionidae were the least speciose, with only a single species each (Table 1). Thirty per cent (19) of the species are solitary, most of which are rare (12), with fewer than 10 individuals found in the past 50 years, or uncommon (3). Most species are tropical or typically found in warm subtropical waters. Exceptions are the introduced temperate-water species Lissoclinum perforatum and Ciona intestinalis. No species has a Patagonian or Magellanic distribution.

Fig. 2. Cumulative number of native, cryptogenic and introduced species of ascidians recorded from São Sebastião, Brazil, from 1945 to present.
Table 1. List of species from the São Sebastião region and introduction status (C, cryptogenic; N, native; I, introduced; ?, no information available).
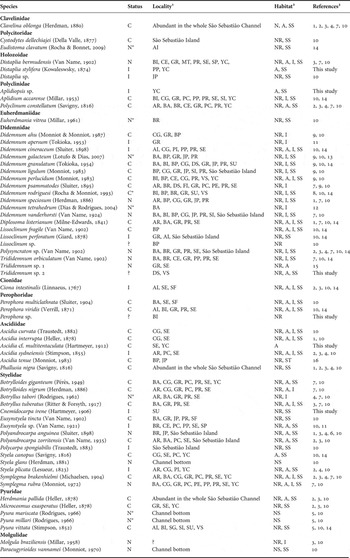
*designates that SS region is the type locality of the species; 1see legend of Figure 1 for localities codes; 2NR, natural rock substrate; NS, natural soft substrate; A, artificial substrate; I, intertidal; SS, shallow subtidal (<20 m); 31, Van Name, Reference Van Name1945; 2, Bjornberg, Reference Bjornberg1956; 3, Millar, Reference Millar1958; 4, Rodrigues, Reference Rodrigues1962; 5, Rodrigues Reference Rodrigues1966; 6, Rodrigues, Reference Rodrigues1977; 7, Rodrigues & Rocha, Reference Rodrigues and Rocha1993; 8, Rocha & Monniot, Reference Rocha and Monniot1993; 9, Rocha & Monniot, Reference Rocha and Monniot1995; 10, Rodrigues et al., Reference Rodrigues, Rocha and Lotufo1998; 11, Lotufo, Reference Lotufo2002; 12, Dias & Rodrigues, Reference Dias and Rodrigues2004; 13, Lotufo & Dias, Reference Lotufo and Dias2007; 14, Rocha & Bonnet, Reference Rocha and Bonnet2009; 15, Dias et al., Reference Dias, Delboni and Duarte2008; 16, Bonnet & Rocha, Reference Bonnet and Rocha2011.
Only 18 species can be definitely considered native to this region, while another 12 are introduced, 29 are cryptogenic and 3 were not classified. Although classified as cryptogenic (Table 1), due to the lack of information about their geographical origin, the species Cystodytes dellechiajei, Ascidia curvata, A. interrupta, A. cf. multitentaculata, A. tenue, Polycarpa spongiabilis, Polyandrocarpa anguinea, Symplegma rubra and Pyura vittata have isolated and small populations and their southernmost geographical limit is in SS or a little further south, suggesting that their presence is due to human transportation. Another group, comprising Polyclinum constellatum, Didemnum perlucidum, Lissoclinum fragile, Diplosoma listerianum, Styela canopus, Microcosmus exasperatus, Herdmania pallida and Pyura vittata has a more widespread distribution, is very common in many ports around the world, and is likely to have been introduced many years ago, probably by navigation in the 16th Century. Collections made by the authors near CEBIMar showed that many species arrived but did not persist in the new environment. One such species was Distaplia sp., common during the 1990s but not retrieved on surveys made in 2008 and 2009. It is worth mentioning also that many species were not even identified, because they were observed only once or twice in subsequent months and they were mostly juveniles, without mature gonads or larvae for complete identification (these species are not listed in Table 1).
