1. Introduction
Darwin famously considered music to be a puzzle for evolutionary theory. Music is universal across human cultures (Brown & Jordania, Reference Brown and Jordania2013; Mehr et al., Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019; Savage, Brown, Sakai, & Currie, Reference Savage, Brown, Sakai and Currie2015), yet its function seems mysterious, because “neither the enjoyment nor the capacity of producing musical notes are faculties of the least use to man in reference to his daily habits of life…” (Darwin, Reference Darwin1871, p. 33). Darwin went on to speculate that music first evolved “for the sake of charming the opposite sex,” after which language “derived from previously developed musical powers.”
Since Darwin there has been no shortage of hypotheses about why and how music evolved (cf. Honing, Cate, Peretz, & Trehub, Reference Honing, Cate, Peretz and Trehub2015; Wallin, Merker, & Brown, Reference Wallin, Merker and Brown2000). The null hypothesis is that music is an evolutionarily “useless” by-product of other evolved capacities, with no adaptive function and involving no direct selection for musical capacities (Pinker, Reference Pinker1997, p. 528). Others hypothesize that musicality evolved for specific adaptive purposes, including signaling mate quality (Miller, Reference Miller, Wallin, Merker and Brown2000), advertising coalitions (Hagen & Bryant, Reference Hagen and Bryant2003; Merker, Reference Merker2000), or soothing infants (Dissanayake, Reference Dissanayake, Wallin, Merker and Brown2000; Falk, Reference Falk2004; Mehr & Krasnow, Reference Mehr and Krasnow2017). Many authors have discussed the evolutionary value of music in facilitating group cohesion (e.g., Benzon, Reference Benzon2001; Brown, Reference Brown, Tonneau and Thompson2000a, Reference Brown2007; Cross & Morley, Reference Cross, Morley, Malloch and Trevarthen2009; Dissanayake, Reference Dissanayake, Malloch and Trevarthen2009; Dunbar, Reference Dunbar and Bannan2012a; Freeman, Reference Freeman, Wallin, Merker and Brown2000; Gioia, Reference Gioia2019; Huron, Reference Huron2001; Loersch & Arbuckle, Reference Loersch and Arbuckle2013; McNeil, Reference McNeil1995; Merker, Morley, & Zuidema, Reference Merker, Morley, Zuidema and Honing2018; Mithen, Reference Mithen2005; Oesch, Reference Oesch2019; Patel, Reference Patel and Honing2018; Roederer, Reference Roederer1984; Schulkin & Raglan, Reference Schulkin and Raglan2014; Trainor, Reference Trainor and Honing2018; Trehub, Becker, & Morley, Reference Trehub, Becker, Morley and Honing2018), sometimes suggesting that musicality may have arisen via group selection (especially Brown, Reference Brown, Tonneau and Thompson2000a). Although such proposals succeed in explaining some properties (or genres) of music, we argue that no single account succeeds as a general explanatory framework for the evolution of human musicality. Our purpose in the current target article is to synthesize and extend previous proposals into a new, parsimonious framework that can explain and predict many aspects of human music-making.
Our argument is that human musicality is a coevolved system for social bonding. Crucially, following Honing (Reference Honing2018) and others, we clearly distinguish between music and musicality. “Music” encompasses the diverse cultural products generated by and for music making: songs, instruments, dance styles, and so on. In contrast, “musicality” encompasses the underlying biological capacities that allow us to perceive and produce music. Distinguishing these clearly is crucial because musical systems are diverse, culture-specific products of cultural development, whereas musicality comprises multiple biological mechanisms, shared across human cultures that enable musical production, perception, and enjoyment.Footnote 1 Musicality is not a monolithic trait evolved to solve one particular problem (coalition signaling, infant mood regulation, sexual attraction, and so on), but rather a set of capabilities that can be used in different ways to support multiple functions, all involving social affiliation, but no one of which is the “primary” or “original” function.
The key phrase “social bonding” refers to the formation, strengthening, and maintenance of affiliative connections (“bonds”) with certain conspecifics (i.e., the set of social processes that engender the bonded relationships that underpin prosocial behavior). As a group-living primate species, such bonds are psychologically and biologically central to human survival and reproduction (e.g., via enhanced predator protection, cooperative child-rearing, collaborative foraging, expansion, and defense of territories; Dunbar, Reference Dunbar2012b; Dunbar & Shultz, Reference Dunbar and Shultz2010; Hrdy, Reference Hrdy2009; Tomasello & Vaish, Reference Tomasello and Vaish2013). For the purpose of this paper, we use “social bonding” as an umbrella term to encompass both bonding processes (over short and longer time scales) and their effects. Consequently, we take “social bonding” to encompass a variety of social phenomena including social preferences, coalition formation, identity fusion, situational prosociality, and other phenomena that bring individuals together. The social functions of music share a general social utility: to forge and reinforce affiliative inter-individual relationships, for example, by synchronizing and harmonizing the moods, emotions, actions, or perspectives of two or more individuals. Crucially, we argue that music achieves this in a variety of situations where language is less effective, and on a scale greater than that achievable by the ancestral bonding mechanisms (ABMs) available to other primates (e.g., grooming). We argue that social bonding promotes, and is the consequence of, interactions not only during music making, but also subsequently via long-lasting changes in affiliative dispositions of group members toward one another, and their associated longer-term prosociality. Because social interactions involve multiple levels of group structure, our conception of social bonding also includes darker phenomena such as out-group exclusion that bring certain individuals closer together by pushing away others (see sect. 6.4).
The final keyword here is “coevolved”: we argue that culturally evolving systems of music (Savage, Reference Savage2019a) have developed in tandem with the human capacity for musicality through a process of gene–culture coevolution. We build on recent arguments by Patel (Reference Patel and Honing2018) and Podlipniak (Reference Podlipniak2017), who suggest that music arose initially as a cultural “invention” that created the context for later selection enhancing human musicality. In much the same way that the use of fire by early hominins provided the preconditions for biological adaptations to cooked food (Wrangham, Reference Wrangham2009), or the invention of dairy farming in some European and African cultures created selection for lactase persistence (Tishkoff et al., Reference Tishkoff, Reed, Ranciaro, Voight, Babbitt, Silverman and Deloukas2007), early instantiations of music provided the selective preconditions for later neurobiological changes underlying human musicality. Notably, both Patel and Podlipniak identified social bonding as a candidate function driving such gene–culture coevolution, with Patel (Reference Patel and Honing2018, p. 118) noting the possibility that “musical behavior first arose as a human invention and then had (unanticipated) beneficial effects on social cohesion.” We argue that because music had multiple adaptive effects on social bonding, this led to subsequent selection (both genetic and cultural) for the ability and motivation to make particular forms of music – music that has features that most effectively function to promote social bonding. This combination of cultural and biological selection led to the particular features and ubiquity of modern human music and musicality.
Our article closely examines this claim, and provides a framework for understanding the biological and cultural evolution of music, taking this argument as foundational. We provide a detailed cross-disciplinary review of the evidence for specific mechanisms by which music functions to enhance social bonding, and consider how some of the mechanisms underlying musicality may have coevolved with music. Similar to Patel, we take for granted the large and sophisticated literature on gene–culture coevolution in general, and will not review it here (cf. Boyd & Richerson, Reference Boyd and Richerson1985; Cavalli-Sforza, & Feldman, Reference Cavalli-Sforza and Feldman1981; Durham, Reference Durham1991; Henrich, Reference Henrich2016; Jablonka & Lamb, Reference Jablonka and Lamb2005; Laland, Odling-Smee, & Feldman, Reference Laland, Odling-Smee and Feldman2000, Reference Laland, Odling-Smee and Myles2010; Richerson et al., Reference Richerson, Boyd and Henrich2010; Tomlinson, Reference Tomlinson2018). However, we do not see the “invention” of music as a unitary event later followed by genetic adaptation, but rather as an iterated process where different proto-musical components of musicality arose over an extended period as behavioral innovations that, because of initial positive effects, generated new cognitive and social niches for subsequent biological adaptations, themselves yielding new innovations, and so on in a virtuous spiral. We thus posit essentially an iterated Baldwin effect (Baldwin, Reference Baldwin1896; Bateson, Reference Bateson2004; Griffiths, Reference Griffiths, Weber and Depew2003; Podlipniak, Reference Podlipniak2017), or more generally, prolonged cognitive “niche construction” (Laland et al., Reference Laland, Odling-Smee and Feldman2000). This mechanism is closely related to many contemporary models of language evolution involving a series of “protolanguages” (Arbib, Reference Arbib2005; Fitch, Reference Fitch2010, Reference Fitch2017). Although hypotheses about the specific ordering of events involved (e.g., Dunbar, Reference Dunbar and Bannan2012a; Mithen, Reference Mithen2005) are useful, it is not our purpose here to propose a specific sequence, but rather to advance a new conception of the entire process.
In their target article, Mehr, Krasnow, Bryant, and Hagen present a contrasting hypothesis for the origins of music. Their hypothesis synthesizes and extends their previous proposals (Hagen & Bryant, Reference Hagen and Bryant2003; Mehr & Krasnow, Reference Mehr and Krasnow2017) into a generalized “credible signaling” hypothesis that incorporates signaling of both coalition strength and parental attention. They also present critiques of the social bonding hypothesis and other candidate hypotheses. The BBS editors decided that publishing these two target articles with contrasting hypotheses would stimulate productive commentary beyond that usually possible for only a single target article. Both target articles originated from the same symposium on “The Origins of Music in Human Society,”Footnote 2 but differ in multiple ways in addition to the focus on social bonding versus credible signaling. In particular, Mehr et al. take an approach grounded in evolutionary psychology, focused on demonstrating domain-specificity and evidence for adaptation. In contrast, our approach emphasizes cultural evolutionary theory, including in particular gene–culture coevolution and cognitive niche construction (cf. Laland & Brown, Reference Laland and Brown2011). We take a pluralistic approach to adaptation and modularity, involve experts from diverse disciplines to synthesize evidence into a single framework, and propose testable predictions for future research. We expand on more detailed contrasts between the two articles in sect. 6.
The following sections lay out the details and implications of the music and social bonding (MSB) hypothesis. Section 2 describes the proposed evolutionary functions and coevolutionary process. Section 3 details cross-disciplinary evidence supporting the MSB hypothesis. Section 4 specifies the neurobiological mechanisms proposed to underlie music's social bonding functions. Section 5 describes testable predictions that follow from the MSB hypothesis. Section 6 addresses a number of potential criticisms of our hypothesis, and sect. 7 provides a brief conclusion.
2. Social bonding as a unifying function in the evolution of musicality
The music and social bonding (MSB) hypothesis posits that core biological components of human musicality evolved as mechanisms supporting social bonding. Musicality relies on multiple neurocognitive components, which likely evolved at different times and for different reasons: musicality is more a cognitive toolkit than a single tool (Fitch, Reference Fitch2015a). Most of the tools in this musical toolkit function to facilitate social bonding, but some may also be used for non-social purposes such as individual mood regulation (see sect. 6.5).
We avoid arguing for one specific single adaptive function for music (e.g., coalition advertisement, courtship, or infant mood regulation) because we think it unlikely that a single “main” evolutionary function for complex, multi-component abilities such as language or music exists. Imagine asking the parallel question “what is vision for?” and coming up with a hypothesis set including “spotting predators,” “judging mate quality,” “finding food,” and “avoiding obstacles.” It seems clear that these are all functions of vision, and all provide potential causal explanations for adaptive improvements in vision during evolution. But the desire to identify ONE function as primary seems misguided. A better approach is mechanistic: we ask “what are lenses for?,” and answer in engineering terms: lenses are for focusing an image on the retina, to enable accurate visual perception. Whether the image is of a predator, mate, or food is not critical, because of improved visual resolution will aid them all.
Turning to music, “social bonding” provides an umbrella explanation analogous to “vision is for seeing.” Particular design features of music (singing discrete pitches, generating an isochronous beat, and use of repetitive patterns based on small-integer ratios) function mechanistically to enhance predictability, aiding synchronization and harmonization when multiple people sing, dance, and play instruments together. Coherent and harmonious merging of sounds and movements during group activity leads to positive feelings of prediction, fulfillment of expectation, and mutual accomplishment. These, through activation of the dopaminergic reward system and other pathways, have affiliative emotional and rewarding effects immediately and also long after music-making ceases (see sect. 4). Crucially, the resulting strengthened social bonds are operative over multiple types and sizes of groups, ranging from dyads (e.g., parent and infant, potential mates) to bands of small coalitions and large groups of unrelated individuals (Fig. 1). Social bonding through music thus produces its ultimate evolutionary dividends in multiple complementary ways, including a larger group of potential allies, increased child rearing success, increased mating success, and better-functioning coalitions.

Figure 1. We propose that supposedly competing hypotheses for the evolution of human music, including mate bonding, infant care, and group cohesion (within both small coalitions and larger groups), are complementary sub-components of a broader social bonding function.
2.1 Ancestral bonding mechanisms
Why was social bonding adaptive for our ancestors, and in what ways does music improve or increase social bonding? Group living comes with costs (e.g., increased local competition for food and mates) and benefits (e.g., safety in numbers and cooperative hunting/defense). Animals that live in groups, particularly primates, have evolved mechanisms that help balance these costs and benefits by forging strong affiliative bonds: good quality, persistent, differentiated inter-individual commitments that require investment of time and energy (Dunbar, Reference Dunbar1991). Strong social bonds enhance individuals' prospects of receiving support through coalitions, which, in certain primate species, influence dominance rank and reproductive performance (Silk, Reference Silk2007). These coalitions form the backbone of successful cooperative hunting, child rearing, and joint defense against predators or competitors (Dunbar & Shultz, Reference Dunbar and Shultz2010). Ecological factors typically constrain the size of a group, but larger groups of well-coordinated, strongly bonded humans enabled exploitation of new forms of resources (e.g., larger prey), and more reliable protection from predators (Dunbar, Reference Dunbar2012b).
ABMs in other primates include grooming, play, and – in some species – non-procreational sex. These ABMs are essentially dyadic (or for play, very small groups mostly limited to young animals), and require substantial time commitments even in small groups if all individuals in the group are to invest in all others. Although vocal duets are present in tropical birds and some primates (Farabaugh, Reference Farabaugh, Kroodsma and Miller1982; Haimoff, Reference Haimoff1986; Mann, Dingess, Barker, Graves, & Slater, Reference Mann, Dingess, Barker, Graves and Slater2009; Thorpe, Reference Thorpe1972), group vocal choruses that are both differentiated and coordinated appear nearly unique to humans (but see Mann, Dingess, & Slater [Reference Mann, Dingess and Slater2006] for the fascinating example of the group-chorusing plain-tailed wren).
