Introduction
Feather stars dominate the diversity of extant crinoids and account for more than 80% of species (Rouse et al., Reference Rouse2013). Unlike other living crinoids, feather stars lose the postlarval stalk and subsequently remain free living and able to crawl and, in some instances, swim. Both behaviors may represent antipredatory strategies as well as a means of relocating to more favorable microhabitats (Meyer and Macurda, Reference Meyer and Macurda1977). These adaptations have been considered important to the evolutionary success of feather stars. Although historically the terms ‘comatulids’ and ‘feather stars’ have been used interchangeably, the two are no longer equivalent as recent work indicates that the crinoid order Comatulida contains the feather stars, a nonmonophyletic group, as well as taxa that retain their stalk as adults (Hemery, Reference Hemery2011; Hess and Messing, Reference Hess and Messing2011; Rouse et al., Reference Rouse2013; Summers et al., Reference Summers, Messing and Rouse2017). In this study, to avoid ambiguity, we use the term ‘feather star’ to refer only to taxa that lose their postlarval stalk and apply the term ‘comatulid’ to all members of the order Comatulida.
The fossil record of feather stars extends back ~200 Myr (Hess, Reference Hess1951, Reference Hess2014; Hagdorn and Campbell, Reference Hagdorn and Campbell1993; Simms et al., Reference Simms, Gale, Gilliland, Rose and Sevastopulo1993), but only 43 genera are known from fossils, and whereas today’s diversity approaches 140 genera, only six of those have a fossil record. Moreover, at no time in the geologic past did recorded diversity exceed a dozen genera (Hess and Messing, Reference Hess and Messing2011). The paucity of feather star fossils is further illustrated by the fact that fewer than 100 occurrences appear in the Paleobiology Database (6 July 2017), which contains over 1,300 total Mesozoic and Cenozoic crinoid occurrences.
The low number of described fossil feather stars could represent a true biological signal and imply a very recent radiation, or, alternatively, it could be an artifact of their poor fossil record that less accurately reflects their past biological diversity. Generally, the latter has been considered more likely (e.g., Howe, Reference Howe1942; Baumiller and Gaździcki, Reference Baumiller and Gaździcki1996; Donovan, Reference Donovan2001). Some of the reasons for this include their low preservation potential due to the gracile skeletons and the high-energy reef environments that feather stars prefer (Meyer and Meyer, Reference Meyer and Meyer1986), taxonomic problems caused by the great rarity of even partially articulated specimens, and inadequate sampling due to the very small size and difficulty of retrieving disarticulated skeletal elements (Howe, Reference Howe1942; Oyen and Portell, Reference Oyen and Portell2001).
Eagle (Reference Eagle2001, Reference Eagle2007, Reference Eagle2008) described a rich Oligocene crinoid fauna from New Zealand, which illustrates the degree to which the fossil record of feather stars may be undersampled. The 11 included taxa were the first Cenozoic fossil feather stars recorded from New Zealand, and all were new to science. In addition, his specimens demonstrated the highly incomplete mode of preservation characteristic of fossil feather stars: all were either isolated centrodorsals or centrodorsals with basal and radial circlets attached. None had articulated brachials, pinnules, or cirri, which are critical to extant feather star taxonomy (Messing, Reference Messing1997). This mode of preservation is a general feature of the feather star fossil record: for Cenozoic genera, ~70% are known only from centrodorsals or centrodorsals with a basal and radial circlet. The lack of data on brachials makes it exceptionally difficult to reconstruct the history of arm-branching transformations as well as the temporal trends in the distribution of various brachial articulations, both of which are deemed critical to this group’s ecology and evolution (e.g., Oji and Okamoto, Reference Oji and Okamoto1994). New finds, especially of intact fossils, are therefore crucial. Here we describe one such fossil from New Zealand of an age contemporaneous with those described by Eagle (Reference Eagle2001, Reference Eagle2007, Reference Eagle2008) that provides information on the morphology of arms and cirri. As it represents the first example of arm autotomy and regeneration in a fossil feather star, it offers evidence of the importance of predation to the evolutionary history of this group. The specimen is also critical for the reassessment of Cypelometra aotearoa Eagle, Reference Eagle2007, which is found to differ significantly from any presently established genus. As such, we propose a new genus, Rautangaroa, to accommodate this species.