The overall abundance of the species registered since the 1980s has not markedly changed since then (Table 2). Nonetheless, some species were only recently found, due to the relatively recent use of SCUBA to explore the subtidal region (i.e. Ascidia multitentaculata, Perophora sp., Cystodytes dellechiajei, Didemnum granulatum, D. ligulum, D. rodriguesi and Lissoclinum perforatum) or because they are very recent introductions (i.e. Distaplia stylifera, Distaplia sp., Eusynstyela sp., Cnemidocarpa irene and Aplidiopsis sp.). Also, two species declined in abundance: Polysyncraton sp. (previously identified as P. amethysteum: see the section about taxonomic issues for details), was reasonably abundant in the low intertidal zone before 2000, after which it declined severely; Didemnum rodriguesi type material was collected in the intertidal zone of Grande Beach, where the species was common. That species then declined precipitously, and between 2001 and 2002, one of us (G.M. Dias, unpublished data) monthly sampled the type locality searching for ascidians on boulders located within 15 randomly selected 1 m2 quadrats and not a single specimen was found. However, it was seen in the subtidal zone elsewhere on São Sebastião Island in December 2008 (region of the wrecks ‘Velasquez' and ‘Dart'), Búzios Island, Sumítica Island and Serraria Island.
Table 2. Relative abundance of ascidians in the São Sebastião Channel. ?, without information; –, not observed; X, rare species; XX, uncommon species; XXX, common species; XXXX, very common species, frequently observed dominating the encrusting community.

In the intertidal zone, exposed vertical surfaces of boulders are dominated by Didemnum psammatodes, while the undersurface of rocks usually harbours a large diversity of didemnid and styelid colonial species, with no single, dominant, species on large areas of rock. In the shallow subtidal zone, Phallusia nigra, Trididemnum orbiculatum, Botrylloides nigrum and Symplegma rubra are the dominant species on the exposed surface of the rocks (Table 2). The solitary P. nigra is probably the most conspicuous ascidian in SS, because it is very abundant and its black colour contrasts against the rocky background. The other two common solitary species are Microcosmus exasperatus and Herdmania pallida, both less abundant and usually camouflaged by epibionts.
Fouling communities associated with artificial substrates (experimental panels) are usually dominated by Diplosoma listerianum, Didemnum perlucidum, Symplegma spp., Botrylloides nigrum, Botrylloides giganteum, Clavelina oblonga, Polyclinum constellatum and Styela canopus (Table 2). All these species, with the exception of B. giganteum, have been abundant in the region for at least the past 30 years.
DISCUSSION
This review shows that, with 62 species, the SS region is a hotspot of ascidian diversity in Brazil, in comparison with species richness in other, well-studied, Brazilian states: Santa Catarina—39 (Rocha et al., Reference Rocha, Moreno and Metri2005b, Reference Rocha, Kremer, Baptista and Metri2009); Paraná—32 (Rocha & Nasser, Reference Rocha and Nasser1998; Rocha & Faria, Reference Rocha and Faria2005; Rocha & Kremer, Reference Rocha and Kremer2005); Rio de Janeiro—47 (Millar, Reference Millar1958; Simões, Reference Simões1981; Lotufo, Reference Lotufo2002; Rocha & Costa, Reference Rocha and Costa2005; Marins et al., Reference Marins, Novaes, Rocha and Junqueira2010); and Ceará—33 (Lotufo, Reference Lotufo2002; Lotufo & Silva, Reference Lotufo, Silva, Cascon and Lotufo2006; Oliveira-Filho, Reference Oliveira-Filho2010). Species richness for ascidians in the SSC is also comparable to that in False Bay (58 species), the richest site in South Africa (Awad et al., Reference Awad, Griffiths and Turpie2002) and Bocas del Toro, Panamá (58 species), considered a very diverse region in the Caribbean (Rocha et al., Reference Rocha, Faria and Moreno2005a). Ascidian richness from SS comprises more than half of all the ascidian species known from Brazil (Rodrigues et al., Reference Rodrigues, Lotufo, Rocha, Migotto and Tiago1999) and 94% of the species of the State of São Paulo (Rocha et al., Reference Rocha, Dias and Lotufo2011). Today, the SSC is apparently a region of high diversity for many groups, as shown by a compilation of a total of 733 species of invertebrates and algae in just one bay (Araçá Bay) within the channel (Amaral et al., Reference Amaral, Migotto, Turra and Schaeffer-Novelli2010).
Three important details contribute to these results. First, the many tunicate specialists working in the region resulted in a sampling effort (both temporally and spatially) that is many times greater than any other region in the country. Second, the region comprises very diverse habitats, including rocky shores, mangroves, rocky and sandy beaches and many islands within a relatively small area. Third, São Sebastião Harbour with international shipping and marinas accounts for the large number of introduced and cryptogenic species. Small boat traffic between SSC and other regions of Brazil can provide an important vector for increasing distribution of non-native species that have been introduced by other vectors such as sea chests of large ships (Wasson et al., Reference Wasson, Zabinc, Bedinger, Diaz and Pearse2001). While some potentially invasive species were observed only once or twice in the proximities of the São Sebastião Harbour, many species persisted and are now established at least on artificial substrates (e.g. Didemnum cineraceum, Eusynstyela sp. and Styela plicata), suggesting that the region has favourable environmental conditions for ascidians.