As Dunbar (Reference Dunbar1993) has argued, the steady increases in group size, complexity, and fluidity that occurred during hominin evolution put increasing strain on ABM-based social bonds. Beyond group sizes of 20 or so, dyadic bonding based on ABMs such as grooming became unsustainably time-consuming, so supra-dyadic bonding mechanisms were needed. Dunbar (Reference Dunbar and Bannan2012a) suggests that another ABM in great apes and humans was laughter (Davila Ross, Owren, & Zimmerman, Reference Davila Ross, Owren and Zimmerman2009), which facilitates social bonds among reasonably large groups. However, there are limits to a bonding mechanism based on laughter: Unlike music, which people can intentionally choose to engage in at any time, large group laughter can be difficult to elicit and to sustain for long periods. Music may have provided our ancestors with a novel system that, like laughter, allowed for simultaneous bonding with a larger group of individuals, but across a broader set of times and contexts, and for longer periods of time than otherwise possible (Dunbar, Reference Dunbar and Bannan2012a; Launay, Tarr, & Dunbar, Reference Launay, Tarr and Dunbar2016). This new system augmented the smaller-scale ABMs that became less robust in larger groups. Specific design features of human musicality – particularly our capacity and proclivity to produce repetitive, synchronized, harmonized music for extended periods – provided a flexible toolkit for bonding, allowing our ancestors to achieve social bonding on a large scale.
2.2 Design features of musicality
2.2.1 Rhythm and dance
Most music has two distinctive rhythmic components: an isochronous (equal-timed) beat, and a metric structure (a hierarchical arrangement of sonic events into small groups with differentially accented constituents; Arom, Reference Arom1991; London, Reference London2004; Savage et al., Reference Savage, Brown, Sakai and Currie2015). These features together provide a predictable, repetitive structure underlying extended, coordinated, and varied group performances, while allowing room for variation and improvisation. Isochronicity and metric structure make the performance predictable, which facilitates planning synchronized and coordinated movements (e.g., dancing). Although synchronization solely to the beat (e.g., in marching or unison chanting) allows large groups to integrate, it tends to submerge individual contributions. Meter solves this problem by allowing many individuals to contribute, out of phase, to the same integrated rhythm. Neither of these core design features of musicality appears well-designed for solo performances, but they support the synchronized and coordinated musical sounds and dance movements of groups that are widespread features of human musical systems (Savage et al., Reference Savage, Brown, Sakai and Currie2015).
Dancing is another intrinsically rhythmic component of human musicality (cf. Fitch, Reference Fitch2015a, Reference Fitch, Toivonen, Csúri and van der Zee2015b; Laland, Wilkins, & Clayton, Reference Laland, Wilkins and Clayton2016). Even newborn infants perceive a musical beat (Winkler, Háden, Ladinig, Sziller, & Honing, Reference Winkler, Háden, Ladinig, Sziller and Honing2009), and dance develops early: Infants hearing music produce spontaneous rhythmic movements during their first year, although the ability to entrain these movements reliably to a beat takes several years to develop (Kim & Schachner, Reference Kim and Schachner2020; McAuley, Jones, Holub, Johnston, & Miller, Reference McAuley, Jones, Holub, Johnston and Miller2006; Merker, Madison, & Eckerdal, Reference Merker, Madison and Eckerdal2009; Zentner & Eerola, Reference Zentner and Eerola2010). The capacity to perceive and move to a beat is a core component of musicality, rare among vertebrates (Patel, Reference Patel2014; Schachner, Brady, Pepperberg, & Hauser, Reference Schachner, Brady, Pepperberg and Hauser2009) but universal across human cultures (Brown, Reference Brown1991). Dance provides an energetic mode of musical participation that is accessible to large numbers of individuals regardless of age, familiarity with the music, or instrumental/singing virtuosity. In addition to its visual effects, dance can also generate an auditory signal, for example, because of foot stamping or hand clapping, and certain styles of dance (such as tap dancing) create their own sonic accompaniment. These factors suggest that dance is a core part of music-making (“musicking”) and not a separate domain (Tarr, Reference Tarr, Enfield and Kockelman2017).
Dance thus expands the potential circle of rhythmically coordinated participants in musical interactions. The inclusive aspect of human musicality provided by dance is predicted by the MSB hypothesis, but poses a challenge to hypotheses seeing music primarily as a signal of virtuosity. Hereafter, we consider dance a core component of musical performance.
2.2.2 Melody, harmony, and vocal learning
The human capacity for song entails vocal production learning: the ability to imitate and learn vocal patterns beyond our species-typical repertoire of screams, laughter, and so on. By about 2 or 3 years of age (often earlier), children reproduce songs that their caregivers sing to them, with intact pitch range and contours (Trehub, Reference Trehub, Hallam, Cross and Thaut2016). Young children commonly exhibit greater fluency in song than in speech (e.g., singing Twinkle Twinkle Little Star from beginning to end with fractured, word-like sounds). This vocal learning ability is highly developed in humans relative to other primates, and the neurobiological mechanisms of its evolution are relatively well-understood, in part because of its convergent evolution in songbirds and other non-human species (Fitch, Reference Fitch2015a; Janik & Slater, Reference Janik and Slater1999; Jarvis, Reference Jarvis2019; Syal & Finlay, Reference Syal and Finlay2011; see sect. 4.4 for details). Vocal learning forms a foundation for group participation in singing culture-specific songs.
In contrast to the continuously varying pitch of normal speech, the discrete pitches used in song and instrumental music generate predictable sequences that enable frequency matching between individuals during group music production (Merker, Reference Merker2002; Savage et al., Reference Savage, Brown, Sakai and Currie2015). Unison performance in which multiple parts produce the same melodies at either the same frequencies (1:1 frequency ratio) or an octave apart (2:1 ratio) is so widespread among humans it is often not even considered a form of harmonization (although cf. Jacoby et al. [Reference Jacoby, Undurraga, McPherson, Valdés, Ossandón and McDermott2019] for evidence that octave equivalence is not completely universal). Octave singing in particular represents the most universal form of musical harmony: different pitches performed simultaneously with maximally overlapping acoustic spectra (cf. Bowling & Purves, Reference Bowling and Purves2015). The common tendency for men and women to sing together in octaves is paralleled by the roughly octave difference in men and women's average vocal pitch, based on vocal anatomy (Titze, Reference Titze1989). This is an unusual feature among primates (and mammals more generally) not observed in chimpanzees (Grawunder et al., Reference Grawunder, Crockford, Clay, Kalan, Stevens, Stoessel and Hohmann2018) – a potential anatomical adaptation for vocal harmonization.
Harmonious overlapping of acoustic spectra also shapes another common design feature: Musical systems around the world restrict pitches to scales containing a limited number of discrete pitch classes (rarely more than seven; Savage et al., Reference Savage, Brown, Sakai and Currie2015). These pitch classes often reflect small-integer frequency relationships which sound consonant together (e.g., the 3:2 frequency ratio underlying musical fifths, 4:3 ratios for fourths, and so on; Bowling, Purves, & Gill, Reference Bowling, Purves and Gill2018; Gill & Purves, Reference Gill and Purves2009; Kuroyanagi et al., Reference Kuroyanagi, Sato, Ho, Chiba, Six, Pfordresher and Savage2019; McDermott, Lehr, & Oxenham, Reference McDermott, Lehr and Oxenham2010; Terhardt, Reference Terhardt1984). By producing pitches that adhere to scales, groups of singing individuals effectively minimize uncertainty in fundamental frequency, thus maximizing harmony via spectral alignment (Sethares, Reference Sethares2004). Coordinating with other individuals musically, by aligning acoustic spectra, can sound pleasing and promote bonding. The specific mechanisms and causal relationships behind this effect remain contested (Bowling, Hoeschele, Gill, & Fitch, Reference Bowling, Hoeschele, Gill and Fitch2017, Reference Bowling, Purves and Gill2018; Bowling & Purves, Reference Bowling and Purves2015; Harrison & Pearce, Reference Harrison and Pearce2020; Jacoby et al., Reference Jacoby, Undurraga, McPherson, Valdés, Ossandón and McDermott2019; Large, Kim, Flaig, Bharucha, & Krumhansl, Reference Large, Kim, Flaig, Bharucha and Krumhansl2016; McBride & Tlusty, Reference McBride and Tlusty2020; McDermott et al., Reference McDermott, Lehr and Oxenham2010, Reference McDermott, Schultz, Undurraga and Godoy2016; Merker et al., Reference Merker, Morley, Zuidema and Honing2018; Pfordresher & Brown, Reference Pfordresher and Brown2017). Nevertheless, scales facilitate harmony, where multiple voices/instruments combine consonantly – another design feature supporting group coordination but not solo performance.
2.2.3 Repetitive structure
The synchronization of rhythms and harmonization of pitches described above is facilitated and enhanced by the widespread use of repetitive musical structures (Savage et al., Reference Savage, Brown, Sakai and Currie2015). Structural building blocks can range from short rhythmic and/or melodic motives of only a few notes, to entire phrases, to large-scale sections or entire works. The level of repetition in music is one of its most striking differences from language (Fitch, Reference Fitch2006; Margulis, Reference Margulis2014), and multiple repetitions of a recording of a spoken phrase cause it to sound sung rather than spoken (Deutsch, Henthorn, & Lapidis, Reference Deutsch, Henthorn and Lapidis2011). Repetition enhances memorization and predictability, allowing multiple performers to engage in long periods of coordinated music-making, with all-night music-and-dance rituals common from contemporary Western nightclub culture to ethnographic descriptions of small-scale societies (Merriam, Reference Merriam1964; Thornton, Reference Thornton1995). In contrast, language and ABMs such as laughter are more difficult to sustain for long periods, making them less suitable for the kind of sustained inclusive interactions that promote the strongest social bonds. However, extreme repetition can lead to boredom and to a dearth of memorable distinguishing features, preventing music from serving as a cue of social identity (see below). Both human and bird songs tend to balance repetition and novelty in the form of repetition with variation (Kroodsma, Reference Kroodsma1978; Lomax, Reference Lomax1968).
2.2.4 Music and social identity
A final potential design feature of culturally-transmitted group music concerns its role in flexibly and hierarchically indicating kinship and group identity (Stokes, Reference Stokes1994; Turino, Reference Turino2008). Because songs are variable, complex, and memorable, two people knowing the same song likely acquired this knowledge via social learning – and thus are likely to share a common socio-cultural history. Thus, shared knowledge of musical repertoire provides information about shared socio-cultural background (Schachner et al., Reference Schachner, Brady, Oro and LeePreprint; Soley & Spelke, Reference Soley and Spelke2016). Musicality may have coevolved in support of this social bonding function: Cultural innovations created a wide variety of musical styles and features, and musical knowledge became a cue to social history and cultural group membership. This created selective feedback favoring individuals who tended to perceive music as a cue to group membership, as they would have more accurate ideas about others' social group membership. This hypothesized combination of cultural and biological evolution would lead to an evolved bias to use music as a cue to guide and facilitate social interactions, consistent with findings that shared musical knowledge serves as a social cue from early in childhood through adulthood (see sects. 3.3 and 3.4).
Synchronized and harmonized group performances help cement group identity, and eventually allow skilled participation in ritualized performances to serve as a hard-to-fake indicator of group membership. Furthermore, the existence of diverse pieces and sub-styles allows subgroups to express their uniqueness within a broader shared musical repertoire or style. Such expressions of identity at multiple hierarchical levels are useful because human biological and cultural evolution has been characterized by increasing complexity of social structure, as exemplified by the large-scale nation-states characteristic of modern human societies (Turchin et al., Reference Turchin, Currie, Whitehouse, François, Feeney, Mullins and Spencer2018). Thus, group musical performance – including dance – facilitates lasting, culturally evolving indicators of group identity and bonds – akin to passwords or shibboleths (cf. Feekes, Reference Feekes1982; Fitch, Reference Fitch, Oller and Griebel2004) – that extend beyond individual recognition and memory, aiding intercultural marriage and trade.
2.3 Gene–culture coevolution
These specific design features and their interactions – dancing to an isochronous beat with a metrical hierarchy, singing learned melodies based on discrete scales in harmony, using predictable, repetitive musical structures, and using musical performances as cues for social identity – are widespread throughout the world's musical systems (Savage et al., Reference Savage, Brown, Sakai and Currie2015; see sect. 3.1). These features have clear functions for group performance, but little or no function in solo performance (hence their rarity in birdsong, whale song, and certain solo human music genres such as lament; Frigyesi, Reference Frigyesi1993; Tolbert, Reference Tolbert1990). These design features are therefore predicted a priori by the MSB hypothesis, but not by solo signaling hypotheses such as sexual selection for mate attraction (Miller, Reference Miller, Wallin, Merker and Brown2000) or maternal singing to infants (Mehr & Krasnow, Reference Mehr and Krasnow2017; Mehr et al., target article). Although these features promote coordination in dyadic music (e.g., duets) and memorability/communicative power in solo music (e.g., lullabies; Cirelli & Trehub, Reference Cirelli and Trehub2020; Corbeil, Trehub, & Peretz, Reference Corbeil, Trehub and Peretz2016), their added value in supporting extended, coordinated group performances is most evident for larger groups.
MSB posits an extended timeline in which different core mechanisms of musicality arose through a coevolutionary “virtuous spiral.” Although many of the specific design features above could in principle function independent of the others, and would prove adaptive independently at any proto-musical stage, over evolutionary time we hypothesize that isochronous beats coevolutionarily enabled meter and dance, and that pitched singing enabled scale-based melody and harmony. Each new feature added value in supporting extended, coordinated, harmonious group performance. Each feature may have been initially based on behavioral innovations involving synchronization of the ancestrally individualistic displays seen in other great apes (e.g., chimpanzee pant-hoot displays and fruit tree “carnival” displays, cf. Merker, Reference Merker1999; Merker et al., Reference Merker, Morley, Zuidema and Honing2018). However, each innovation opened a new cognitive/musical niche selecting for independent specialization of relevant neural circuitry (see sect. 4).
Early instantiations of music provided selective preconditions for later cognitive and neurobiological changes underlying human musicality, analogous to the well-documented examples of gene–culture coevolution involving fire and dairy farming. Cultural innovations created a variety of proto-musical behaviors, with musical knowledge becoming a potential cue to social history and cultural (sub-)group membership. For example, this could have created selective feedback favoring individuals who used music as cues to group membership. Together, biological and cultural coevolution created a framework for the coordinated, harmonious, emotional group performances that are evident today throughout the world's musical cultures. The major inter-relationships among these components of human musicality are summarized in Figure 2 (but see sect. 6.3 for caveats regarding causality in our proposed coevolutionary mechanisms).