Geologic setting
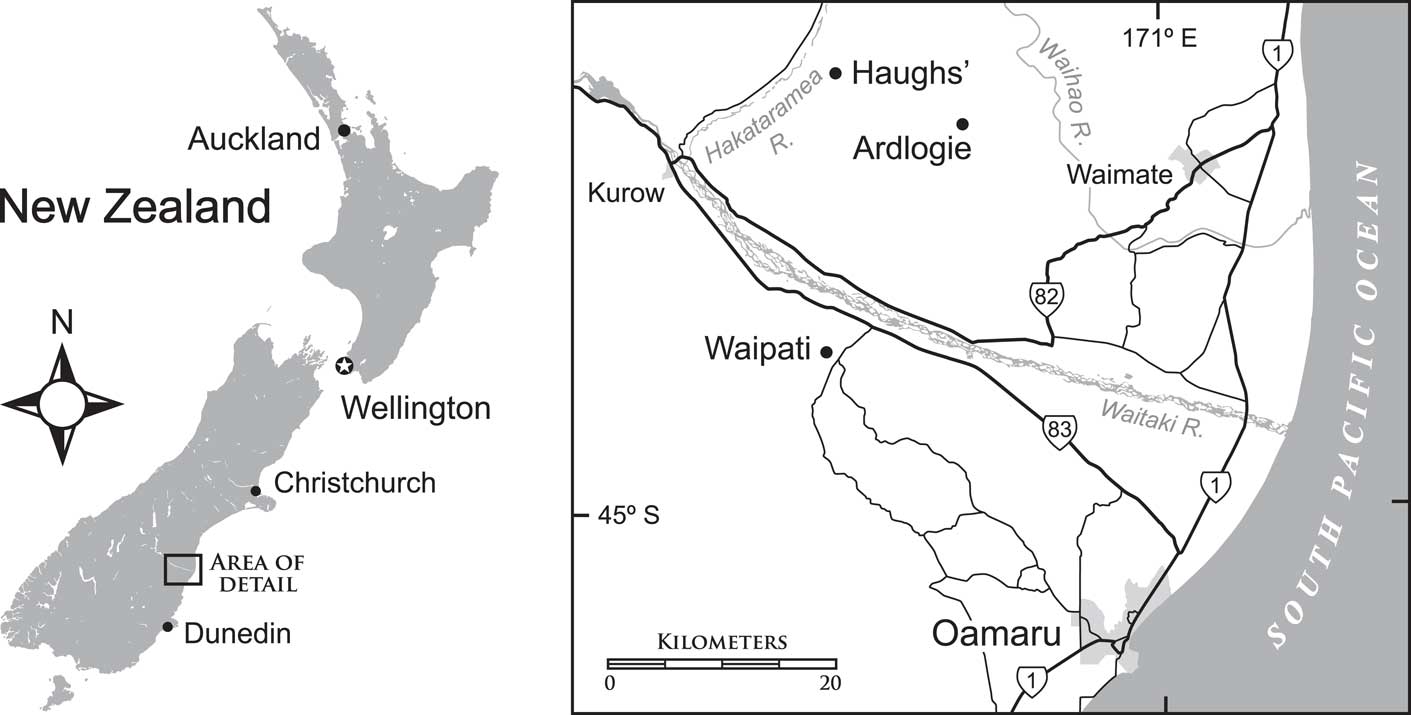
Localities and horizons for specimens discussed here are uniquely numbered in the New Zealand Fossil Record Electronic Database (see www.fred.org.nz). Centrodorsals assigned by Eagle to Cypelometra aotearoa Eagle, Reference Eagle2007 were all collected at two localities in South Canterbury, South Island, New Zealand. For both localities, Eagle (Reference Eagle2007) reported the age as Waitakian Stage, although age-diagnostic fossils were not cited. These localities are: (1) Ardlogie (or Pentland Hills) quarry (44.70277S, 170.78937E; Fossil Record numbers J40/f053A, J40/fl12, J40/f6636), and (2) the informally named Haughs’ (or Hurstlea, or Hakataramea) Quarry (44.6627S, 170.65021E; Fossil Record number I40/f324).
