INTRODUCTION
Aesthetic values of wildlife and coastal seascape, high biodiversity and increased environmental awareness can contribute to the promotion of tourism activities along the coast (Badalamenti et al. Reference Badalamenti, Ramos, Voultsiadou, Sanchez-Lisazo, D'Anna, Pipitone, Mas, Ruiz Fernandez, Whitmarsh and Riggio2000; Milazzo et al. Reference Milazzo, Chemello, Badalamenti, Camarda and Riggio2002; Cater & Cater Reference Cater and Cater2007). This is most evident within marine protected areas (MPAs) where ecotourism is an important component of the socioeconomy of the area (Sorice et al. Reference Sorice, Oh and Ditton2007; Parsons & Thur Reference Parsons and Thur2008). An increasing number of MPAs have been designated around the world (Ballantine Reference Ballantine, Shackell and Martin Willison1995; Agardy Reference Agardy, Bridgewater, Crosby, Day, Dayton, Kenchington, Laffolley, McConney, Murray, Parks and Peau2003); this has contributed to the exponential increase of marine tourism (Hawkins et al. Reference Hawkins, Roberts, Kooistra, Buchan and White2005; Cater & Cater Reference Cater and Cater2007).
Technical advances in equipment, in addition to a rising interest in nature, conservation and environmental matters have resulted in the increased popularity of marine recreation, particularly scuba diving (Zakai & Chadwick-Furman Reference Zakai and Chadwick-Furman2002; Barker & Roberts Reference Barker and Roberts2004). Diving tourism is one of the major forms of commercial use of MPAs throughout the world (Rouphael & Inglis Reference Rouphael and Inglis2001; Parsons & Thur Reference Parsons and Thur2008). MPAs provide goods and services (i.e. attractive underwater flora and fauna, reef structures and seascapes) that attract tourists and often represent the reason for which scuba divers choose to visit an area (Hawkins et al. Reference Hawkins, Roberts, Kooistra, Buchan and White2005; Dearden et al. Reference Dearden, Bennett and Rollins2006).
Owing to increasing recreational use of coastal areas, there is a general consensus about the potential impacts of these activities on the marine environment (Dearden et al. Reference Dearden, Bennett and Rollins2007; Uyarra & Côtè Reference Uyarra and Côté2007) and many papers have dealt with the impact of diving activity within MPAs (Hawkins & Roberts Reference Hawkins, Roberts, Lessios and Macintyre1997; Rouphael & Inglis Reference Rouphael and Inglis1997, Reference Rouphael and Inglis2001; Garrabou et al. Reference Garrabou, Sala, Arcas and Zabala1998; Hawkins et al. Reference Hawkins, Roberts, Kooistra, Buchan and White2005). Local biological effects can be dramatic (Rouphael & Inglis Reference Rouphael and Inglis2001; Walters & Samways Reference Walters and Samways2001; Rudd & Tupper Reference Rudd and Tupper2002; Zakai & Chadwick-Furman Reference Zakai and Chadwick-Furman2002; Barker & Roberts Reference Barker and Roberts2004; Coma et al. Reference Coma, Pola, Ribes and Zabala2004); scuba divers may affect organisms in several ways, both intentionally and unintentionally (Milazzo et al. Reference Milazzo, Chemello, Badalamenti, Camarda and Riggio2002; Uyarra & Côtè Reference Uyarra and Côté2007). Damage is mainly due to physical contact with organisms (i.e. by divers' fins, body and scuba gear), air bubbles becoming trapped within marine caves, increased sediment resuspension potentially affecting sessile organisms (see Milazzo et al. Reference Milazzo, Chemello, Badalamenti, Camarda and Riggio2002 for a review) and human presence in the water leading to changes in animal behaviour (Kulbicki Reference Kulbicki1998; Milazzo et al. Reference Milazzo, Anastasi and Willis2006).
Until now, research has mainly focused on: (1) impact assessment comparing frequented with less-frequented sites, usually with the aim of assessing the carrying capacity of a given area (Prior et al. Reference Prior, Ormond, Hitchen and Wormald1995; Hawkins & Roberts Reference Hawkins, Roberts, Lessios and Macintyre1997; Hawkins et al. Reference Hawkins, Roberts, Van't Hof, De Meyer, Tratalos and Aldam1999; Zakai & Chadwick-Furman Reference Zakai and Chadwick-Furman2002), and (2) direct observations of scuba diver behaviour, generally resulting in management recommendations of best practices designed to reduce impacts (Medio et al. Reference Medio, Ormond and Pearson1997; Barker & Roberts Reference Barker and Roberts2004).