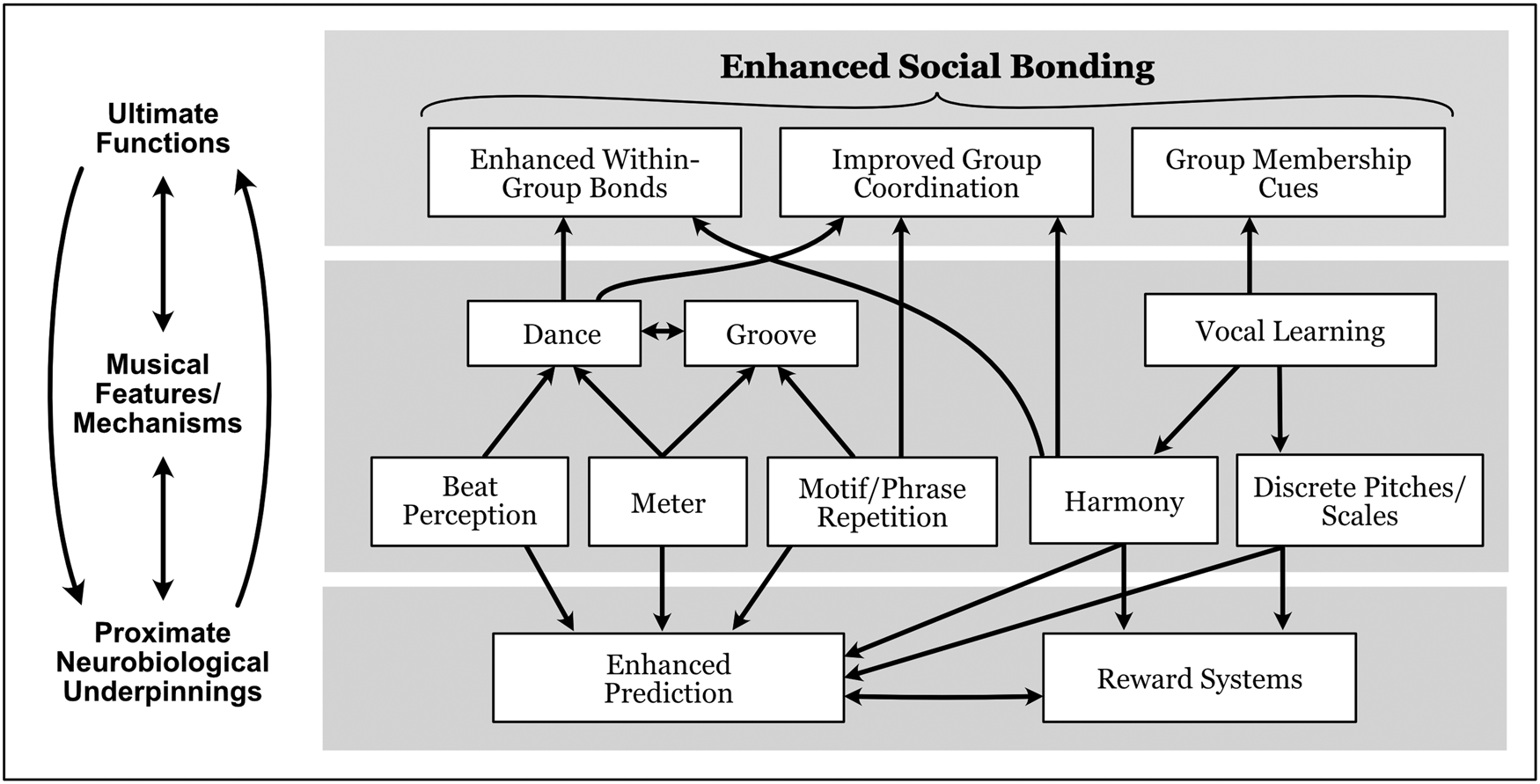
Figure 2. Proposed coevolutionary relationships among multiple musical features and mechanisms, indicating their contributions to ultimate functions by facilitating social bonding in multiple ways, their proximate neurobiological underpinnings in prediction and reward systems, and feedback loops among these different levels.
2.4 Benefits of social bonding
We hypothesize that musicality increased the number of “simple” relationships (e.g., “friends”), and increased the quality (depth and complexity) of existing relationships. The opportunity for many individuals to participate productively in social interaction through proto-musical behaviors facilitates an efficient bonding mechanism for groups of varying sizes, thereby conferring associated benefits (as outlined in sect. 2.1). However, we must consider the nature of the subsidiary relationships and social structures in which they operate. Many vertebrate species live in large groups (e.g., fish schools, bird flocks, and ungulate herds), but do not exhibit strong social bonds with more than a small number of individuals, and/or the relationships are undifferentiated. Indeed, the “number of differentiated relationships” (Bergman & Beehner, Reference Bergman and Beehner2015) can vary independently from raw group size. For example, a monogamous pair with bi-parental care involves two differentiated relationships (sexual mate, and caregiving partner) or even three (adding joint territory defense), a situation typical in many birds. The social bonding design features we have identified can operate at multiple levels simultaneously, in the same way that a couple dancing at a party can intensify their own relationship, and their relationship with the broader social group.
2.5 Participatory versus presentational music
For most of hominin evolution, the only way to experience music was to make it oneself, or to observe others making music in real time. But as music-making technology culturally evolved, opportunities for solo listening increased (e.g., recording technology and personal music-playing devices) and individual virtuosity became increasingly emphasized. Cross-cultural analyses suggest that forms of music-making coevolved in parallel with social structures: larger-scale, more hierarchical societies tend to emphasize “presentational” music made by small numbers of performers for large numbers of passive (or virtual) audiences. Conversely, smaller-scale, more egalitarian societies tend to emphasize “participatory” music in which large groups sing, dance, and play instruments together with little or no distinction between performers and audience (Lomax, Reference Lomax1968; Turino, Reference Turino2008). Once group size increases substantially, it may not be feasible for all individuals to participate actively in a coordinated manner, but music can facilitate bonding via passive (including digital) participation. This enables music (e.g., national anthems) to help construct social identities even among massive “imagined communities” (Anderson, Reference Anderson1991) whose members may never physically interact with one another.
The participatory mode of musical performance is hypothesized to be the ancestral one that operated over long time scales. It is imperative to avoid conflating pervasive technology-driven aspects of contemporary musical practice (e.g., static audiences, solo listening, and control by global corporations) with the conditions under which humans experienced music during most of our evolutionary history. As a result, testing predictions of the MSB hypothesis should favor contexts such as drumming circles, campfire singalongs, and folk dances over solo-listening via headphones, or collective, static listening at a Mozart performance. Even in societies dominated by presentational music, participatory contexts retain their social and emotional potency, as highlighted by the collective singing of Italians from their balconies during the coronavirus lockdown (Grahn, Bauer, & Zamm, Reference Grahn, Bauer and Zamm2020; Horowitz, Reference Horowitz2020; Kornhaber, Reference Kornhaber2020).
2.6 Summary
Summarizing, the MSB hypothesis argues that music is a derived bonding mechanism, akin to but augmenting previous ABMs such as grooming and laughter. This augmentation occurs via the provision of a shared framework for individual participants to establish and maintain strong bonds with more than one individual (or a small group of individuals) at a time, thus bridging the “bonding gap” problem posed during human evolution by increasing group size and complexity (Dunbar, Reference Dunbar1993, Reference Dunbar2012b). Proto-musical features may initially have arisen as behavioral innovations that later initiated a process of gene–culture coevolution. Crucially, the design features of musicality discussed above make music better suited than ABMs or language for coordinating behavior and facilitating social bonding in larger and more complex groups.
3. Cross-disciplinary evidence
Evidence in support of the MSB hypothesis comes from cross-cultural, historical/archeological, developmental, and social psychological research.
3.1. Cross-cultural evidence
One line of evidence for the MSB hypothesis comes from the study of cross-cultural musical universals (Brown & Jordania, Reference Brown and Jordania2013; Lomax, Reference Lomax1968; Mehr et al., Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019; Nettl, Reference Nettl2015; Savage, Reference Savage2018, Reference Savage and Sturman2019b; Savage & Brown, Reference Savage and Brown2013; Stevens & Byron, Reference Stevens, Byron, Hallam, Cross and Thaut2016; Trehub et al., Reference Trehub, Becker, Morley and Honing2018). Music, like language, is a human universal found in all known cultures (Brown, Reference Brown1991; Mehr et al., Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019). Few if any specific musical features are found in all known musics, just as few specific linguistic features are found in all known languages (Evans & Levinson, Reference Evans and Levinson2009). However, researchers have identified dozens of “statistical universals” that predominate throughout diverse samples of the world's music, relating both to functional context and to musical structure (Mehr et al., Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019; Savage et al., Reference Savage, Brown, Sakai and Currie2015; Table 1). These cross-cultural similarities suggest selection by biological and/or cultural evolution.
Table 1. Cross-culturally widespread musical structures and functions
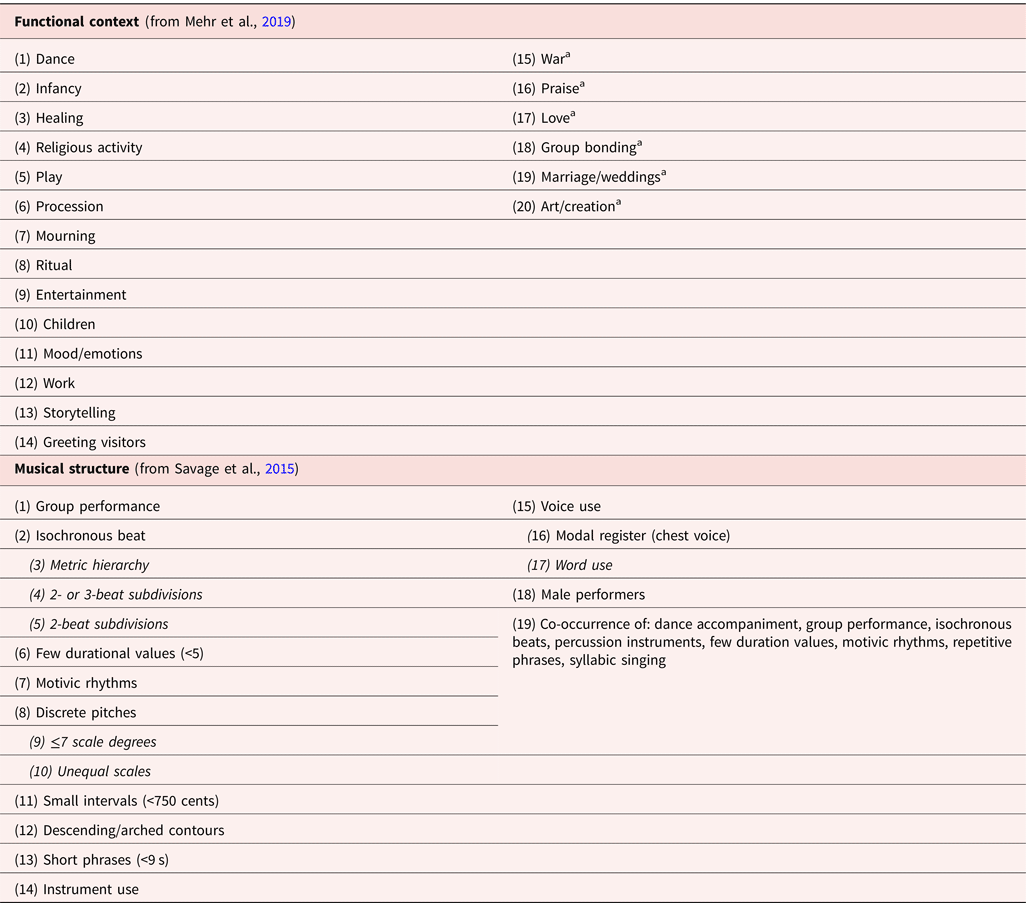
Functional contexts were found by Mehr et al. (Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019) to be associated with singing in ethnographic descriptions of the 60 societies from the Human Relations Area Files Probability Sample (Lagacé, Reference Lagacé1979). Musical structures were found by Savage et al. (Reference Savage, Brown, Sakai and Currie2015) to predominate (items 1–18) or to co-occur (item 19) consistently in each of nine world regions across a sample of 304 audio recordings from the Garland Encyclopedia of World Music (Nettl, Stone, Porter, & Rice, Reference Nettl, Stone, Porter and Rice1998–2002). Nested relationships are indicated with indented italics.
a Indicates associations that were only significant using one of the two methods reported by Mehr et al. (Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019) (Mehr et al. [Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019] used two methods to examine universal associations with singing: “topic annotations from the Outline of Cultural Materials [‘OCM identifiers’] and automatic detection of related keywords.” The second method was needed “because some hypotheses correspond only loosely to the OCM identifiers (e.g., ‘love songs’ is only a partial fit to ARRANGING A MARRIAGE [the OCM identifier used] and not an exact fit to any other identifier).” Similarly, “group bonding” is only a partial fit to the OCM identifier “SOCIAL RELATIONSHIPS AND GROUPS,” which covers a broader range of social behaviors than simply “group bonding.” After adjusting for ethnographer bias and multiple comparisons, Mehr et al. found “support from both methods for 14 of the 20 hypothesized associations between music and a behavioral context, and support from one method for the remaining six.” See Mehr et al. [Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019] for further details).
Crucial to our hypothesis, music performs similar social bonding functions across cultures. All of the 20 widespread functional contexts supported by at least one analysis in Mehr et al. (Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019) summarized in Table 1 relate to social bonding, particularly through the ubiquitous use of music in communal ceremonies and rituals (e.g., healing, procession, mourning, storytelling, greeting visitors, praise/religion, and weddings). Even the secular use of music as art or entertainment is itself often a form of communal ritual. For example, aspects of Western art music concert attendance function to cement social bonds between participants and exclude non-participants in similar ways to other elite rituals throughout history (Nooshin, Reference Nooshin2011; Small, Reference Small1998). Other non-ritual contexts have social bonding functions in bringing together parents and infants (lullabies and play songs), mates (love songs), or coordinating activities among multiple individuals (work songs and dance music). Finally, regulation of moods/emotions is one of the key components of our definition of social bonding (“…synchronizing and harmonizing the moods, emotions, actions, or perspectives of two or more individuals”). Even mood regulation via solo music can support social functions or evoke social contexts. For example, people may ease the pain of separation from loved ones by listening to or playing music that evokes shared memories (Kornhaber, Reference Kornhaber2020), or use music to prepare their mood for an effective social interaction, allowing them to regulate their behavior and behave in the socially-expected manner (Erber, Wegner, & Therriault, Reference Erber, Wegner and Therriault1996; Greenwood & Long, Reference Greenwood and Long2009).
Similarly, most of the widespread structural aspects of music support coordinated music-making. Throughout the world, humans tend to sing, play percussion instruments, and dance to simple, repetitive music in groups, and this is facilitated by the widespread use of simple-integer pitch and rhythm ratios, scales based on a limited number of discrete pitches (≤7), and isochronous beats grouped in multiples of two or three (Bowling & Purves, Reference Bowling, Hoeschele, Gill and Fitch2015; Jacoby & McDermott, Reference Jacoby and McDermott2017; Jacoby et al., Reference Jacoby, Polak, Grahn, Cameron, Lee, Godoy and McDermottPreprint; Kuroyanagi et al., Reference Kuroyanagi, Sato, Ho, Chiba, Six, Pfordresher and Savage2019; Ravignani, Delgado, & Kirby, Reference Ravignani, Delgado and Kirby2017; Savage et al., Reference Savage, Brown, Sakai and Currie2015). The widespread use of simple, discrete meters and scales also enables multiple people to memorize and coordinate their performances. These widespread musical properties have few direct parallels in language. Group coordination provides a common purpose that unifies the cross-cultural structural regularities of human music (Savage et al., Reference Savage, Brown, Sakai and Currie2015).