Changes in the ascidian assemblage have been reported from different locations in the Atlantic. In Jamaica, Goodbody (Reference Goodbody1993) found important shifts in the ascidian assemblage dwelling on mangrove roots after 30 years of monitoring, with eight species disappearing, four clearly declining in abundance and two new arrivals, for a total of 16 from the initial 22 species. In the east Atlantic, examination of samples from the Azores, Cape Verde, Canary Islands, Sierra Leone and Senegal in the 1990s found 12 new records, mainly of cosmopolitan species commonly found in the western Atlantic or Mediterranean (Monniot & Monniot, Reference Monniot and Monniot1994). These observations, with results described here, point to the need for more detailed monitoring of faunal shifts, as changes may happen over a temporal scale of a few years to decades.
As is the case for many marine invertebrate taxa, identification of ascidian species is often challenging, and taxonomic difficulties in some SSC species persist. Indeed, most taxonomic issues comprise didemnids, partly because of the reduced size of the zooids and comparatively few distinguishing characters. The complex taxonomic identity of the Trididemnum genus in SSC is a good example of this problem. Trididemnum orbiculatum has two morphotypes (intertidal and subtidal) that were considered by Monniot (Reference Monniot1983) as synonyms. However, Dias et al. (Reference Dias, Abreu, Silva and Solferini2009) showed that the two morphotypes of T. orbiculatum are distinct species (using both genetic and morphological criteria). Because the subtidal morphotype corresponds to the original description by Van Name (Reference Van Name1902), Dias et al. (Reference Dias, Abreu, Silva and Solferini2009) suggested that only this morphotype should keep the name T. orbiculatum, while the intertidal morphotype may be one or two species.
The only species of Polysyncraton in the SSC was originally identified as Polysyncraton amethysteum by Moure et al. (Reference Moure, Björnberg and Loureiro1954) and Millar (Reference Millar1958) based only on the description published by Van Name (Reference Van Name1945). Recent analysis of the holotype (AMNH 1304) by Lotufo (Reference Lotufo2002) revealed important differences in the SS specimens. The holotype has larger systems, with a single cloacal opening on the colony, a unique arrangement of the spicules on the tunic and larger larvae, and so the Brazilian animals should be described as a new species. The genus Diplosoma, especially Diplosoma listerianum, also needs revision. Rowe (Reference Rowe1966) reviewed the genus and found that D. macdonaldi and D. rayneri are junior synonyms of D. listerianum. Subsequently, Lafargue (Reference Lafargue1968) discussed the status of some Diplosoma with bilobed testicles and recognized D. macdonaldi as a distinct species, based on the absence of a muscular process. Kott (Reference Kott2001), however, agreed with Rowe (Reference Rowe1966) and lists the Atlantic records as D. listerianum, without specific remarks. We recognize that the species has an unexpected distribution both in cold and tropical waters and might comprise a complex of cryptic species, but here we retain D. listerianum, while awaiting clarification of this matter.
Another taxonomic conundrum is Distaplia spp. from the Atlantic. Van Name (Reference Van Name1945) recognized the synonymy proposed by Michaelsen (Reference Michaelson1930) between D. bursata (Van Name, 1921) and D. stylifera (Kowaleswsky, 1874) and considered the latter to be the valid name for the western Atlantic specimens. Distaplia stylifera has a broad range of variation in its characters and is probably a complex of cryptic species that needs urgent revision. The original record by Rodrigues et al. (Reference Rodrigues, Rocha and Lotufo1998) of a recently introduced species in SS used the name Distaplia stylifera, but we now recognize that this was a misidentification and further work may reveal that those animals are indeed a new species. Samples of a different Distaplia that more closely match the loose description of D. stylifera were recently (December 2009) collected at the Petrobras piers and at the Yacht Club of Ilha Bela on São Sebatião Island.
There are currently two species of Symplegma reported for the SSC: Symplegma rubra and Symplegma brakenhielmi. However, Couto (Reference Couto2003) reviewed the genus and recognized, but did not describe, two additional new species. Both S. rubra and S. brakenhielmi are widespread in warm waters and have very similar counterparts in the Indo-Pacific—the first with S. bahraini Monniot & Monniot, 1987 and the second with Symplegma stuhlmanni (Michaelsen, 1904). A revision of this genus including molecular analysis is necessary to understand the identity and geographical distribution of the species.