Most studies have focused on tropical systems, mainly coral reefs (Plathong et al. Reference Plathong, Inglis and Huber2000; Rouphael & Inglis Reference Rouphael and Inglis2001; Zakai & Chadwick-Furman Reference Zakai and Chadwick-Furman2002; Barker & Roberts Reference Barker and Roberts2004), while less is known about the effects of recreational diving activities on temperate subtidal habitats (Walters & Samways Reference Walters and Samways2001), particularly in the Mediterranean (Sala et al. Reference Sala, Garrabou and Zabala1996; Garrabou et al. Reference Garrabou, Sala, Arcas and Zabala1998; Coma et al. Reference Coma, Pola, Ribes and Zabala2004).
Mediterranean subtidal habitats are characterized by the presence of different communities, mainly related to variation in light exposure (Peres & Picard Reference Peres and Picard1964). Upper subtidal assemblages are dominated by photophilic algae (such as Cystoseira spp., Dyctiotales, Sargassum spp.) comprising a canopy that shelters many benthic encrusting species. Deeper assemblages are composed mainly of encrusting flora and fauna, with the former (for example Peyssonnelia spp., Stypocaulon spp., Anadyomene stellata) constituting an understorey from which slow-growing species, mainly gorgonians (for example Eunicella spp., Paramuricea clavata) and bryozoans (for example Pentapora fascialis) emerge, constituting the main feature of Mediterranean coralligenous communities (Ballesteros Reference Ballesteros2006).
Coralligenous and pre-coralligenous communities are characterized by the presence of calcareous benthic organisms in shaded environments, and thus are highly vulnerable to the impacts of scuba diving (Sala et al. Reference Sala, Garrabou and Zabala1996; Lloret et al. Reference Lloret, Marin, Marin-Guirao and Carreño2006) because of the presence of species with high fragility and low growth rate (Ballesteros Reference Ballesteros2006). Despite the relevance of recreational activities in the Mediterranean Sea (see Zabala Reference Zabala and Briand1999), there is lack of knowledge regarding the effects of diving disturbance on subtidal low dynamic systems like coralligenous and pre-coralligenous communities (Garrabou Reference Garrabou1999; Garrabou & Harmelin Reference Garrabou and Harmelin2002), with few studies aimed at specifically assessing scuba diving effects; most focus on vulnerable coralligenous communities (Sala et al. Reference Sala, Garrabou and Zabala1996; Garrabou et al. Reference Garrabou, Sala, Arcas and Zabala1998; Coma et al. Reference Coma, Pola, Ribes and Zabala2004), with less information presently available on other subtidal habitats (but see Lloret et al. Reference Lloret, Marin, Marin-Guirao and Carreño2006).
The present study aimed to investigate the behaviour of divers and their potential immediate effects on benthic organisms in different Mediterranean subtidal habitats within a MPA. Our specific objectives were to (1) define scuba diver behaviour in terms of habitat selection (time spent by divers in each habitat), (2) quantify scuba diver habitat use (number of contacts made by divers with the environment) and (3) assess the immediate damage caused to sessile benthic organisms. Time spent by each group of divers in each habitat was used as a proxy for habitat selection. This variable may be affected by both the actual availability of the habitat along the scuba trail and the presence of particular biological attributes. We controlled for both sources of variation by quantifying the percentage coverage of each habitat in the field and assessing divers' preferences for specific biological attributes (such as the number and the diversity of the species associated with a given habitat).
METHODS
Study area
The Capo Gallo-Isola delle Femmine MPA covers an area of 2173 ha. It was established in 2002 and is devoted to biodiversity conservation, educational activities and research. The MPA is divided into three zones with different levels of protection: zone A (fully protected), and zones B and C (partially protected) (Fig. 1). The two small areas of zone A are no-take and no-entry areas where only scientific research is allowed. In all zones uncontrolled boat anchoring is denied, and scuba diving is permitted only in zones B and C in the company of certified diving centres or if authorized by the MPA management body.