The sequence at Ardlogie comprises lower Kokoamu Greensand, grading up to Otekaike Limestone; these strata span the Duntroonian and Waitakian Stages so that surface-collected crinoids could represent either formation or stage. A sample from in situ transitional glauconitic limestone (J40/f0021) included the Waitakian Stage index species Globoquadrina dehiscens (Chapman, Parr, and Collins, Reference Chapman, Parr and Collins1934) (N. de B. Hornibrook, personal communication to Fordyce, 1978).
At Haughs’ Quarry, the main upper fossil horizon is a diffuse para-autochthonous shell bed with conspicuous Protula tubes. Fossils are readily surface collected. For this bed (I40/f0219B), Tanaka and Fordyce (Reference Tanaka and Fordyce2015) reported the planktic foraminiferan Globoturborotalita woodi (Jenkins, Reference Jenkins1960), a zonal indicator for middle Waitakian Stage, equivalent to upper Chattian. Tanaka and Fordyce (Reference Tanaka and Fordyce2015) also cited a strontium (87Sr/86Sr) date, from just above the Protula bed, of 22.28±0.13 Ma, or basal Aquitanian.
The nearly complete crinoid, OU46680, described in this study comes from a third locality: (3) Waipati (44.87833S, 170.65115E), west-southwest of the town of Duntroon, in North Otago, ~20 km south of the two South Canterbury localities (Fig. 1). Specimen OU46680 (Fossil Record number I40/f0407) is from the Otekaike Limestone, probably high in the Duntroonian Stage, older than 25.2 Ma, roughly mid-Chattian. The crinoid matrix was not dated directly; rather, the age is from a nearby sample (I40/f0408) from the same horizon. A Duntroonian age is based on the presence of the foraminiferan Notorotalia spinosa (Chapman, Reference Chapman1926) and absence of Globoquadrina dehiscens from an otherwise diverse planktic assemblage.

Figure 1 Map of New Zealand and the three localities (Haughs’, Ardlogie, Waipati) where specimens of Rautangaroa aotearoa n. gen. n. comb. were collected. Detailed coordinates of localities can be found in the text.
The geology of this region of Canterbury Basin has been described in numerous publications, notably by Gage (Reference Gage1957), Forsyth (Reference Forsyth2001), Fordyce and Maxwell (Reference Fordyce and Maxwell2003), Eagle (Reference Eagle2007), and Gottfried et al. (Reference Gottfried, Fordyce and Rust2012). During the late Oligocene, this part of New Zealand was experiencing an interval of relative tectonic quiescence between an earlier phase of submergence associated with the break-up of Gondwana and the subsequent emergence associated with plate-boundary tectonics of the early Miocene. Sediments deposited at this time include a thin, calcareous glauconitic sandstone (Kokoamu Greensand) overlain gradationally by a massive, low to moderately cemented, bioclastic limestone (Otekaike Limestone). The former represents a period of terrigenous-sediment starvation, distant shorelines, and/or low-lying landmasses, whereas the latter is interpreted as having been formed as flat-lying, loose bioclastic sediments deposited on a shelf, generally below storm wave base.
The crinoids reported here are from the widespread dominant facies of the limestone, the Maerewhenua Member; this massive, little-cemented, light brown-yellow, bioclastic sand consists of fragments of mollusks, brachiopods, echinoderms, bryozoans, foraminiferans, and other invertebrates. (The Maerewhenua Member appears to be a senior synonym for the Meyers Pass Limestone Member of Eagle, Reference Eagle2007).