3.2 Fossil and archeological evidence
Although music itself leaves no fossil record, inferences can be drawn from evidence about the evolution of musicality, the role this played in early human society, and its relationship to other evolutionary developments such as brain size, language, group size, and sociality (Mithen, Reference Mithen2005; Morley, Reference Morley2013). The fossil record for human evolution indicates that capacities for sophisticated and diverse vocalizations and body language, including dancing, were present before there is credible evidence for compositional language (as reviewed in Mithen, Reference Mithen2005). Archeological evidence from the Paleolithic indicates increasing group size and long-distance contacts (Gamble, Reference Gamble, Dunbar, Gamble and Gowlett2010; Read & Van der Feeuw, Reference Read, Van der Feeuw, Coward, Hosfield, Pope and Wenban-Smith2015), suggesting that ABMs had become insufficient by at least 2 million years ago. The earliest surviving musical instruments – bone flutes – have been dated to over 35,000 years ago and are speculated to have functioned to support larger social networks (Conard, Malina, & Münzel, Reference Conard, Malina and Münzel2009). Prehistoric rock art often appears to be positioned with regard to the acoustic properties of either the cave or cliff face on which it is located (e.g., Fazenda et al., Reference Fazenda, Scarre, Till, Pasalodos, Guerra, Tejedor and Foulds2017; Rainio, Lahelma, Aikas, Lassfolk, & Okkonen, Reference Rainio, Lahelma, Aikas, Lassfolk and Okkonen2018), suggesting that music played a role in the social-bonding rituals associated with that art. Similarly, prehistoric and early historic architecture used for social-bonding ceremonies often appears to have been designed with regard to its acoustic properties and to facilitate music making (e.g., Göbekli Tepe: Notroff, Dietrich, & Schmidt, Reference Notroff, Dietrich, Schmidt, Renfrew, Boyd and Morely2015; Stonehenge and other Neolithic monuments in Britain: Banfield, Reference Banfield2009; Watson & Keating, Reference Watson and Keating1999; and Ancient Mayan temples: Sanchez, Reference Sanchez2007).
3.3. Developmental evidence
Extensive evidence demonstrating spontaneous and early development of social functions of music also supports the MSB hypothesis. Adults around the world produce infant-directed songs, such as lullabies, with similar, cross-culturally recognizable acoustic features (Mehr, Singh, York, Glowacki, & Krasnow, Reference Mehr, Singh, York, Glowacki and Krasnow2018; Trehub, Unyk, & Trainor, Reference Trehub, Unyk and Trainor1993). Song is highly effective at emotional modulation in infants – reliably more effective than speech, with infants exhibiting longer visual fixations and greater reductions in stress and body movement to maternal singing than to speaking (Cirelli & Trehub, Reference Cirelli and Trehub2020; Corbeil et al., Reference Corbeil, Trehub and Peretz2016; Ghazban, Reference Ghazban2013; Nakata & Trehub, Reference Nakata and Trehub2004; Trehub, Reference Trehub, Hallam, Cross and Thaut2016). Infants also respond differently to songs sung in different styles (e.g., lullaby vs. playsong; Cirelli, Jurewicz, & Trehub, Reference Cirelli, Jurewicz and Trehub2019; Rock, Trainor, & Addison, Reference Rock, Trainor and Addison1999). Singing to infants thus appears to serve a communicative function, allowing parents to communicate specific emotional messages to infants before they can understand the semantic content of language (Rock et al., Reference Rock, Trainor and Addison1999; Trainor, Clark, Huntley, & Adams, Reference Trainor, Clark, Huntley and Adams1997; Trehub et al., Reference Trehub, Unyk, Kamenetsky, Hill, Trainor, Henderson and Saraza1997). Singing and musical interactions also directly improve parent–infant social bonds: Interventions promoting singing and musical interaction between parents and infants strengthen parents' attachment to their infants, more so than nonmusical play (Vlismas, Malloch, & Burnham, Reference Vlismas, Malloch and Burnham2013). Music thus facilitates both parent–infant communication and parent–infant bonding from early in life, before extensive experience or opportunities for learning.
Beyond infancy, musical activities continue to promote bonding: Across a range of tasks, group musical involvement increases children's prosocial behavior. Thus, young children act more prosocially (in terms of sharing and fairness) after a musical game than a similar non-musical game (Kirschner & Tomasello, Reference Kirschner and Tomasello2010); after group singing than group art or competitive games (Good & Russo, Reference Good and Russo2016); and after joint synchronized, rhythmic movement than non-synchronized movement (Rabinowitch & Meltzoff, Reference Rabinowitch and Meltzoff2017).
Children (like adults) choose to affiliate with members of their own social group (Bigler, Jones, & Lobliner, Reference Bigler, Jones and Lobliner1997). From early infancy, music serves as a marker of social group membership, allowing for the identification of preferred social partners (Cirelli, Trehub, & Trainor, Reference Cirelli, Trehub and Trainor2018). Shared knowledge of specific songs serves as a particularly informative signal of common group membership: because of the wide range of forms a song can take, knowledge of a particular song implies common social or cultural background (Soley & Spelke, Reference Soley and Spelke2016). Infants accordingly treat shared musical knowledge as socially meaningful from early in life: 5-month-old infants prefer to look at people who sing melodies previously sung by a parent, over people who sing melodies previously sung by an unfamiliar adult (Mehr, Song, & Spelke, Reference Mehr, Song and Spelke2016). These early preferences appear to form the foundation for selective social affiliations based on music: At preschool age, children use knowledge of a familiar song as a social cue to select friends (Soley & Spelke, Reference Soley and Spelke2016), and by 14 months exhibit more prosocial behavior (helping) toward an unfamiliar woman who sings a familiar song (previously sung by a parent) than an unfamiliar song (Cirelli & Trehub, Reference Cirelli and Trehub2018). Together, these results suggest that musical knowledge shapes the formation of children's social bonds, and that the link between shared musical knowledge and social connection is rooted in early infancy.
3.4. Social psychological evidence
Behavioral experiments from social psychology support the MSB hypothesis, suggesting that musical behavior is not only associated with, but may causally support, social bonding. In particular, music provides a foundation for synchronized behavior in large groups (as argued above), and a number of experiments and meta-analyses show that rhythmic synchronization with other individuals promotes increased prosocial behavior (i.e., actions that increase others' well-being; Mogan, Fischer, & Bulbulia, Reference Mogan, Fischer and Bulbulia2017; Rennung & Göritz, Reference Rennung and Göritz2016). Synchrony has been empirically linked to cooperation in economic games (Lang, Bahna, Shaver, Reddish, & Xygalatas, Reference Lang, Bahna, Shaver, Reddish and Xygalatas2017; Launay, Dean, & Bailes, Reference Launay, Dean and Bailes2013; Reddish, Bulbulia, & Fischer, Reference Reddish, Bulbulia and Fischer2014; Wiltermuth & Heath, Reference Wiltermuth and Heath2009), entitativity (feelings of being on the same team; Lakens & Stel, Reference Lakens and Stel2011; Reddish, Fischer, & Bulbulia, Reference Reddish, Fischer and Bulbulia2013), rapport and interpersonal liking (Hove & Risen, Reference Hove and Risen2009; Miles, Nind, & Macrae, Reference Miles, Nind and Macrae2009; Valdesolo & Desteno, Reference Valdesolo and Desteno2011), and helping behavior (Cirelli, Einarson, & Trainor, Reference Cirelli, Einarson and Trainor2014; Kokal, Engel, Kirschner, & Keysers, Reference Kokal, Engel, Kirschner and Keysers2011; Valdesolo & Desteno, Reference Valdesolo and Desteno2011). Similarly, dancing in synchrony increases participants' feelings of connectedness to the group with which they are dancing, as well as their liking and assessment of similarity with co-dancers (Tarr, Launay, Cohen, & Dunbar, Reference Tarr, Launay, Cohen and Dunbar2015; Tarr, Launay, & Dunbar, Reference Tarr, Launay and Dunbar2016). These prosocial effects of synchrony are robust in different contexts (Mogan et al., Reference Mogan, Fischer and Bulbulia2017). Although demand characteristics have been suggested as possible confounds underlying these effects (Atwood, Mehr, & Schachner, Reference Atwood, Mehr and Schachner2020; Rennung & Göritz, Reference Rennung and Göritz2016), significant prosocial effects of synchrony remain after potential confounds of suggestion, competence, and shared intention are eliminated (e.g., in a virtual reality setting; Tarr, Slater, & Cohen, Reference Tarr, Slater and Cohen2018). However, meta-analyses implied inconclusive results regarding the precise roles of “music” and of synchrony to an isochronous beat, as opposed to more generally synchronized or coordinated non-musical behaviors such as gaze synchrony, affect synchrony, and motor synchrony (Mogan et al., Reference Mogan, Fischer and Bulbulia2017; Rennung & Göritz, Reference Rennung and Göritz2016). In sect. 5, we propose clearer predictions and tests of specific mechanisms by which music promotes social bonding.
More broadly, behavioral studies indicate varied social bonding effects associated with music-based activities, even those that do not explicitly involve constant synchrony. Young children randomly assigned to activities incorporating music exhibit elevated levels of empathy compared to non-musical controls in longitudinal studies (Rabinowitch, Cross, & Burnard, Reference Rabinowitch, Cross and Burnard2013), and adults singing in regular group sessions develop feelings of social closeness toward co-participants more quickly than people engaged in other (non-musical) group activities (Pearce, Launay, & Dunbar, Reference Pearce, Launay and Dunbar2015). Feelings of inclusion, connectivity, and positive affect emerge in small and large singing groups, with participants in large choirs (>80 participants) reporting greater changes in these measures compared to smaller choirs (Weinstein, Launay, Pearce, Dunbar, & Stewart, Reference Weinstein, Launay, Pearce, Dunbar and Stewart2016). These findings highlight the relevance of music-based activities for large-scale social bonding.
4. Neurobiological mechanisms
The MSB hypothesis proposes that social bonding is the ultimate, functional explanation of the evolution of musicality. We now propose specific hypotheses about underlying neurobiological proximate mechanisms underpinning music's social effects (Fig. 3). In brief, music involves predictable combinations of rhythms and pitches, activating neural mechanisms for perception that are tightly coupled with the motor system. Learning to form predictions about these features activates the dopaminergic reward system, which synchronizes its activity with distal regions within the brain. Crucially, predictability also supports synchronization of homologous regions in other individuals' brains. This “neural resonance” (synchronous brain activity across individuals) facilitates social bonding through shared experience, joint intentionality, and “self-other merging.” Through the production of oxytocin and endogenous opioids, neural resonance also facilitates prosociality by associating the rewarding musical experience with specific co-experiencers. Furthermore, because these prosocial experiences are themselves rewarding, we seek them out by attending to and learning more musical features/experiences, updating our predictions (e.g., through statistical learning, by performing and/or experiencing new music), and closing the mechanistic cycle. This proposed mechanistic cycle is detailed below.
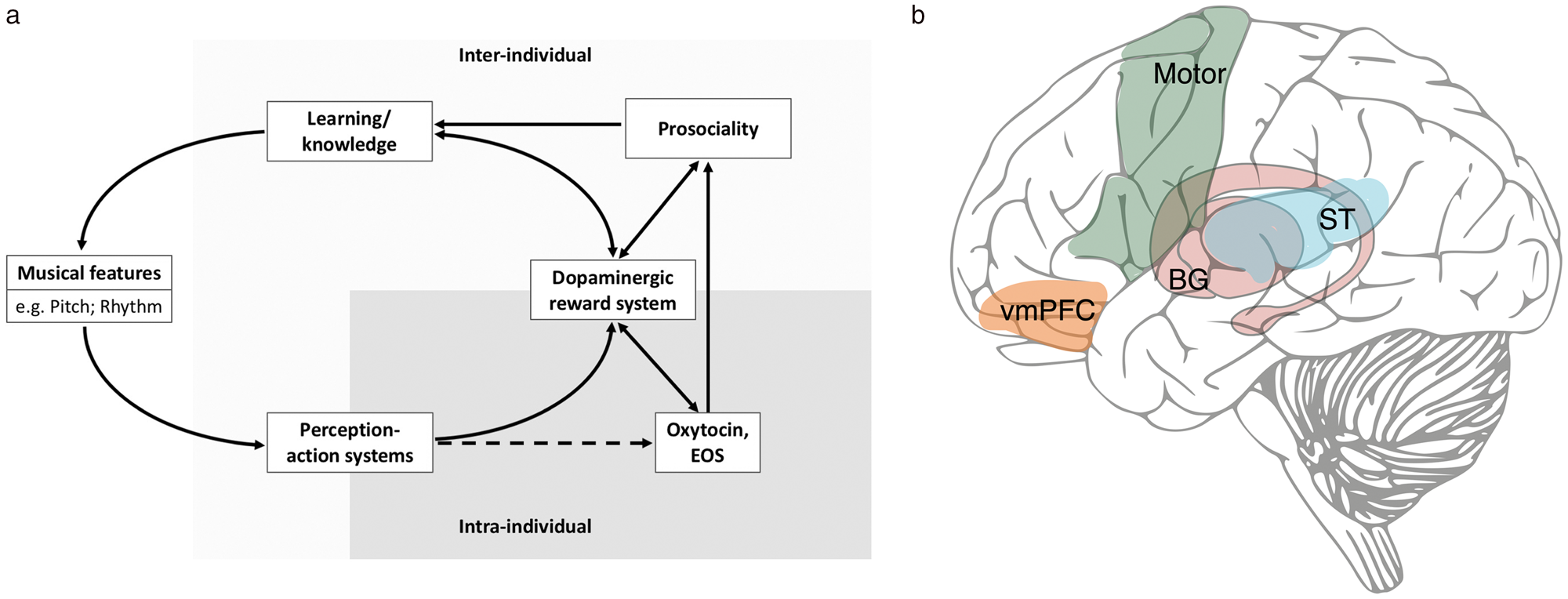
Figure 3. (a) Proposed neurobiological mechanisms underlying music's social bonding functions, showing intra- and inter-individual levels. We propose that the dopaminergic reward system interacts with the endogenous opioid system (EOS) and the release of oxytocin, ultimately providing opportunities for individuals to synchronize their moods, emotions, actions, and/or perspectives through musical engagement (dashed arrow indicates need for more evidence to confirm that the perception/production of music stimulates this pathway). (b) Key neuroanatomical regions in the human brain underlying the MSB hypothesis. ST: superior temporal lobe structures important for auditory perception including Heschl's gyrus, planum temporale, superior temporal gyrus, superior temporal sulcus, middle temporal gyrus. Motor: frontal lobe structures crucial for action planning and execution including premotor and supplementary motor areas as well as primary motor cortex. BG: basal ganglia and related structures, including amygdala, striatum, ventral tegmental area/substantia nigra, caudate, putamen, globus pallidus, and nucleus accumbens. vmPFC: ventromedial prefrontal cortex.