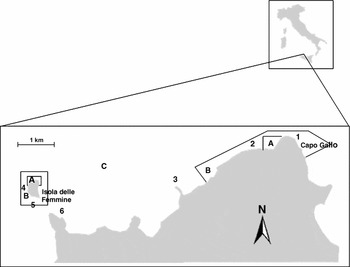
Figure 1 Capo Gallo-Isola delle Femmine MPA with zonation. Letters indicate different zones (see text for details). Numbers indicate dive trails: 1 = Capo Gallo, 2 = Cunicoli-Olio, 3 = Punta Barcarello, 4 = Lavaria Nord, 5 = Lavaria Sud and 6 = Punta Matese.
Data collection
Data on scuba diver behaviour and its effects on the biota were recorded during 14 experimental dives in each period July–October 2005 and June–September 2006, both periods encompassing the high tourist season. During the sampling, six different dive trails were followed by divers, namely Capo Gallo, Punta Matese, Cunicoli-Olio, Lavaria Nord, Lavaria Sud and Punta Barcarello (Fig. 1).
We accompanied guests diving with a dive company based within the Capo Gallo-Isola delle Femmine MPA. Dive staff were asked to treat the observer as any other guest so that the researcher remained anonymous, to prevent any change in the behaviour of the divers due to the observer's presence (Medio et al. Reference Medio, Ormond and Pearson1997; Rouphael & Inglis Reference Rouphael and Inglis1997, Reference Rouphael and Inglis2001; Barker & Roberts Reference Barker and Roberts2004).
Observations started from the time divers entered the water and ended at the time they began their ascent to the surface. Visitors that enquired about the observer's note-taking underwater were told that information was being collected on the fauna and flora of the MPA. During each dive we followed a maximum of five divers (minimum two) to ensure correct observation of each diver's behaviour. Divers were followed by an observer from a distance of 3–5 m.
Diver duration in each habitat (in minutes, subsequently transformed into a percentage) and number of contacts (both intentional and unintentional) (Medio et al. Reference Medio, Ormond and Pearson1997; Rouphael & Inglis Reference Rouphael and Inglis2001) were quantified. Contacts were defined as intentional when a diver made voluntary contact with the substrate or with living organisms; they were defined as unintentional in the case of involuntary contact; total contacts were the sum of intentional and unintentional contacts. Contact rates were standardized using seven minute observation periods (Medio et al. Reference Medio, Ormond and Pearson1997). Data on intentional, unintentional and total contacts rates were independently collected, not recording more than one type of contact rate for the same diver during a single dive.
Seven different benthic infralittoral habitats were considered, namely algae on horizontal substratum, algae on vertical substratum, Posidonia oceanica, encrusted walls, submerged caves, sandy bottoms and pebbles. The actual availability of each habitat for the divers in each dive trail was assessed by a second observer, considering the whole trail as a transect and quantifying the percentage coverage of each habitat.
Damage was assessed by evaluating the direct effect of each contact. When contact with a living organism occurred, it was noted whether the organism was damaged upon contact (Walters & Samways Reference Walters and Samways2001), defined as injury (i.e. total or partial breaking, abrasion, detaching or crushing) to the organism (Rouphael & Inglis Reference Rouphael and Inglis1997). The taxa were identified to species level.
Each diver was classified by his/her diver certification: Open Water diver, Advanced Open Water diver and Higher levels.
Diver preference survey
We surveyed the preference of divers for dive attributes by means of interviews conducted with 69 divers at Capo Gallo-Isola delle Femmine MPA. Random divers were interviewed at two diving centres operating inside the MPA about the features preferred during their dive. Answers concerning three attributes, namely fish abundance and diversity, presence of unusual benthic species and reef structure, were rated on a scale from 1 (most preferred) to 5 (not important).
Data analyses
Univariate analyses
Analyses of variance were used to test for differences in contact rates. We used three different two-way ANOVA models. The first model evaluated differences in the intentional, unintentional and total contact rates between different diver certification levels during the two years of experimental observations. Certification (CE) was treated as a fixed factor with three levels: Open Water Diver, Advanced Open Water Diver and Higher Levels; sampling year (YE) was treated as a fixed and orthogonal factor with two levels: first year (1) and second year (2). For each condition seven replicates were considered, defining as a replicate the rate of contact of a single diver during a dive.
A second ANOVA model evaluated differences in intentional, unintentional and total contact rates among the three different certifications and in four of the habitats most commonly used by the divers. This was necessary in order to balance the design, as not all the habitats considered were available at accessible depths for Open Water divers. Certification (CE) was treated as a fixed factor with three levels: Open Water Diver, Advanced Open Water Diver and Higher Levels; habitat (H) was treated as a fixed and orthogonal factor with four levels: caves (CA), encrusted walls (EW), algae growing on horizontal substrate and algae on vertical substrate (VA). For each condition four replicates were considered.