Eusynstyela sp. appeared for the first time during a monthly survey of rocky walls in Jarobá point in 1995. It was initially identified as E. floridana because of its small zooids, general colour and aspect of the colony. The study of the holotype (USNM 5969) revealed that both species differ in colony organization (Lotufo, Reference Lotufo2002). Eusynstyela floridana forms clusters of zooids without apparent ordering, while in the SS the colonies are organized in a single layer of adjoined zooids, and the testis follicles are long and usually of the same size, while E. floridana has one dorsal globular follicle and one ventral and elongated follicle. Eusynstyela sp. resembles E. hartmeyeri Michaelsen, 1904, that is endemic to the Indian Ocean, and introduced in New Caledonia and Hong Kong (Monniot, Reference Monniot2002). In SSC, the colonies of Eusynstyela sp. are very conspicuous, in shallow waters and unlikely to remain undetected, and were not found in the region before 1995, suggesting that the species is also introduced in the SSC.
Despite the increase in species richness during the past 50 years due to the arrival of many alien species (ten reported during the past 15 years), important changes in species abundances were not noted during the same period. There are, however, exceptions, such as Styela plicata, Polysyncraton sp. and D. rodriguesi that decreased in abundance. Nonetheless, it is important to emphasize that abundance data are not precise enough to detect any but very large changes. Styela plicata is not native in the region and thus population fluctuations are expected. Causes of the decline in the two colonial species are unclear. However, both inhabit the intertidal zone, where conditions are naturally stressful for ascidians and where the first effects of environmental degradation are frequently observed during oil-leaks or by sewage release (Peterson et al., Reference Peterson, Rice, Short, Esler, Bodkin, Ballachey and Irons2003).
The SSC is sheltered, protected by São Sebastião Island, has many marinas and is one of the most important oil terminals in Brazil. By the end of 2000, 220 oil leaks were reported for the SSC, and during a single event in May 1994, 2.6 million l of oil leaked from the oil terminal into the SSC. These constant accidents, along with untreated domestic pollution that enters the waters, increase the organic load and thereby affect the local fauna (Cunha, Reference Cunha2003). Reports from the state environmental agency, CETESB, from 1991 to 2008 showed a trend of an increasing number of beaches with coliform indices above the legal limits. For instance, in 1999, 65% of the beaches were considered adequate in the SSC, while in 2008 that had declined to only 40% of the beaches (CETESB, 2001, 2009). Impacts on the biota have been shown in molluscs, where the number of faecal coliform bacteria increases during the summer (vacation period) and this increase is associated with reduced mollusc species richness and diversity (Denadai et al., Reference Denadai, Amaral and Turra2001). While we have no data for ascidians, we infer that these sessile animals, with their filter feeding habits, are affected by eutrophication. In Jamaican lagoons, eutrophication was the main reason for the changes in the ascidian fauna during the course of 30 years (Goodbody, Reference Goodbody1993). On the other hand, bacterial increase was mentioned as the main cause of a population explosion of Trididemnum solidum in Curaçao that culminated in reduction of local species richness (Bak et al., Reference Bak, Lambrechts, Joenje, Nieuwland and Van Veghel1996).