4.1 Perception–action coupling
Perception–action coupling refers to anatomical and/or functional connectivity between brain regions involved in sensory perception (e.g., of pitch or rhythm) and those that are involved in movement (e.g., vocalization and dance). Specifically, auditory–motor coupling is a key neural mechanism that underlies social bonding through music because it enables individuals to synchronize and/or harmonize their own music and actions with others, which is crucial for coordinated group music making. Even during the perception of solo music, the tight coupling between perceptual and motor regions leads to spontaneous and obligatory activity in premotor and supplementary motor areas, classic motor areas that are also part of the action observation network that drives physical and observational learning (Cross, Kraemer, Hamilton, Kelley, & Grafton, Reference Cross, Kraemer, Hamilton, Kelley and Grafton2008).
Rhythm and beat consistently activate the premotor area, supplementary motor area, and basal ganglia, regions commonly thought to belong to the motor system (Grahn & Brett, Reference Grahn and Brett2007). Furthermore, the auditory system is strongly coupled with areas in the motor system during rhythm perception (Grahn & Rowe, Reference Grahn and Rowe2009), and rhythmic oscillatory activity in both the auditory and motor systems tracks the rhythm of music (Fujioka, Ross, & Trainor, Reference Fujioka, Ross and Trainor2015). Some observations show that neural phase-locking activity is even higher in music than in speech (Vanden Bosch der Nederlanden, Joanisse, & Grahn, Reference Vanden Bosch der Nederlanden, Joanisse and Grahn2020). This process of “neuronal entrainment” (neural activity changing its frequency, amplitude, and/or phase in response to external stimulation) is a proposed mechanism through which rhythm in sensory stimuli affects the brain by coordinating activity between separate neuronal populations, such as between the auditory and motor systems (Jones, Reference Jones2018; Morillon & Baillet, Reference Morillon and Baillet2017). This neuronal entrainment enables selective attention to specific points in time (Lakatos et al., Reference Lakatos, Karmos, Mehta, Ulbert and Schroeder2008; Large & Jones, Reference Large and Jones1999). In particular, auditory–motor coupling is strongest when perceiving “high groove” music that elicits the pleasurable drive toward action such as in dance (Janata, Tomic, & Haberman, Reference Janata, Tomic and Haberman2012). Groovy music elicits the urge to dance by increasing the auditory cortex's sensitivity and its coupling with the motor cortex (Stupacher, Hove, Novembre, Schutz-Bosbach, & Keller, Reference Stupacher, Hove, Novembre, Schutz-Bosbach and Keller2013), which is particularly evident with medium levels of rhythmic complexity and expectation violation (Koelsch et al., Reference Koelsch, Vuust and Friston2019; Witek et al., Reference Witek, Clarke, Wallentin, Kringelbach and Vuust2014). In this respect, dance – or any movement to music – is inextricably linked to musical experiences. Note, however, that similar to many of the mechanisms proposed here, coding of value in sensory cortices (i.e., a stronger sensory response to more important or rewarding stimuli) is not unique to the auditory domain but is also evident in other sensory domains such as vision (Koelsch et al., Reference Koelsch, Vuust and Friston2019).
An important pathway underlying perception–action coupling is the arcuate fasciculus, a bundle of axonal connections between frontal lobe (including motor areas) and superior temporal lobe (including auditory areas). Abundant neuroimaging evidence supports the role of the arcuate fasciculus in music making, specifically in auditory perception–action coupling (Halwani, Loui, Rüber, & Schlaug, Reference Halwani, Loui, Rüber and Schlaug2011; Loui, Alsop, & Schlaug, Reference Loui, Alsop and Schlaug2009, Reference Loui, Li and Schlaug2011; Moore, Schaefer, Bastin, Roberts, & Overy, Reference Moore, Schaefer, Bastin, Roberts and Overy2017; Sammler, Grosbras, Anwander, Bestelmeyer, & Belin, Reference Sammler, Grosbras, Anwander, Bestelmeyer and Belin2015). This same pathway also plays a role in social functions: more emotionally empathic people have higher microstructural integrity within the arcuate fasciculus (Parkinson & Wheatley, Reference Parkinson and Wheatley2014). In contrast, people on the autism spectrum, who have known impairments in social bonding, have less connectivity in the arcuate fasciculus (Fletcher et al., Reference Fletcher, Whitaker, Tao, DuBray, Froehlich, Ravichandran and Lainhart2010; Wan, Demaine, Zipse, Norton, & Schlaug, Reference Wan, Demaine, Zipse, Norton and Schlaug2010). By enabling perception–action coupling, the arcuate fasciculus thus provides one possible shared neural mechanism between music and social bonding.
4.2 Prediction and the dopaminergic reward system
Musical perception–action coupling sets up repeated cycles of prediction, expectation violation, and resolution (Huron, Reference Huron2006). In these hierarchical perception–action trajectories, the predictive context surrounding pitch and rhythm are established, violated, and then resolved (Clark, Reference Clark2013; Fitch, von Graevenitz, & Nicolas, Reference Fitch, von Graevenitz, Nicolas, Skov and Vartanian2009). Successful predictions become rewarding to the brain by activating neurons of the dopaminergic system and its related areas (caudate, nucleus accumbens, amygdala, and ventromedial prefrontal cortex) that code for fundamental evolutionary rewards such as food and sex, and also learned rewards such as money (Friston, Reference Friston2010; Knutson, Westdorp, Kaiser, & Hommer, Reference Knutson, Westdorp, Kaiser and Hommer2000; Schultz, Dayan, & Montague, Reference Schultz, Dayan and Montague1997). The same dopaminergic reward system is also active during the anticipation and perception of pleasurable music (Blood & Zatorre, Reference Blood and Zatorre2001; Blood, Zatorre, Bermudez, & Evans, Reference Blood, Zatorre, Bermudez and Evans1999; Cheung et al., Reference Cheung, Harrison, Meyer, Pearce, Haynes and Koelsch2019; Salimpoor, Benovoy, Larcher, Dagher, & Zatorre, Reference Salimpoor, Benovoy, Larcher, Dagher and Zatorre2011, Reference Salimpoor, Zald, Zatorre, Dagher and McIntosh2015; Zatorre, Reference Zatorre2018; Zatorre & Salimpoor, Reference Zatorre and Salimpoor2013), supported by the functional coupling between auditory areas in the superior temporal lobe and reward-sensitive areas such as the nucleus accumbens (Salimpoor et al., Reference Salimpoor, van den Bosch, Kovacevic, McIntosh, Dagher and Zatorre2013). Manipulating expectations for pitch-related musical features, such as consonance and dissonance, can modulate activity in the nucleus accumbens and amygdala. Thus, music can provide its own reward prediction error and motivate learning (Cheung et al., Reference Cheung, Harrison, Meyer, Pearce, Haynes and Koelsch2019; Gold et al., Reference Gold, Mas-Herrero, Zeighami, Benovoy, Dagher and Zatorre2019). Additionally, people who frequently experience chills when listening to music show high white matter connectivity between auditory, social, and reward-processing areas (Sachs, Ellis, Schlaug, & Loui, Reference Sachs, Ellis, Schlaug and Loui2016). Chills from music are also related specifically to increased binding to dopamine receptor D2 (Salimpoor et al., Reference Salimpoor, Benovoy, Larcher, Dagher and Zatorre2011). In contrast, people with musical anhedonia, who find music unrewarding, have decreased functional connectivity and altered structural connectivity between auditory and reward-related areas (Loui et al., Reference Loui, Patterson, Sachs, Leung, Zeng and Przysinda2017; Martínez-Molina, Mas-Herrero, Rodríguez-Fornells, Zatorre, & Marco-Pallarés, Reference Martínez-Molina, Mas-Herrero, Rodríguez-Fornells, Zatorre and Marco-Pallarés2016; Mas-Herrero, Zatorre, Rodriguez-Fornells, & Marco-Pallarés, Reference Mas-Herrero, Zatorre, Rodriguez-Fornells and Marco-Pallarés2014).
Because humans are social animals, the predictions we make and the rewards we receive are often tied to social stimuli. Thus, the brain has to learn from social cues by associating social stimuli with reward predictions (Atzil, Gao, Fradkin, & Barrett, Reference Atzil, Gao, Fradkin and Barrett2018). Indeed, the same areas in the dopaminergic reward system – the caudate, nucleus accumbens, and ventromedial prefrontal cortex – are causally linked to cooperative behavior as well as prediction and reward. The reward system is activated when we share information with others about ourselves (Tamir & Mitchell, Reference Tamir and Mitchell2012), when we view loved ones (Bartels & Zeki, Reference Bartels and Zeki2004), and when mothers bond with their infants (Atzil et al., Reference Atzil, Touroutoglou, Rudy, Salcedo, Feldman, Hooker and Barrett2017). Prosocial behaviors commonly engage the reward system (Zaki & Mitchell, Reference Zaki and Mitchell2013); these include cooperating (Decety, Jackson, Sommerville, Chaminade, & Meltzoff, Reference Decety, Jackson, Sommerville, Chaminade and Meltzoff2004), perspective taking (Mitchell, Banaji, & Macrae, Reference Mitchell, Banaji and Macrae2005), and empathizing with others (Beadle, Paradiso, & Tranel, Reference Beadle, Paradiso and Tranel2018). Together, these results suggest that the dopaminergic reward system is involved causally in the link between music and social bonding through the mechanism of prediction.
4.3 Oxytocin and the endogenous opioid system (EOS)
We propose that opioids released in the EOS, and oxytocin, are also part of the mechanistic underpinnings linking prediction, reward, and social bonding (Chanda & Levitin, Reference Chanda and Levitin2013; Launay et al., Reference Launay, Tarr and Dunbar2016; Tarr, Launay, & Dunbar, Reference Tarr, Launay and Dunbar2014). The nucleus accumbens and ventral tegmental area are key regions that overlap between the dopaminergic reward system and the EOS (Dölen, Darvishzadeh, Huang, & Malenka, Reference Dölen, Darvishzadeh, Huang and Malenka2013; Le Merrer, Becker, Befort, & Kieffer, Reference Le Merrer, Becker, Befort and Kieffer2009), and dopamine is thought to be a salience processing mechanism regulated by oxytocin (Love, Reference Love2014; Shamay-Tsoory & Abu-Akel, Reference Shamay-Tsoory and Abu-Akel2016).
The EOS likely plays a mechanistic role in music-related prosociality. This system has been implicated in the maintenance of social bonds in primate social networks (Keverne, Martensz, & Tuite, Reference Keverne, Martensz and Tuite1989; Maestripieri, Reference Maestripieri, Platt and Ghazanfar2010; Ragen, Maninger, Mendoza, Jarcho, & Bales, Reference Ragen, Maninger, Mendoza, Jarcho and Bales2013; Schino & Troisi, Reference Schino and Troisi1992). Intervention studies in humans indicate that, compared to a placebo, naltrexone (an opioid blocker) can reduce feelings of social connections with others (e.g., Inagaki, Reference Inagaki2018; Inagaki, Ray, Irwin, Way, & Eisenberger, Reference Inagaki, Ray, Irwin, Way and Eisenberger2016), and lower affiliative behavior and desire for interpersonal closeness (Tchalova & Macdonald, Reference Tchalova and Macdonald2020). Listening to music influences mu-opiate receptor expression in the EOS (Stefano, Zhu, Cadet, Salamon, & Mantione, Reference Stefano, Zhu, Cadet, Salamon and Mantione2004) and can reduce the need for pain medicationFootnote 3 (e.g., Bernatzky, Presch, Anderson, & Panksepp, Reference Bernatzky, Presch, Anderson and Panksepp2011; Lepage, Drolet, Girard, Grenier, & DeGagné, Reference Lepage, Drolet, Girard, Grenier and DeGagné2001). Elevated pain thresholds are experienced after singing (Pearce et al., Reference Pearce, Launay and Dunbar2015; Weinstein et al., Reference Weinstein, Launay, Pearce, Dunbar and Stewart2016) and synchronized dancing (Tarr et al., Reference Tarr, Launay, Cohen and Dunbar2015, Reference Tarr, Launay and Dunbar2016), but not after administration of naltrexone (Tarr, Launay, Benson, & Dunbar, Reference Tarr, Launay, Benson and Dunbar2017), suggesting that pain threshold is an appropriate proxy-measure of endorphin uptake in these experiments. There is some evidence of endorphin-mediated synchrony effects on cooperation (e.g., when dancing; Lang et al., Reference Lang, Bahna, Shaver, Reddish and Xygalatas2017), further demonstrating links between music, the EOS, and social bonding.
Although more empirical research is needed, there is evidence that oxytocin levels are elevated after taking part in a singing class (Grape, Sandgren, Hansson, Ericson, & Theorell, Reference Grape, Sandgren, Hansson, Ericson and Theorell2003), or following a group jam session of improvised singing (Keeler et al., Reference Keeler, Roth, Neuser, Spitsbergen, Waters and Vianney2015). Elevated oxytocin levels have been correlated with increased generosity (Fujii, Schug, Nishina, Takahashi, & Okada, Reference Fujii, Schug, Nishina, Takahashi and Okada2016; Zak, Stanton, & Ahmadi, Reference Zak, Stanton and Ahmadi2007), empathy (Domes, Heinrichs, Michel, Berger, & Herpertz, Reference Domes, Heinrichs, Michel, Berger and Herpertz2007; Hurlemann et al., Reference Hurlemann, Patin, Onur, Cohen, Baumgartner, Metzler and Kendrick2010), and possibly trust (Kosfeld, Heinrichs, Zak, Fischbacher, & Fehr, Reference Kosfeld, Heinrichs, Zak, Fischbacher and Fehr2005; Zak, Kurzban, & Matzner, Reference Zak, Kurzban and Matzner2005; but see Nave, Camerer, & McCullough [Reference Nave, Camerer and McCullough2015] and Declerck, Boone, Pauwels, Vogt, & Fehr [Reference Declerck, Boone, Pauwels, Vogt and Fehr2020]). Furthermore, intranasal administration of oxytocin promotes in-group cooperation (e.g., De Dreu and Kret, Reference De Dreu and Kret2016) and increases synchrony in dancing (Josef, Goldstein, Mayseless, Ayalon, & Shamay-tsoory, Reference Josef, Goldstein, Mayseless, Ayalon and Shamay-tsoory2019) and finger-tapping behavior (Gebauer et al., Reference Gebauer, Witek, Hansen, Thomas, Konvalinka and Vuust2016), suggesting a reciprocal feedback loop between music-based activity and social cohesion. Although evidence linking oxytocin specifically with music remains limited, and the strength of oxytocin's relationship with cooperation more generally is debated (particularly studies based on administering intranasal oxytocin; e.g., Walum, Waldman, & Young, Reference Walum, Waldman and Young2016), current evidence suggests that music engages the oxytocin and EOS systems in ways that facilitate social bonding, as predicted by the MSB hypothesis. Combined with the reward system, these pathways offer a positive-feedback loop following music engagement, enabling groups of individuals to synchronize their moods, emotions, actions and/or perspectives, and providing motivation to continue engaging with others in social and musical contexts.