A third ANOVA model evaluated differences in unintentional contact rates between two of the three different diver levels during three intervals of each dive time. Certification (CE) was treated as a fixed factor with two levels: Open Water Diver and Advanced Diver; time interval (TI) was treated as a fixed and orthogonal factor with three levels: from 1st to 8th minute of dive time (1°), 9th to 16th minute of dive time (2°), and 17th to 24th minute of dive time (3°). For each condition five replicates were considered. A replicate is defined here as the rate of unintentional contact during eight minutes of the dive time of a single diver.
Cochran's test was used to check for homogeneity of variance (Winer Reference Winer1971), with data transformed when necessary. When appropriate, Student–Newman–Keuls' (SNK) tests were employed to separate means (p = 0.05). GMAV 5.0 software was used to perform statistics.
Strauss index
The Strauss index (S; Unger & Lewis Reference Unger and Lewis1983) was used to describe habitat selection by divers using the formula:
where S ranges from 1 to −1, Tr is the percentage amount of time spent by divers on each habitat and Ar is the percentage availability of habitat in each dive trail. S was calculated for each habitat of each dive, and was then expressed as a mean value for each habitat. An S value of +1 indicates active selection for a given habitat, −1 indicates habitat avoidance and 0 indicates diver selection of a habitat in the same proportion as it is available along a scuba trail.
Diver interviews
To assess divers' preferences we used the average rating given to each of the attributes considered. Given that the data were not normally distributed, the non-parametric Mann-Whitney test was used to identify significant differences in median rating between attributes (2-tailed, p < 0.025) (see also Williams & Polunin Reference Williams and Polunin2000).
RESULTS
Observational dives lasted on average 31.6 minutes (± 1.3 SE, n = 28). The habitats most frequented by the 105 observed divers were algae on horizontal substrate (c. 72% of dive time), sand (c. 9%), algae on vertical substrate (c. 7%) and encrusted walls (c. 7%) (Fig. 2), these habitats together accounting for an average of 95% of the dive trails' surface area (Table 1). S was positive for algae on vertical substrate, caves, encrusted walls, Posidonia oceanica and sand, and negative for algae on horizontal substrate and pebbles. However, the S values were close to zero for each habitat, hence there was no active habitat selection/avoidance by the divers (Fig. 3).

Figure 2 Mean (± SE) diver duration (%) in the seven subtidal habitats considered at Capo Gallo-Isola delle Femmine MPA: HA = algae on horizontal substrate, VA = algae on vertical substrate, PO = Posidonia oceanica, EW = encrusted walls, CA = caves, SA = sand and PE = pebbles. n = the number of experimental dives within each habitat.
Table 1 Habitat coverage (%) along each scuba trail, number of experimental dives (n) in each trail, average habitat coverage for the 28 experimental dives and presence of vulnerable species (see Lloret et al. Reference Lloret, Marin, Marin-Guirao and Carreño2006; Di Franco et al. Reference Di Franco, Marchini, Baiata, Milazzo and Chemello2009) in each of the seven subtidal habitats along the dive trails. ES = Eunicella singularis; AC = Astroides calycularis; PC = Paramuricea clavata. HA = algae on horizontal substrate, VA = algae on vertical substrate, PO = Posidonia oceanica, EW = encrusted walls, CA = caves, SA = sand and PE = pebbles.


Figure 3 Mean Strauss Index (± SE) for each habitat at Capo Gallo-Isola delle Femmine MPA. HA = algae on horizontal substrate, VA = algae on vertical substrate, PO = Posidonia oceanica, EW = encrusted walls, CA = caves, SA = sand and PE = pebbles. n = the number of experimental dives within each habitat.
According to the diver preference surveys, the most important attributes were reef structure (mean 2.59), fish abundance and diversity (mean 2.65) and the presence of unusual benthic species (mean 2.97). None of these attributes had a greater median rating than any other (Mann-Whitney test, p > 0.025), so benthic and fish attributes seem to have the same importance.
Total and unintentional contact rates differed between years irrespective of the different diver certification levels, while intentional contact rates did not differ (Table 2). These differences are probably due to the different type of dives observed during the two years of sampling; in the first year the number of divers spending time in caves was higher than in the second year.