Among the introduced species present in the SS region, some were reported elsewhere as invasive, for instance, Ciona intestinalis (Braithwaite & McEvoy, Reference Braithwaite and McEvoy2005; Howes et al., Reference Howes, Herbinger, Darnell and Vercaemer2007), Styela plicata (Rocha et al., Reference Rocha, Kremer, Baptista and Metri2009) and Didemnum perlucidum (Lambert, Reference Lambert2002). Ciona intestinalis and S. clava are causing considerable damage to mussel farms in Canada because of high costs associated with the cleaning of shells and submerged structures (Carver et al., Reference Carver, Chisholm and Mallet2003; Davis & Davis, Reference Davis and Davis2009). Rodrigues et al. (Reference Rodrigues, Rocha and Lotufo1998) included C. intestinalis in the ascidian guide for the State of São Paulo, but only one animal had been collected at that time. Vieira et al. (Reference Vieira, Duarte and Dias2012) during an experiment conducted in 2007 at Segredo Beach found several specimens of C. intestinalis on PVC plates protected against predation. Inspections along the beach did not find evidence that the species occurred on natural substrates. Similar effects of predation on C. intestinalis were reported in Guanabara Bay, Brazil (Marins et al., Reference Marins, Oliveira, Maciel and Skinner2009) and Chile (Dumont et al., Reference Dumont, Gaymer and Thiel2011). However, one individual was found on a natural substrate on a more distant island near SS (Rocha & Bonnet, Reference Rocha and Bonnet2009). Styela plicata was very common on natural substrates in shallow waters in the 1960s (Rodrigues, Reference Rodrigues1962) but it became uncommon and restricted to artificial, usually hanging, substrates. Both species usually live in waters colder than the usual water temperatures in SS (Yamaguchi, Reference Yamaguchi1975) and peaks of high water temperature may control their populations. For instance, high mortality of S. plicata occurred in oyster farms in Santa Catarina, south of Brazil, in March 2008 when surface water temperatures reached 29–30oC for several consecutive days (Nelson Silveira Jr, personal communication). Didemnum perlucidum is abundant on artificial substrates in the SSC with very small colonies on the underside of boulders, suggesting that the species might have been controlled by predation in natural habitats. Given that D. perlucidum has a great capability to colonize new substrates (Kremer et al., Reference Kremer, Rocha and Roper2010) and that other didemnid species are known for their rapid growth rates and space domination, causing a decrease in local species richness (Didemnum vexillum—Bullard et al., Reference Bullard, Lambert, Carman, Byrnes, Whitlatch, Ruiz, Miller, Harris, Valentine, Collie, Pederson, McNaught, Cohen, Asch, Dijkstra and Heinonen2007; Trididemnum solidum—Bak et al., Reference Bak, Lambrechts, Joenje, Nieuwland and Van Veghel1996; Diplosoma similis—Vargas-Ángel et al., Reference Vargas-Ángel, Godwin, Asher and Brainard2009), D. perlucidum and other introduced didemnids in the region may be a threat for natural communities and should be monitored for any signs of population increase.
Speculation about temporal variation in populations of species occurring in deep waters is not possible because most of the sampling efforts during the past 50 years were concentrated on rocky shores from the intertidal to 10 m depth and hence many species that occur outside that zone were rarely observed. Euherdmania vitrea is one of these species that occurs in areas deeper than 10 m and so was only occasionally found, mainly by dredging studies. Yet, the species is common in shallow (6–8 m) waters of Santa Catarina, southern Brazil (Rocha et al., Reference Rocha, Moreno and Metri2005b), which suggests that the SS population could be a marginal, northern-most geographical limit for this species. In addition, species living in soft bottoms, such as Paraeugyrioides vannamei, have certainly been underestimated by our studies.
Any monitoring in the region must also take into account that the abundance of many species, such as Clavelina oblonga and Diplosoma listerianum, oscillates throughout the year and sometimes a marked seasonal decline occurs in winter. Clavelina oblonga can dominate the encrusting community at the end of summer and beginning of autumn (March–April) on both artificial and natural substrates. Diplosoma listerianum has a fragile tunic, rapid colony growth and early maturation of gonads (Rocha, Reference Rocha1991). During the spring and summer D. listerianum can become very common and form large colonies, but usually has high mortality soon after larval recruitment and colony growth, disappearing in subsequent months when small colonies hidden in protected places are occasionally found.
In conclusion, while the SS region has one of the best-known ascidian faunas in Brazil, it is also one of the most challenging in terms of marine conservation. Difficulties arise from its location in the most urbanized and industrialized state in Brazil, and because the SSC is considered a key piece in the economic development of the region. Urban and industrial pollution, biological invasions and construction of coastal facilities and structures may be considered the main threats to marine organisms in general, and to ascidians in particular. This study provides a current detailed description of the ascidian species in the area and a baseline for future taxonomic studies, conservation of native species and management of introduced species. To the best of our knowledge, the data reported here comprise the first account of a long-term ascidian survey in the south-western Atlantic, an area which is still relatively poorly known.
ACKNOWLEDGEMENTS
The authors thank Dr Sérgio de Almeida Rodrigues (in memoriam) who introduced R.M.R., T.M.C.L. and G.M.D. to the study of ascidians, the Center of Marine Biology (CEBIMar-USP) and Yatch Club Ilhabela for field support and James J. Roper for his review of the English. We also thank CNPq for a research grant (R.M.R.) and financial support, FAPESP for providing grants for G.M.D. and T.M.C.L. and for financing field trips in the region coordinated by Dr Roberto G.S. Berlinck and CAPES who provided graduation grants for G.M.D. and who financed the December 2009 RAS in the region.