4.4 Learning and vocal imitation
The capacity to learn and reproduce complex motor movements, including vocalizations (songs), is central to the cultural transmission of music. Although humans are the only primates capable of learning complex, novel vocalizations, this ability has evolved independently at least seven times in evolutionary history (Fitch & Jarvis, Reference Fitch, Jarvis and Arbib2013; Nowicki & Searcy, Reference Nowicki and Searcy2014; Syal & Finlay, Reference Syal and Finlay2011), allowing us to make inferences about how and why it evolved. Some vocal learning clades (seals, baleen whales, and some songbirds) show a strong male bias in vocal learning abilities consistent with sexual selection. However, such a bias is absent in most other vocal learners (parrots, elephants, toothed whales, many tropical bird species, and humans), suggesting that sexual selection cannot be the only factor driving the evolution of vocal learning (Fitch, Reference Fitch2006). Instead, learned animal songs (solo or duet) appear to serve multiple evolutionary functions within the umbrella of social bonding, including mate attraction, cementing and affirming social bonds within pairs or groups, and territorial functions including advertising the bonded group's ability to repel outsiders (Geissmann, Reference Geissmann1999; Haimoff, Reference Haimoff1986; Wickler, Reference Wickler1980).
In vocal learning species, vocal imitation and song production are likely based on similar neurobiological mechanisms (Mercado, Mantell, & Pfordresher, Reference Mercado, Mantell and Pfordresher2014). Learning to reproduce pitches and rhythms accurately engages reward mechanisms, as shown by evidence that dopamine neurons encode performance error in songbirds (Gadagkar et al., Reference Gadagkar, Puzerey, Chen, Baird-Daniel, Farhang and Goldberg2016). Furthermore, the presence of a conspecific (of the opposite sex in this case) leads the male zebra finch to decrease variability of sung syllables; this syllabic structure is attributed to perception–action circuits analogous to the human superior temporal and motor structures (Fitch & Jarvis, Reference Fitch, Jarvis and Arbib2013; Sakata & Brainard, Reference Sakata and Brainard2008). Once individuals learn to produce musical features, they not only reproduce learned patterns of features, but also deviate from predicted combinations of features, for example by inventing new melodies (Wiggins, Tyack, Scharff, & Rohrmeier, Reference Wiggins, Tyack, Scharff, Rohrmeier and Honing2018).
5. Predictions for future research
The MSB hypothesis predicts that core design features of music make it particularly well-suited to facilitate social bonding, and particularly effective in the bonding of large, complex groups. This leads to the following testable predictions.
5.1. Cross-domain predictions (e.g., music, language, ritual)
The MSB hypothesis predicts that music (including dance) is better-suited to social bonding of large, complex groups than ABMs (grooming and laughter), language, or other non-acoustic bonding mechanisms such as shared decorations or non-musical ritual behaviors (e.g., praying together without music). Music should be more effective and/or efficient relative to other methods as group size and complexity increase, such that while making music in pairs might only produce a small increase in dyadic bonding relative to conversation, making music in larger, more complex groups of people (dozens or hundreds organized into differentiated sub-groups) should be more effective for collective bonding than language, laughter, grooming, and so on.
In a social species such as humans, many activities can develop and enhance social bonding, but we predict that bonding via non-musical methods such as language, ritual, or sports should be enhanced by the addition of musical components (e.g., religious services with group singing will result in stronger bonding than those that only involve group prayer). Different musical components are predicted to have synergistic effects such that – all things being equal – including more of these components (e.g., synchronized, harmonized singing and dancing in groups) will tend to increase bonding more than activities that only use one or a few (e.g., conversations or recitation in pairs).Footnote 4 We also predict that participatory musical performances will have significantly stronger effects than either non-participatory (e.g., performance for a static audience) or solo musical experiences (e.g., listening alone to recordings). Group size and complexity should have independent effects (e.g., singing in large choirs should produce greater bonding than singing in small choirs).
These predictions can be tested in controlled experiments and/or field studies along the lines of those discussed in sect. 3. Designing studies that control for specific similarities and differences between closely related domains such as music, language, and dance is challenging but not impossible. For example, to control for the fact that languages have their own (non-isochronous) rhythms, Savage et al. (Reference Savage, Yamauchi, Hamaguchi, Tarr, Kitayama and Fujii2020) had groups of participants simultaneously recite the lyrics to “Twinkle, Twinkle, Little Star” to an isochronous beat or in non-isochronous free rhythm. Savage et al. (Reference Savage, Yamauchi, Hamaguchi, Tarr, Kitayama and Fujii2020) also propose additional manipulations that would allow this paradigm to test other specific predictions of the MSB hypothesis regarding the social bonding effects of melody, harmony, and dance (cf. Fig. 3 in Savage et al., Reference Savage, Yamauchi, Hamaguchi, Tarr, Kitayama and Fujii2020).
5.2. Cross-cultural predictions
The MSB hypothesis predicts that music's social bonding functions should be distributed widely in space and time. Hence, the kinds of predictions described in sect. 5.1 regarding music's superior social bonding power in large groups should apply consistently across cultures and throughout history. Furthermore, it predicts that musical contexts and structures that promote social bonding (e.g., coordinated, participatory group performances) will be more common across cultures than music produced by and for individuals. At the same time, the relative importance of participatory versus presentational music-making is predicted to vary cross-culturally as a function of social structure (because of limitations on simultaneous coordinated performance discussed in sect. 2.5). Smaller-scale, more egalitarian cultures should thus perform and value participatory music more than larger-scale, hierarchical cultures where presentational music should be more common and valued. Participatory versus presentational distinctions are analogous to those found in “imagistic” (high-intensity, small-scale) versus “doctrinal” (low-intensity, large-scale) religious rituals, respectively (Whitehouse, Reference Whitehouse2004), and are predicted to covary cross-culturally with these modes of religiosity. Even in cultures where music is often consumed passively by individuals (e.g., in Western culture, over headphones on personal listening devices), MSB predicts that music will be more effective than non-musical alternatives for social bonding purposes (cf. Rentfrow & Gosling, Reference Rentfrow and Gosling2006). These predictions about cross-cultural use of music for social bonding could be tested in cross-cultural behavioral experiments (cf. Henrich et al., Reference Henrich, Boyd, Bowles, Camerer, Fehr, Gintis, McElreath, Alvard, Barr, Ensminger, Henrich, Hill, Gil-White, Gurven, Marlowe, Patton and Tracer2005; Jacoby et al., Reference Jacoby, Polak, Grahn, Cameron, Lee, Godoy and McDermottPreprint; Polak et al., Reference Polak, Jacoby, Fischinger, Goldberg, Holzapfel and London2018) or analysis of cross-cultural databases of recordings, artifacts, ethnographies, or questionnaires (cf. Lomax, Reference Lomax1968; Mehr et al., Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019; Savage, Reference Savage, Shanahan, Burgoyne and Quinn2019c; Savage et al., Reference Savage, Brown, Sakai and Currie2015; Whitehouse et al., Reference Whitehouse, François, Savage, Hoyer, Feeney, Cioni and Turchinpreprint; Wood et al., Reference Wood, Kirby, Ember, Silbert, Daikoku, McBride and SavagePreprint).
5.3. Cross-species predictions
The MSB hypothesis proposes that human musicality has been shaped by biological and cultural selection, and that the features of music are particularly well-suited for social bonding functions because they support extended, coordinated group performances on a large scale. The MSB account does not claim that music's social bonding function is a unique biological adaptation specific to human musicality. Instead, it argues that music-like behaviors should enhance existing bonding mechanisms in other species as well. Thus, it predicts that, rather than an all-or-nothing divide between human and non-human “music,” species will vary continuously in the degree to which they share specific features of human musicality. The social bonding functions associated with different components of musicality should operate similarly across species, depending on the specific subcomponent, its suitability for group coordination, and the importance of social bonding to their species.
Thus, melodic, learned songs among songbirds, whales, or other vocal learners are predicted to enhance social bonding in these species in a manner analogous to song in humans. These effects may be limited in many non-human species by their lack of ability and/or interest in performing in coordinated groups (e.g., some primates appear motivated to conduct group displays but are unable to synchronize to a beat, whereas some birds appear able to move to a beat but are unmotivated to do so in groups in the wild; Hoeschele, Merchant, Kikuchi, Hattori, & ten Cate, Reference Hoeschele, Merchant, Kikuchi, Hattori, ten Cate and Honing2018). However, such effects should be pronounced in species that perform duets (e.g., many birds, and duetting primates such as gibbons or titi monkeys; Haimoff, Reference Haimoff1986; Hall, Reference Hall2004). Conversely, social primates that do not typically perform in coordinated groups may nonetheless experience social bonding effects of “group” music when exposed to versions of their own vocalizations that have been artificially manipulated to be in synchrony/harmony. Such production/perception dissociations and other nuances of musicality could be tested in controlled cross-species experiments (cf. Hoeschele et al., Reference Hoeschele, Merchant, Kikuchi, Hattori, ten Cate and Honing2018; Merchant, Grahn, Trainor, Rohrmeier, & Fitch, Reference Merchant, Grahn, Trainor, Rohrmeier and Fitch2015).
The MSB hypothesis posits that music and musicality provided a major means by which humans could coordinate behavior on a larger scale than dyads or small groups, allowing for the formation of larger socio-cultural groups. If true, and if different species share components of musicality to differing degrees, then across species, production or proficiency in “musical” behaviors should predict both the number and complexity of social bonds. For example, gelada baboons live in unusually large and complex groups for primates, and they also exhibit rhythmic and melodic vocal features that are unique among primates (Bergman, Reference Bergman2013; Gustison, Aliza, & Bergman, Reference Gustison, Aliza and Bergman2012; Richman, Reference Richman1978, Reference Richman1987). Similar to geladas, many parrot species live in large fission–fusion social groups, and members of the parrot clade show vocal imitation, call convergence, duetting, and the capacity for rhythmic synchronization (Balsby & Scarl, Reference Balsby and Scarl2008; Bradbury, Reference Bradbury2001; Scarl & Bradbury, Reference Scarl and Bradbury2009; Schachner et al., Reference Schachner, Brady, Pepperberg and Hauser2009). In both of these clades, pairs or mating “harems” form stronger bonds than those they share with the larger groups in which they are embedded (cf. Balsby & Scarl, Reference Balsby and Scarl2008; Wanker, Sugama, & Prinage, Reference Wanker, Sugama and Prinage2005). Other species that live in complex fission–fusion groups and could provide evidence of specific design features are elephants and some odontocetes (e.g., orcas and bottlenose dolphins). Such species live in large, complex fission–fusion groups, and are documented vocal learners, but their possession of other design features of music (e.g., synchronization) have not been tested rigorously.
For many species, evidence for design features of musicality would count as evidence against our hypotheses. Examples include solitary species (e.g., many reptiles), species for whom groups consist only of mothers and dependent young (e.g., many carnivores), or group living species that do not have differentiated social bonds with other group members (e.g., schooling fish, larger herds, and swarming insects).
The MSB hypothesis further predicts that if a species does not follow this pattern (e.g., by having a larger social group size than predicted by their features of musicality), then that species will have evolved other non-musical but effective means of coordinating behavior that likely do not appear in human behavior (e.g., reproductive suppression in naked mole rats or pheromonal queen control in eusocial insects; Alaux, Maisonnasse, & Le Conte, Reference Alaux, Maisonnasse and Le Conte2010; Dengler-Crish & Catania, Reference Dengler-Crish and Catania2007). Thus, although the social bonding design features seen in human musicality are not the only way to achieve large, well-bonded groups, they are effective enough that we predict them to evolve convergently (cf. Fitch, Reference Fitch2006).
5.4. Neurobiological predictions
The MSB hypothesis predicts that each of the mechanistic factors proposed above (Fig. 3) contributes to the effects of music on social bonding. Alterations of these mechanistic pathways should therefore produce specific, quantifiable results on bonding. For example, music's perceived social bonding functions should correlate with oxytocin/EOS production, and disrupting the oxytocin/EOS pathway via blocking oxytocin or opioid receptors should disrupt its social bonding effects. Furthermore, because of the dopaminergic reward system is at the center of prediction for musical features, populations with deficient dopaminergic activity may have impaired predictions, which could affect their ability to synchronize or harmonize with others. On the contrary, drugs that restore dopaminergic functions are hypothesized to restore these abilities, and because of the reciprocal nature of these interactions, activities that enhance predictions (such as dancing and harmonizing) may in turn restore dopaminergic functions. These predictions are being tested in the case of Parkinson's disease, which is a special population with deficient dopaminergic activity (Cameron et al., Reference Cameron, Pickett, Earhart and Grahn2016; Grahn & Rowe, Reference Grahn and Rowe2009).
Another prediction is that special populations with high sociability may respond well to musical features especially when coupled with social stimuli, as in the case of children with Williams syndrome (Järvinen-Pasley et al., Reference Järvinen-Pasley, Vines, Hill, Yam, Grichanik, Mills and Bellugi2010; Lense, Gordon, Key, & Dykens, Reference Lense, Gordon, Key and Dykens2014). At a neural level, music's social bonding function should correlate with the degree of neural connectivity between the perception–action and prediction–reward networks, and disruptions to this network (e.g., lesions or genetic syndromes) should accordingly disrupt music's social bonding effect. For example, people with musical anhedonia, who have disrupted connectivity between auditory prediction and reward networks (Belfi & Loui, Reference Belfi and Loui2020), are predicted to have weaker social bonds, and genetic differences (e.g., in DRD2) may predict variation in bonding experienced through musical activities. Although some of these predictions may be difficult to test ethically in humans through controlled experiments, many can be tested using neuroimaging combined with neuropsychological testing in special populations, as well as correlational, longitudinal, or intervention (including brain-stimulation) studies, genome-wide association studies, and/or animal models that share specific neurobiological endophenotypes (Finlay, Darlington, & Nicastro, Reference Finlay, Darlington and Nicastro2001; Fitch & Jarvis, Reference Fitch, Jarvis and Arbib2013; Gingras, Honing, Peretz, Trainor, & Fisher, Reference Gingras, Honing, Peretz, Trainor, Fisher and Honing2018; Hoeschele et al., Reference Hoeschele, Merchant, Kikuchi, Hattori, ten Cate and Honing2018; Niarchou et al., Reference Niarchou, Sathirapongsasuti, Jacoby, Bell, McArthur, Straub and Gordon2019).Footnote 5
6. Potential criticisms
Having detailed our social bonding hypothesis and its predictions, we wish to preempt several potential criticisms.