Both the greensand and the limestone include notable scattered shell beds. The highly friable limestone weathers easily, and fossils, most of which show little evidence of deformation or compaction, are easily extracted. Important fossils of marine vertebrates have been recovered from these units, including those of cetaceans (e.g., Fordyce and Marx, Reference Fordyce and Marx2016), penguins (e.g., Ksepka et al., Reference Ksepka, Fordyce, Ando and Jones2012), and fish (e.g., Gottfried et al., Reference Gottfried, Fordyce and Rust2012). The units are also rich in marine macroinvertebrates, including bryozoans, corals, serpulids, brachiopods, mollusks, and echinoderms (see Eagle, Reference Eagle2007 for review), while common microfossils (foraminiferans and ostracods) provide excellent biostratigraphic constraints (Hornibrook et al., Reference Hornibrook, de, Brazier and Strong1989; Ayress, Reference Ayress1993). According to microfossils and Sr/Sr dates, the Kokoamu Greensand and Otekaike Limestone span the local upper Whaingaroan, Duntroonian, and Waitakian stages, from low in the Chattian (27.82 Ma to 23.03 Ma) to possibly basal Aquitanian (23.03 Ma to 20.44 Ma).
Materials and methods
Repositories and institutional abbreviations
Specimens described by Eagle (Reference Eagle2007), all centrodorsals, collected at Ardlogie include AU19053-E868, AU19053-E869, GS11338.119–EC1162, GS11156.49-EC1163, GS11156.69-EC1164, GS11156.67-EC1165, GS11156.71-EC1166, and GS11156.18-EC1167, and at Haughs’ Quarry AU19054-E870A. A specimen (centrodorsal) collected at Haughs’ Quarry by A. Grebneff, OU44147; an intact specimen collected at ‘Waipati’ by R.E. Fordyce, OU46680. Four specimens of the type species of Cypelometra, Cypelometra iheringi (de Loriol, Reference de Loriol1902): MACN4567—two specimens with same number collected at ‘Golfo San Jorge, Patagonia,’ one consisting of centrodorsal, the other of a centrodorsal and a radial ring; MACN4568 collected at ‘Bajo San Julian,’ consisting of a centrodorsal and a radial ring; MACN4569 collected at ‘Bajo San Julian,’ consisting of a centrodorsal and a radial ring.
All specimens are housed in research collections in museums in the following institutions: Department of Geology in the School of Geography, Geology and Environmental Science, University of Auckland, NZ (AU); Institute of Geological and Nuclear Sciences, Lower Hutt, NZ (GS); Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia,’ Buenos Aires, ARG (MACN); Geology Museum, University of Otago, Dunedin, NZ (OU).
Anatomical terms and abbreviations used follow Hess and Messing (Reference Hess and Messing2011).
Systematic paleontology
Class Crinoidea Miller, Reference Miller1821
Subclass Articulata Zittel, Reference Zittel1879
Order Comatulida Clark, Reference Clark1908
Superfamily Tropiometroidea Clark, Reference Clark1908
Family Conometridae Gislén, Reference Gislén1924
Genus Rautangaroa new genus
Type species
Cypelometra aotearoa Eagle, Reference Eagle2007, here designated by monotypy.
Included species
Cypelometra aotearoa Eagle, Reference Eagle2007.
Diagnosis
Genus of Conometridae with a large, pentastellate, truncated conical centrodorsal. Aboral pole large (40%–70%), flat to slightly concave cirrus-free rugose or granulated. Adoral outline of centrodorsal deeply notched radially with triangular to blunt rectangular interradial projections. Subradial cleft present. Cirral sockets concave, moderately deep, and covered with radiating crenelae along the margins. Arms more than 20 divided at primibrachial 2 (IBr2) and secundibrachial 2 (IIBr2); may divide further at tetribrachial 2 (IVBr2). Synarthries between brachials 1 and 2 of brachitaxes. Syzygies at IIIBr1–2 or IIIBr3–4, and, when tetribrachials present, at IVBr1–2.
Etymology
From the Māori words rau, meaning frond or feather, and tangaroa, meaning sea.
Occurrence
Maerewhenua Member of the Otekaike Limestone, Oligocene: South Canterbury and North Otago, South Island, New Zealand.