Table 2 Analysis of variance (ANOVA) on total, unintentional and intentional contact rates. ** = p < 0.01, ns = not significant; 1 = 2005, 2 = 2006.

Over the two years of sampling, each diver made contact with the environment on average 2.52 times every seven minutes. This contact was unintentional in 76% of all cases. Although diver duration in caves was low, we recorded the highest rate of both total (4.69 ± 1.27 contacts/diver/7 min; Fig. 4a) and unintentional (3.96 ± 1.01 contacts/diver/7 min; Fig. 4b) contact in this habitat. On encrusted walls the rate of total contact was 3.26 ± 0.74 contacts/diver/7 min (Fig. 4a), while the lowest rate of total contact was recorded on Posidonia oceanica and algae on vertical substrate (Fig. 4a). The highest rate of intentional contact was made on encrusted walls (1.04 ± 0.39 contacts/diver/7 min; Fig. 4c).
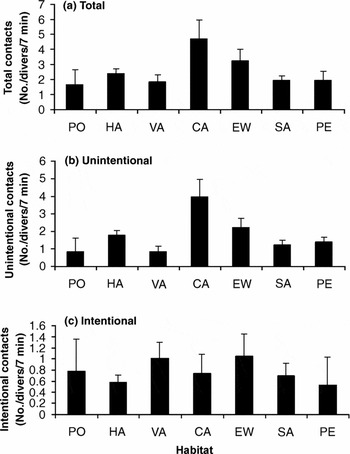
Figure 4 Mean rate of (a) total, (b) unintentional and (c) intentional contacts (number of contacts per diver per 7 minutes) (± SE) in each habitat at Capo Gallo-Isola delle Femmine MPA. HA = algae on horizontal substrate, VA = algae on vertical substrate, PO = Posidonia oceanica, EW = encrusted walls, CA = caves, SA = sand and PE = pebbles.
Contact rates of divers did not differ among diving certification levels, the interaction between certification and habitat factors also being non-significant (Table 3). However, the highest rate of total contact was by divers with the higher dive certification levels within caves (10.5 ± 3.5 contacts/diver/7 min). The highest rates of contact for open water divers (5.26 ± 2.27 contacts/diver/7 min) and advanced divers (2.89 ± 1.23 contacts/diver/7 min) were also recorded in caves.
Table 3 Analysis of variance (ANOVA) on total, unintentional and intentional contact rate. ns = not significant; *** = p < 0.001. Habitat labels: algae on horizontal substrate (HA), algae on vertical substrate (VA), encrusted walls (EW) and caves (CA).

Only total and unintentional contact rates differed among habitats (Table 3). Rates of both total and unintentional contacts were higher in caves and on encrusted walls than the other habitats (Table 3), and no differences were recorded in the rate of intentional contacts.
Most of the unintentional contacts were recorded during the initial eight minutes of the dive time regardless of diver level (Table 4), probably owing to the difficulty for divers of correcting buoyancy at the start of the dive. Immediate injuries mainly consisted of colony breakage, detachment and tissue abrasions. The species most frequently damaged by diver contact were the branching sea fan Eunicella singularis (c. 8% of total contacts) and the massive Astroides calycularis colonies (c. 0.7%). About 5% of contacts with E. singularis caused immediate and evident tissue abrasion, the remaining 95% of contacts did not cause any immediate damage. All the contacts made on Astroides calycularis caused evident injuries to the colonies in the form of detaching one or more corallites. No contacts were recorded with the sea fan Paramuricea clavata.
Table 4 Analysis of variance (ANOVA) on unintentional contact rate. ns = not significant; ** = p < 0.01; 1° = 1st to 8th minute of dive, 2° = from 9th to 16th minute of dive, 3° = from 17th to 24th minute of dive.

DISCUSSION
At Capo Gallo-Isola delle Femmine MPA divers spent more time on algae growing on horizontal substrate, algae on vertical substrate, encrusted walls and sand than on any other habitat. With the exception of the encrusted wall habitat, dominated by colourful invertebrates, the aesthetic value of these habitats is regarded as very low. Indeed, in addition to the high coverage of these habitats within the diving trails considered, the reason why divers spent so much time there may be the great species richness of the associated fauna, including reef associated fish. Moreover, the high amount of time spent by divers on sand, a habitat with very low aesthetic value and low fish biomass at shallow depths (Guidetti Reference Guidetti2000), could be related to the presence of colourful starfishes and large shells that attract the interest of scuba divers frequenting Capo Gallo-Isola delle Femmine MPA in the same measure as the presence of eye-catching features such as large fish; this idea is sustained by the high rate of intentional contacts recorded on sand. In other marine regions, attributes relating to fish (such as large size, variety, abundance and presence of unusual species) are usually more appreciated by divers than those relating to reef structure and benthos (such as reef structure, variety of corals, large corals, coral cover, unusual corals, sponges and unusual algae; Williams & Polunin Reference Williams and Polunin2000).