6.1 Music, language, and domain-specificity
The key criticism that we anticipate regards the degree to which the evolution of musicality and social bonding are uniquely and causally linked. Few would deny that music can facilitate social bonding via neurobiological mechanisms that are evolutionarily adaptive. However, whether music is a domain-specific evolutionary adaptation for social bonding, as opposed to a byproduct of the evolution of other adaptations, is open to debate. Language, in particular, has been proposed as an evolutionary adaptation that led to musicality as a byproduct (Pinker, Reference Pinker1997).Footnote 6 Importantly, many researchers have noted that, although there are clear differences in the structure and processing of music and language, there is extensive overlap ranging from structural content (e.g., “musilinguistic continua” between speech and song including intermediate forms like poetry and chant) to neurobiological substrates (e.g., similar neural substrates for processing of pitch, rhythm, and syntax; Brown, Reference Brown, Wallin, Merker and Brown2000b, Reference Brown2017; Fitch, Reference Fitch2006; Patel, Reference Patel2008; Peretz & Coltheart, Reference Peretz and Coltheart2003; Peretz, Vuvan, Lagrois, & Armony, Reference Peretz, Vuvan, Lagrois, Armony and Honing2018; Savage, Merritt, Rzeszutek, & Brown, Reference Savage, Merritt, Rzeszutek and Brown2012). Indeed, many have proposed that the evolution of musicality may have paved the way for the evolution of language (Brown, Reference Brown, Wallin, Merker and Brown2000b, Reference Brown2017; Darwin, Reference Darwin1871; Fitch, Reference Fitch2010; Mithen, Reference Mithen2005; Shilton, Breski, Dor, & Jablonka, Reference Shilton, Breski, Dor and Jablonka2020).
We accept that our present level of understanding is insufficient to demonstrate conclusively that music coevolved uniquely with social bonding independent from language or other social behaviors. Accordingly, in sect. 5, we proposed future investigations of such relationships. However, the fact that music and language are both found universally in all known societies (Brown, Reference Brown1991; Mehr et al., Reference Mehr, Singh, Knox, Ketter, Pickens-Jones, Atwood and Glowacki2019) suggests that both music and language independently fulfill more fundamental adaptive functions than technologies or cultural artifacts that are not cross-culturally universal.
We make no claim that the mechanisms discussed here are entirely specific to music, or that “musicality” is modular in either the cognitive or neuroscientific senses of this term. For example, prediction and predictive coding are ubiquitous features of vertebrate brains (Clark, Reference Clark2013; Schultz & Dickinson, Reference Schultz and Dickinson2000), by no means specific to musicality. However, music affords a uniquely effective scaffolding framework, including rhythm and harmony, within which neural prediction (and occasional expectation violations) can unfold (Fitch et al., Reference Fitch, von Graevenitz, Nicolas, Skov and Vartanian2009; Hanslick, Reference Hanslick1858; Huron, Reference Huron2006; Koelsch, Vuust, & Friston, Reference Koelsch, Vuust and Friston2019). Similarly, synchrony is widespread in human sociality (including phenomena such as gaze synchrony, affect synchrony, the chameleon effect, and others), but the isochrony of musical rhythm provides an unusually effective affordance for synchronization. Furthermore, phenomena such as “groove” seem to be mainly evoked by musical stimuli, and therefore are relatively domain-specific. Thus, musicality encompasses multiple mechanisms that vary in their domain-specificity, but combines them into a uniquely effective package.
6.2 Group selection
Most previous social bonding theories of music evolution have relied on an evolutionary mechanism incorporating some form of group selection, in which genetic variants are selected for because of their effects on the reproductive success of entire groups (e.g., Brown, Reference Brown, Tonneau and Thompson2000a; Wiltermuth & Heath, Reference Wiltermuth and Heath2009). Group selection has been largely dismissed for decades (Williams, Reference Williams1966), and while it is re-emerging in the form of multi-level selection (Traulsen & Nowak, Reference Traulsen and Nowak2006; Wilson & Wilson, Reference Wilson and Wilson2007) and cultural group selection (Richerson et al., Reference Richerson, Baldini, Bell, Demps, Frost, Hillis and Zefferman2016), it remains controversial (Pinker, Reference Pinker2012; see also commentary accompanying Richerson et al., Reference Richerson, Baldini, Bell, Demps, Frost, Hillis and Zefferman2016).
The MSB hypothesis does NOT require group selection (any more than grooming, play, or laughter do): fitness advantages accrue to individuals who are able to bond more effectively with larger numbers of individuals. Although there are often advantages to well-bonded groups for various activities (e.g., group hunting or foraging, jointly repelling enemies), even for such activities the key fitness advantages accrue to individuals.
6.3 Gene–culture coevolution and causality
Some evolutionary psychologists have been critical of social bonding theories of music evolution because they consider them circular arguments that fail to explain the ultimate causal mechanism by which music could have evolved as a biological adaptation:
Perhaps singing lullabies soothes babies; perhaps dancing relieves tension; perhaps shared stories bond the community. The question is, why would anyone have predicted, a priori, that people would be constituted in such a way that these things would happen? (Pinker, Reference Pinker2007, pp. 170–171)
Several have posited an adaptive function for music in enhancing “cohesion” or “bonding”…. But this reasoning is circular: it takes as a given the fact that music performance and listening produces reliable effects… and then argues that one or more parts of the music faculty evolved in order to produce these effects. But why should music produce these effects and not others? … accounts invoking cohesion and/or bonding as an adaptive target provide neither a specific account of the ultimate functional mechanism by which music should increase cohesion, nor an account of how that cohesion would produce fitness advantages. And if cohesion is indeed fitness enhancing, why should individuals wait for music-making to produce that cohesion? Why not just be cohesive without music? (Mehr & Krasnow, Reference Mehr and Krasnow2017, p. 676)
music does not directly cause social cohesion: rather, it signals existing social cohesion that was obtained by other means (Mehr et al., target article, sect. 4.2.1, para. 14 [emphasis in original]; paraphrasing Hagen & Bryant, Reference Hagen and Bryant2003, p. 30)
Our preceding account provides a priori arguments detailing why and how specific design features of human musicality have social bonding effects, the mechanisms underlying these effects, and how and why these may have evolved. In particular, we provided specific reasons that behaviors with the design features of music would have social bonding effects: because such behaviors allow people to predict, synchronize, share goals, distinguish individual contributions, experience shared positive emotions, and make social decisions more than other human behaviors (ABMs or language). This explains why music should produce “[social bonding] effects and not others”: behaviors that allow us to align in time and frequency, coordinate behaviors in large groups while distinguishing individual contributions, share emotions and goals, and choose appropriate social partners have tangible and predictable social bonding effects. Music is a particularly effective cognitive “technology” (Patel, Reference Patel2008, Reference Patel and Honing2018) that fulfills these design criteria, making musicality an effective toolkit for social bonding functions, shaped by both biological and cultural evolution.
Our hypothesis differs from most traditional social bonding theories because we do not argue that musicality necessarily originated as a biological adaptation. Instead, components of musicality may have arisen initially as cultural inventions and/or byproducts of other adaptations, later exapted and modified through gene–culture coevolution for their social bonding functions in a musical context (e.g., beat synchronization initially as a byproduct of the evolution of vocal learning, as argued by Patel, Iversen, Bregman, & Schulz [Reference Patel, Iversen, Bregman and Schulz2009] and Schachner et al. [Reference Schachner, Brady, Pepperberg and Hauser2009]; although cf. Merker et al. [Reference Merker, Morley, Zuidema and Honing2018] for an alternative interpretation). The initial social cohesion functions may not have begun as genetic adaptations. In this sense, we largely agree with Mehr et al., who write:
We also agree with the proponents of the social bonding hypothesis that musical abilities evolved because musical performances played an important role in cooperative sociality. But given the issues described above, we find it more likely that music evolved to credibly signal decisions to cooperate that were already reached by other means, not to determine them. (Mehr et al., target article, sect. 4.2, para. 2)
But in a social environment in which social bonding already enhanced individual reproductive fitness, the subsequent cultural evolution of musical behaviors would lead to biological selection on musicality (e.g., to promote motivation to engage in/attend to musical behaviors), because of the adaptive consequences of musicality for social bonding. In this way, just as social bonding is crucial in most primates, generating selection on the mechanisms that achieve it, social bonding functions of “proto-musical” mechanisms may have played important roles in hominin evolution long before today's full-blown musicality evolved.
We emphasize that past adaptive function, although important, should not be the sole criterion by which to judge theories of the evolution of musicality. As previously argued at length (e.g., Fitch, Reference Fitch2006, Reference Fitch, Toivonen, Csúri and van der Zee2015b; Honing et al., Reference Honing, Cate, Peretz and Trehub2015), Tinbergen's (Reference Tinbergen1963) multi-factorial perspective, which seeks understanding of traits at the four interlinked explanatory levels of mechanism, ontogeny, phylogeny, and adaptive function, is a fruitful method for understanding the evolution of musicality. We may never know with certainty the precise ancestral adaptive conditions or specific genetic mutations involved in the evolution of musicality. Even so, the comparative method provides a key tool for empirically testing evolutionary hypotheses (Fitch, Reference Fitch, Toivonen, Csúri and van der Zee2015b). Section 5 lists a variety of testable empirical predictions of the MSB hypothesis.
6.4. Parochial altruism and out-group exclusion
Enhanced social bonding between some individuals inevitably means a relative decrease between others. In-group social bonding has a dark side of increasing hostility toward out-groups (Gelfand, Caluori, Jackson, & Taylor, Reference Gelfand, Caluori, Jackson and Taylor2020; Whitehouse, Reference Whitehouse2018), as exemplified in the use of music in warfare by the Nazis and other groups throughout history (Brown & Volgsten, Reference Brown and Volgsten2006). The traditional Māori haka “Ka Mate” is famously used by New Zealand's national rugby team to simultaneously bind team-mates together and intimidate the opposing team through coordinated dancing and vocalization (Jackson & Hokowhitu, Reference Jackson and Hokowhitu2002). The ability of music to exclude out-group members might appear to be an argument against its function in bonding in-group members, but out-group exclusion is entirely consistent with the social bonding hypothesis. Because the creation or strengthening of a social bond between some (participating) individuals by definition excludes others, the observation that particular forms of music can cause emotional dissonance or fear in others is compatible with a social bonding function.
Earlier expositions of the social bonding hypothesis (Brown, Reference Brown, Tonneau and Thompson2000a; Freeman, Reference Freeman, Wallin, Merker and Brown2000) noted that “bonding is always exclusionary” and “individuals who do not ‘belong’ become enemies … The process is similar to sexual jealousy, which manifests the exclusionary nature of the pair bond” (Freeman, Reference Freeman, Wallin, Merker and Brown2000, pp. 421–422). This observation is mirrored in the recent literature on oxytocin which, far from being an indiscriminate “love drug,” simultaneously exerts affiliative effects among in-group members and exclusionary effects toward out-group individuals (cf. Beery, Reference Beery2015; Shamay-Tsoory & Abu-Akel, Reference Shamay-Tsoory and Abu-Akel2016). The use of music to exclude others is no argument against its social bonding origins.
6.5. Solo music, sexual selection, and individual signaling
Although coordinated group performances predominate throughout the world, various widespread musical genres are not necessarily performed in coordinated groups. In particular, lullabies and love songs are found throughout the world and are often performed by a lone singer (Mehr & Singh et al., Reference Mehr, Singh, York, Glowacki and Krasnow2018; Trehub et al., Reference Trehub, Unyk and Trainor1993). This is perfectly consistent with the MSB hypothesis, as lullabies and love songs are often dyadic: sung by a soloist to bond with another person (by soothing an infant or wooing a potential mate).
More generally, some may wonder why, if social bonding is so important to the evolution of musicality, do people enjoy playing or listening to music alone? We emphasize that even solo music listening can support social bonding goals (Trehub et al., Reference Trehub, Becker, Morley and Honing2018). A young adult meeting a new person in an online chat discusses music preferences more often than other topics, and based on music preferences alone, people draw social inferences about others (Rentfrow & Gosling, Reference Rentfrow and Gosling2006). Thus, music preferences developed during solo listening can be used as social cues, displayed and evaluated when establishing new social bonds.
Solo listening may serve other, non-social functions (e.g., mood regulation, staying awake while driving; DeNora, Reference DeNora2000; North, Hargreaves, & Hargreaves, Reference North, Hargreaves and Hargreaves2004; Sloboda, O'Neill, & Ivaldi, Reference Sloboda, O'Neill and Ivaldi2001). We do not argue that social bonding is the only possible function of music. By analogy, language's primary function may be to communicate information between people, but it is also useful in private thought, or to allow one to preserve thoughts for the future (particularly after the invention of writing). Similarly, the same auditory–motor–reward connections that make music so socially powerful also allow people to enjoy playing or listening to music alone. Often, solo music was experienced previously in a social context, which is re-evoked by solo listening/playing.
Related to the idea of virtuosic solo music-making is the distinction between social bonding and theories such as sexual selection or honest signaling that emphasize music as a signal of individual fitness. The MSB hypothesis does not reject such theories. Instead, it emphasizes that individual signaling theories are insufficient to explain all of the broader social functions of music, whereas social bonding provides more explanatory power (although we concede that the MSB hypothesis cannot explain all possible functions of music; Oesch, Reference Oesch2019). For example, in contemporary Western night clubs and traditional non-Western societies, all-night music and dance rituals function both to bond participants and as opportunities to find potential mates (Merriam, Reference Merriam1964; Thornton, Reference Thornton1995). In such contexts, dancing, singing, and/or playing instruments can function to bond with same and opposite-sex partners and to advertise evolutionary fitness to potential mates. Bonding and signaling hypotheses are not mutually exclusive, but rather complementary.
The complementarity of the MSB and alternative hypotheses makes it challenging to falsify the MSB hypothesis. However, we have provided a number of specific predictions, each of which is potentially falsifiable and would count as evidence against the MSB hypothesis, particularly if alternative hypotheses better predict the data. For example, our hypothesis and Hagen and Bryant's (Reference Hagen and Bryant2003) coalitional signaling hypothesis make predictions regarding synchrony: we argue that synchrony should enhance social bonding, whereas Hagen and Bryant argue that synchrony should enhance perceived coalitional quality. To differentiate between these and other competing hypotheses, our predictions regarding the effects of synchrony (or other aspects of musicality) on social bonding could be compared directly against perceived coalition quality or other competing predictions (e.g., attractiveness; Miller, Reference Miller, Wallin, Merker and Brown2000, parental investment; Mehr & Krasnow, Reference Mehr and Krasnow2017; Mehr et al., target article) in future research. If synchrony increases perceived bonding relative to perceived coalition quality, attractiveness, or parental investment, it would constitute evidence favoring the MSB hypothesis over competing alternatives. Another example of predictions that differentiate among alternative hypotheses is the MSB prediction that social bonding functions will be common cross-culturally but the relative frequencies of specific genres and sub-functions (e.g., lullabies vs. love songs vs. group dancing) will vary across societies. In contrast, theories that focus on infant-directed song or sexual selection predict instead that these categories should be more common and consistent cross-culturally than the other categories of social bonding. Furthermore, phylogenetic or other cross-species analyses (e.g., Hoeschele et al., Reference Hoeschele, Merchant, Kikuchi, Hattori, ten Cate and Honing2018; Schruth, Templeton, & Holman, Reference Schruth, Templeton and HolmanIn press; Shultz, Opie, & Atkinson, Reference Shultz, Opie and Atkinson2011) could allow us to quantify the relative effects of group size, sexual competition, parental investment strategies, or other factors on the evolution of vocal learning, beat perception, or other aspects of musicality. We encourage tests of MSB predictions against those of competing hypotheses.