Rates of contact recorded in the present study are appreciably higher than those recorded in other marine regions (Medio et al. Reference Medio, Ormond and Pearson1997; Barker & Roberts Reference Barker and Roberts2004; but see Uyarra & Côtè Reference Uyarra and Côté2007), and are particularly elevated in caves and encrusted walls, which represent two of the habitats most vulnerable to scuba diving activity (Lloret et al. Reference Lloret, Marin, Marin-Guirao and Carreño2006) since they host many slow-growing sessile species with fragile skeletons, such as gorgonians and bryozoans (Ballesteros Reference Ballesteros2006; Bussotti et al. Reference Bussotti, Terlizzi, Fraschetti, Belmonte and Boero2006).
In particular the highest rate of contact was by divers with higher certification levels in caves. The higher level divers being more experienced and better trained probably exhibit greater confidence than open water and advanced divers in a constricted habitat like a marine cave, the narrow caves of the Capo Gallo-Isola delle Femmine MPA requiring particular attention to buoyancy (A. Di Franco, personal observation 2006). The observed differences in the rate of contact could also be related to the number of visits to each habitat made by divers with different scuba certification levels, higher level divers being fewer, and some habitats being more common at greater depths, making them less accessible to such as open water divers. However no differences in contact rate were recorded among diver certification levels.
Based on the conservative assumption that 9 divers/day have a dive lasting 30 minutes, over 80 days/year of activity by diving centres (data from interview with diving centre owners), recreational scuba divers in a year contact substratum about 7560 times, and bump benthic sessile organisms about 740 times, causing 100 permanent injuries.
Generally, the risk of long term degradation of a particular diving spot by scuba activity should be determined by the rate at which new damage occurs and how quickly it is restored by the recruitment and regrowth of a particular species. The rate at which new damage occurs is determined by both social and environmental features, whilst the rate of recovery is principally a function of the life history traits of the affected species (Rouphael & Inglis Reference Rouphael and Inglis1997). The probability of divers coming into contact with benthic organisms will be also determined by a range of personal attributes that may strongly influence the behaviour of the divers in the water, including buoyancy, use of limbs, sudden movements, the activities they pursue whilst at the site (such as photography or training; Rouphael & Inglis Reference Rouphael and Inglis2001; but see Uyarra & Côtè Reference Uyarra and Côté2007) and their awareness of the environmental consequences of their actions (Medio et al. Reference Medio, Ormond and Pearson1997; Dearden et al Reference Dearden, Bennett and Rollins2007). Moreover, both physical conditions (i.e. reef topography, waves and currents) and ecological characteristics of the site (i.e. fragile species density) may also influence how often divers damage organisms (Rouphael & Inglis Reference Rouphael and Inglis1997).
Rate of damage to species contacted depends on species morphology (see Zakai & Chadwick-Furman Reference Zakai and Chadwick-Furman2002), with ramified species (i.e. Eunicella singularis) damaged less frequently than massive species, and these last being subject to detachment of entire corallites. However our observations may underestimate damage caused to ramified organisms, since micro-fractures of the tissue are not evident in the field, yet can be followed by the attachment of numerous epibionts and subsequent weakening of the sea fan skeleton, increasing the likelihood of breakage (see Bavestrello et al. Reference Bavestrello, Cerrano, Zanzi and Cattaneo-Vietti1997).
For the benthic species damaged by divers during the surveys, diver impact can be considered a chronic disturbance since their slow growth rates do not allow individuals and colonies to recover from year to year (Coma Reference Coma1994; Sala et al. Reference Sala, Garrabou and Zabala1996; Cocito et al. Reference Cocito, Sgorbini and Bianchi1998). The negative effect on the massive colonies of the ahermatypic scleractinian Astroides calycularis, a species protected by the Environmental Protection Act (EPA), the Bern Convention (Convention on the Conservation of European Wildlife and Natural Habitats) and included in the list of the endangered and threatened species of the ASPIM protocol concerning specially protected areas and biological diversity in the Mediterranean (UNEP [United Nation Environment Programme] 1995), is of particular interest for conservation purposes. This species is endemic to the Mediterranean Sea and is placed at risk by its ecological characteristics and distribution. It is very likely that its attractive colour and the fragility of its colony make it an easy ‘target’ for divers (Milazzo et al. Reference Milazzo, Chemello, Badalamenti, Camarda and Riggio2002). No contacts were recorded on P. clavata that is probably, along with the red coral Corallium rubrum, the Mediterranean sessile species most popular and appreciated among scuba divers (Harmelin & Marinopoulos Reference Harmelin and Marinopoulos1994; Milazzo et al. Reference Milazzo, Chemello, Badalamenti, Camarda and Riggio2002; Coma et al. Reference Coma, Pola, Ribes and Zabala2004). Differences in contact frequency between the two sea fan species (P. clavata and E. singularis) are probably due to their different abundance along the trails and their different degree of exposure (Table 1; Di Franco et al. Reference Di Franco, Marchini, Baiata, Milazzo and Chemello2009). At the Capo Gallo-Isola delle Femmine MPA, P. clavata is mainly restricted to deep waters (>40 m), and is considered rare at shallower depths where diving activity is mostly concentrated. Where the density of gorgonians and the frequency of divers are considerably higher (Coma Reference Coma1994; Garrabou et al. Reference Garrabou, Sala, Arcas and Zabala1998), greater mortality of P. clavata is expected in highly frequented locations than control areas (Coma & Zabala Reference Coma and Zabala1994).
The management of this recreational activity needs more than simple control of the number of divers visiting a diving spot and probably should be focused on the reduction of the levels of damage on fragile organisms (Hawkins & Roberts Reference Hawkins, Roberts, Lessios and Macintyre1997; Rouphael & Inglis Reference Rouphael and Inglis1997; Zakai & Chadwick-Furman Reference Zakai and Chadwick-Furman2002). This goal could be reached through correct briefing before any diving takes place (Medio et al. Reference Medio, Ormond and Pearson1997) specifically targeting diver behaviour, localizing high risk activity (i.e. the first open water sessions of most training courses) to appropriate and less vulnerable habitats (Rouphael & Inglis Reference Rouphael and Inglis1997; Tratalos & Austin Reference Tratalos and Austin2001; Lloret et al. Reference Lloret, Marin, Marin-Guirao and Carreño2006).
Diver selection of habitats in the same proportion as their availability along the scuba trail reflects a priori selection by diving guides of the most attractive diving spots, usually areas with high habitat diversity. Immediate damage to some benthic species could be ecologically relevant where many divers concentrate in habitats hosting highly vulnerable species (Lloret et al. Reference Lloret, Marin, Marin-Guirao and Carreño2006). Since the greatest number of contacts recorded during dives in the present MPA were unintentional, correct buoyancy could be crucial to minimizing these impacts (Medio et al. Reference Medio, Ormond and Pearson1997). More experienced and better trained divers who are better able to control their own buoyancy should have less impact on marine species (Davis & Tisdell Reference Davis and Tisdell1995), but neither formal diver certification level nor the number of dives previously undertaken always represent a true indication of diver skills, as the findings of this and other studies clearly indicate (Harriott et al. Reference Harriott, Davis and Banks1997; Rouphael & Inglis Reference Rouphael and Inglis2001; Uyarra & Côtè Reference Uyarra and Côté2007). This is a pertinent matter for MPA managers because there is widespread belief that more experienced divers have less impact on benthic communities than beginners. Management restrictions based on diver certification level could be relatively unproductive. A possible approach to reduce potential impacts could be to concentrate divers in low vulnerability habitats (Di Franco et al. Reference Di Franco, Marchini, Baiata, Milazzo and Chemello2009; but see Lloret et al. Reference Lloret, Marin, Marin-Guirao and Carreño2006) during the initial minutes of the dive and to proceed to more vulnerable habitats only when divers have reached correct buoyancy and/or environmental awareness.
ACKNOWLEDGEMENTS
The authors thank dive operators in the Capo Gallo-Isola delle Femmine MPA for collaboration in field, the MPA management authority for funding this research and the anonymous reviewers and editors for critical review.