7. Conclusion
Social bonding has long been acknowledged as an important function of contemporary music, but its causal role in the evolution of music has often been dismissed as a naïve application of group selection theory. Recent advances in gene–culture coevolution theory allow us to provide a more nuanced model of music evolution that does not rely on group selection. Our argument has focused on social bonding as the primary factor shaping the evolution of human musicality. This MSB hypothesis provides a framework for understanding the past evolution of musicality, and a starting point for the future cultural evolution of new forms of music that harness the social power of music to bring people together. Music may not be a “universal language” (Longfellow, Reference Longfellow1835; Savage, Reference Savage and Sturman2019b), but music's universal power to bring people together across barriers of language, age, gender, and culture sheds light on its biological and cultural origins, and provides humanity with a set of tools to create a more harmonious future – both literally and figuratively.
Acknowledgments
This paper originated in an invited symposium entitled “The origins of music in human society” held in December 2017 at the Abbaye de Royaumont, France, organized by Paul Seabright, Francis Maréchal, Marie-Hélène Cassar, and Juliette Lobry. We gratefully acknowledge funding for the workshop from the French Agence Nationale de la Recherche (under the Investissement d'Avenir program, ANR-17-EURE-0010). We thank the organizers, sponsors, and other invited participants (in addition to the seven of us, these were: Simha Arom, Kofi Asante, Jean-Julien Aucouturier, Greg Bryant, François Cam, Didier Demolin, Edward Hagen, Nori Jacoby, Martin Lang, Jacques Launay, Samuel Mehr, Marcel Peres, Benjamin Purzycki, Lauriane Rat-Fischer, Nick Rawlins, Sandra Trehub, Connor Wood, and the Ensemble Organum) for this stimulating interdisciplinary workshop and discussion leading to this article and a separate article by Mehr, Bryant, Hagen, and Max Krasnow. We thank Greg Bryant, Shinya Fujii, Eva Jablonka, Nori Jacoby, Kimberly Kobayashi-Johnson, Samuel Mehr, Bjorn Merker, Aniruddh Patel, Peter Pfordresher, Manvir Singh, Dor Shilton, Parker Tichko, Sandra Trehub, the students of Keio University's CompMusic and NeuroMusic Labs, and of Oxford University's Institute of Cognitive and Evolutionary Anthropology, and four anonymous reviewers for comments on earlier versions of this manuscript.
Author contributions
PES proposed the article concept and author list with feedback from WTF and LG. PES, WTF, and PL drafted the abstract and outline, with feedback from LG, AS, and BT. WTF, PES, and PL drafted sects. 1 and 2; PL and BT drafted sect. 4; PES, WTF, and PL drafted sects. 5–7. All authors drafted sub-sects. relevant to their own expertise, which were then edited and synthesized into sects. 3 and 4 by PES, PL, BT, WTF, and AS. All authors edited and approved the final manuscript.
Financial support
PES was supported by Grant-in-Aid no. 19KK0064 from the Japan Society for the Promotion of Science and startup grants from Keio University (Keio Global Research Institute, Keio Research Institute at SFC, and Keio Gijuku Academic Development Fund). PL was supported by the National Science Foundation NSF-STTR no. 1720698 NSF-CAREER #1945436, NSF-STTR #2014870, the Grammy Foundation and startup funds from Northeastern University. BT was supported by funding from the French Agence Nationale de la Recherche (under the Investissement d'Avenir program, ANR-17-EURE-0010) while on a Visiting Fellowship at the Institute of Advanced Study Toulouse. AS was supported by the National Science Foundation under NSF-BCS no. 1749551. WTF was supported by Austrian Science Fund (FWF) DK Grant “Cognition & Communication” (W1262-B29).
Conflict of interest
None.
Treatment Index
TARGET ARTICLES
Savage, Patrick E., Psyche Loui, Bronwyn Tarr, Adena Schachner, Luke Glowacki, Steven Mithen, and W. Tecumseh Fitch, Music as a coevolved system for social bonding, 1
Mehr, Samuel A., Max M. Krasnow, Gregory A. Bryant, and Edward H. Hagen, Origins of music in credible signaling, 23
COMMENTARIES
Akkermann, Miriam, Ugur Can Akkaya, Cagatay Demirel, Dirk Pflüger, and Martin Dresler, Sound sleep: Lullabies as a test case for the neurobiological effects of music, 95
Ashley, Richard D., Music, groove, and play, 39
Atzil, Shir, and Lior Abramson, Musicality was not selected for, rather humans have a good reason to learn music, 41
Belfi, Amy M., Social bonding and music: Evidence from lesions to the ventromedial prefrontal cortex, 43
Benítez-Burraco, Antonio, Evolutionary linguistics can help refine (and test) hypotheses about how music might have evolved, 44
Bowling, D. L., M. Hoeschele, and J. C. Dunn, Progress without exclusion in the search for an evolutionary basis of music, 96
Brown, Steven, Music and dance are two parallel routes for creating social cohesion, 46
Cross, Ian, Music, attachment, and uncertainty: Music as communicative interaction, 48
Del Mastro, Maria Chiara, Maria Rosaria Strollo, and Mohamad El Haj, Music as a social bond in patients with amnesia, 49
Dissanayake, Ellen, Ancestral human mother–infant interaction was an adaptation that gave rise to music and dance, 51
Dubourg, Edgar, Jean-Baptiste André, and Nicolas Baumard, The evolution of music: One trait, many ultimate-level explanations, 98
Eirdosh, Dustin, and Susan Hanisch, The music and social bonding hypothesis does require multilevel selection, 52
Fritz, Jonathan B., Is the MSB hypothesis (music as a coevolved system for social bonding) testable in the Popperian sense?, 53
Gabriel, Shira, and Elaine Paravati, If music be the food of love, play on: Four ways that music may lead to social connection, 55
Gardiner, Martin F., Human evolution of gestural messaging and its critical role in the human development of music, 99
Gingras, Bruno, Music's putative adaptive function hinges on a combination of distinct mechanisms, 57
Grahn, Jessica A., Anna-Katharina R. Bauer, and Anna Zamm, Is neural entrainment to rhythms the basis of social bonding through music?, 58
Hannon, Erin E., Alyssa N. Crittenden, Joel S. Snyder, and Karli M. Nave, An evolutionary theory of music needs to care about developmental timing, 60
Hansen, Niels Chr. and Peter E. Keller, Oxytocin as an allostatic agent in the social bonding effects of music, 61
Harrison, Peter M. C. and Madeleine Seale, Against unitary theories of music evolution, 64
Hattori, Yuko, Bonding system in nonhuman primates and biological roots of musicality, 65
Hilton, Courtney B., Rie Asano, and Cedric Boeckx, Why musical hierarchies?, 101
Honing, Henkjan, Unravelling the origins of musicality: Beyond music as an epiphenomenon of language, 66
Iyer, Vijay, What's not music, but feels like music to you?, 68
Juslin, Patrik N., Mind the gap: The mediating role of emotion mechanisms in social bonding through musical activities, 69
Kasdan, Anna, Reyna L. Gordon, and Miriam D. Lense, A neurodevelopmental disorders perspective into music, social attention, and social bonding, 103
Kennedy, Patrick, and Andrew N. Radford, Credible signalling and social bonds: Ultimately drawing on the same idea, 105
Killin, Anton, Carl Brusse, Adrian Currie, and Ronald J. Planer, Not by signalling alone: Music's mosaicism undermines the search for a proper function, 107
Kraus, Nils, and Guido Hesselmann, Musicality as a predictive process, 71
Leivada, Evelina, The origins of music in (musi) language, 108
Levitin, Daniel J., Knowledge songs as an evolutionary adaptation to facilitate information transmission through music, 109
Lieberman, Debra, and Joseph Billingsley, If it quacks like a duck: The by-product account of music still stands, 111
Lumaca, Massimo, Elvira Brattico, and Giosuè Baggio, Signaling games and music as credible signal, 112
Margulis, Elizabeth Hellmuth, Pluralism provides the best chance for addressing big questions about music, 73
Merker, Bjorn, Music, bonding, and human evolution: A critique, 74
Morrison, Steven J., Bonds and signals underlie the music learning experience, 76
Moser, Cody, Jordan Ackerman, Alex Dayer, Shannon Proksch, and Paul E. Smaldino, Why don't cockatoos have war songs?, 113
Patel, Aniruddh D., Chris von Rueden, Where they sing solo: Accounting for cross-cultural variation in collective music-making in theories of music evolution, 77
Pfordresher, Peter Q., Music production deficits and social bonding: The case of poor-pitch singing, 79
Pinker, Steven, Sex and drugs and rock and roll, 115
Popescu, Tudor, Nathan Oesch, and Bryony Buck, Musical features emerging from a biocultural musicality, 80
Ravignani, Andrea, Isochrony, vocal learning, and the acquisition of rhythm and melody, 82
Rendell, Luke, Emily L. Doolittle, Ellen C. Garland, and Alex South, A boldly comparative approach will strengthen co-evolutionary accounts of musicality's origins, 84
Sachs, Matthew E., Oriel FeldmanHall, and Diana I. Tamir, Clarifying the link between music and social bonding by measuring prosociality in context, 86
Scott-Phillips, Thom, Atsuko Tominaga, and Helena Miton, Ecological and psychological factors in the cultural evolution of music, 116
Sievers, Beau R. and Thalia Wheatley, Rapid dissonant grunting, or, but why does music sound the way it does?, 117
Snyder, Kate T., and Nicole Creanza, Functional and evolutionary parallels between birdsong and human musicality, 119
Stewart-Williams, Steve, Making music: Let's not be too quick to abandon the byproduct hypothesis, 120
Számadó, Szabolcs, Pre-hunt charade as the cradle of human musicality, 121
Tichko, Parker, Kevin A. Bird, and Gregory Kohn, Beyond “consistent with” adaptation: Is there a robust test for music adaptation?, 123
Trainor, Laurel J., Understanding the origins of musicality requires reconstructing the interactive dance between music-specific adaptations, exaptations, and cultural creations, 124
Trehub, Sandra E., Challenging infant-directed singing as a credible signal of maternal attention, 126
Trevor, Caitlyn, and Sascha Frühholz, The evolutionary benefit of less-credible affective musical signals for emotion induction during storytelling, 127
van Mulukom, Valerie, The evolution of music as artistic cultural innovation expressing intuitive thought symbolically, 87
Verpooten, Jan, and Marcel Eens, Singing is not associated with social complexity across species, 89
Wald-Fuhrmann, Melanie, Lara Pearson, Tina Roeske, Christian Grüny and Rainer Polak, Music as a trait in evolutionary theory: A musicological perspective, 90
Wood, Connor, Musical bonds are orthogonal to symbolic language and norms, 129
Zentner, Marcel, Social bonding and credible signaling hypotheses largely disregard the gap between animal vocalizations and human music, 130
Zhang, Qing, and Edward Ruoyang Shi, Why language survives as the dominant communication tool: A neurocognitive perspective, 92
Zou, Ivan Yifan, and William S.-Y. Wang, Music as social bonding: A cross-cultural perspective, 93
AUTHORS' RESPONSES
Savage, Patrick E., Psyche Loui, Bronwyn Tarr, Adena Schachner, Luke Glowacki, Steven Mithen, and W. Tecumseh Fitch, Toward inclusive theories of the evolution of musicality, 132
Mehr, Samuel A., Max M. Krasnow, Gregory A. Bryant, and Edward H. Hagen, Toward a productive evolutionary understanding of music, 140



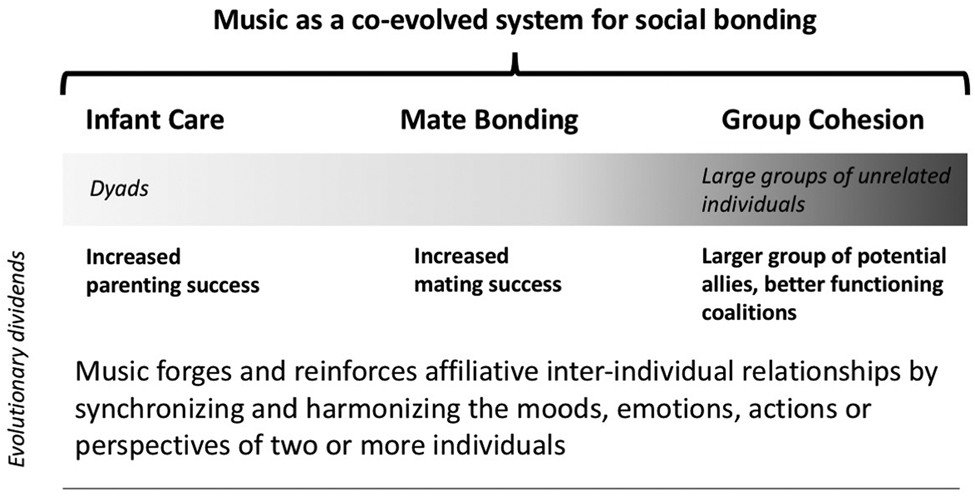




Target article
Music as a coevolved system for social bonding
Related commentaries (24)
A boldly comparative approach will strengthen co-evolutionary accounts of musicality's origins
A neurodevelopmental disorders perspective into music, social attention, and social bonding
Beyond “consistent with” adaptation: Is there a robust test for music adaptation?
Clarifying the link between music and social bonding by measuring prosociality in context
Ecological and psychological factors in the cultural evolution of music
Evolutionary linguistics can help refine (and test) hypotheses about how music might have evolved
Human evolution of gestural messaging and its critical role in the human development of music
If it quacks like a duck: The by-product account of music still stands
Is neural entrainment to rhythms the basis of social bonding through music?
Is the MSB hypothesis (music as a coevolved system for social bonding) testable in the Popperian sense?
Isochrony, vocal learning, and the acquisition of rhythm and melody
Music and dance are two parallel routes for creating social cohesion
Music as a social bond in patients with amnesia
Music as a trait in evolutionary theory: A musicological perspective
Not by signalling alone: Music's mosaicism undermines the search for a proper function
Oxytocin as an allostatic agent in the social bonding effects of music
Pre-hunt charade as the cradle of human musicality
Progress without exclusion in the search for an evolutionary basis of music
Rapid dissonant grunting, or, but why does music sound the way it does?
Sex and drugs and rock and roll
Social bonding and music: Evidence from lesions to the ventromedial prefrontal cortex
The evolution of music as artistic cultural innovation expressing intuitive thought symbolically
Where they sing solo: Accounting for cross-cultural variation in collective music-making in theories of music evolution
Why don't cockatoos have war songs?
Author response
Toward a productive evolutionary understanding of music
Toward inclusive theories of the evolution of musicality