Remarks
The assignment of Rautangaroa to the Conometridae is based on the size and shape of its centrodorsal and the arrangement, size, and shape of its cirrus sockets. Its truncated conical centrodorsal with a flattened aboral apex, absence of a dorsal star, cirrus sockets arranged in 10 more or less distinct vertical columns of two to four sockets, separated by a naked space in the radii, a narrow centrodorsal cavity, concealed basals, and visible dorsal, free surface of radials are all traits found in Conometridae (Hess and Messing, Reference Hess and Messing2011).
Among the Conometridae, Rautangaroa most closely resembles Cypelometra, but a detailed analysis of the type species of Cypelometra, C. iheringi (de Loriol, Reference de Loriol1902), reveals significant differences.
Gislén (Reference Gislén1924) established the genus Cypelometra with Antedon iheringi de Loriol, Reference de Loriol1902 as the type species. In the original description, de Loriol relied on six specimens collected in Patagonia by C. Ameghino (Ameghino, Reference Ameghino1906, p. 171) that had been sent to him by Ihering (de Loriol, Reference de Loriol1902, p. 3, 23). de Loriol’s description included drawings of two specimens (de Loriol, Reference de Loriol1902, figs. 3, 4). Until Eagle’s Reference Eagle2007 publication, C. iheringi (de Loriol, Reference de Loriol1902) remained the only species of the genus, and specimens mentioned by de Loriol (Reference de Loriol1902) were the only ones reported in the literature. It is highly unlikely that subsequent to de Loriol’s Reference de Loriol1902 publication, specimens of C. iheringi (de Loriol, Reference de Loriol1902) had been reexamined since de Loriol did not specify where the types were deposited, and all subsequent authors relied solely on de Loriol’s descriptions, often republishing his figures.
One of the original illustrations (de Loriol, Reference de Loriol1902, fig. 3a) shows deep radial pits or impressions on the adoral side of the centrodorsal. Surprisingly, this striking feature is not mentioned in subsequent publications, including in the one by Gislén (Reference Gislén1924) that established the genus Cypelometra with Antedon iheringi de Loriol, Reference de Loriol1902 as the type species. We located four specimens of C. iheringi (de Loriol, Reference de Loriol1902) in the collections of Museo Argentino de Ciencias Naturales ‘Bernardino Rivadavia,’ Buenos Aires (MACN, catalog numbers 4567 [two specimens, herein referred to as ‘A’ and ‘B’], 4568, and 4569) collected from the same Patagonian localities as those described by de Loriol (Reference de Loriol1902). Of these four, only one specimen (MACN 4567 ‘A’) lacks radials, thus exposing the adoral surface of the centrodorsal. MACN 4567 ‘A’ has five cavernous radial pits or impressions, which are separated from each other by tall, narrow ridges that extend interradially from the thin, external wall of the centrodorsal to a small, relatively deep central cavity, as in the specimen figured by de Loriol (Reference de Loriol1902, fig. 3a). Deep radial pits separated by interradial ridges have been noted in several other feather stars, including Notocrinus (Messing, Reference Messing2003, fig. 3a), Microcrinus (Ciampaglio and Weaver, Reference Ciampaglio and Weaver2004, fig. 2b), and Jaekelometra (Messing, Reference Messing2003, fig. 3b), yet in details they all differ substantially from each other. The presence of deep radial pits in the type species of Cypelometra clearly distinguishes this taxon from the New Zealand specimens whose adoral surface is “circular, smooth, concave, with five long, wide, convex-floored, smooth, raised margins above reduced V-shaped radial surface” (Eagle, Reference Eagle2007, p. 95–96, fig. 12).
Although similar in size, the shapes of the centrodorsals are also distinctly different: in profile, those of C. iheringi (de Loriol, Reference de Loriol1902) are hemispherical, whereas in the New Zealand specimens they are truncated conical, with a large flat to slightly concave aboral pole. In adoral view, the latter is deeply notched radially, with triangular to blunt rectangular interradial projections, whereas the radial margin of C. iheringi (de Loriol, Reference de Loriol1902) is gently curved. Cirrus sockets of C. iheringi (de Loriol, Reference de Loriol1902) have smooth margins, whereas in the New Zealand specimens, margins are crenulated.
Rautangaroa aotearoa (Eagle, Reference Eagle2007)
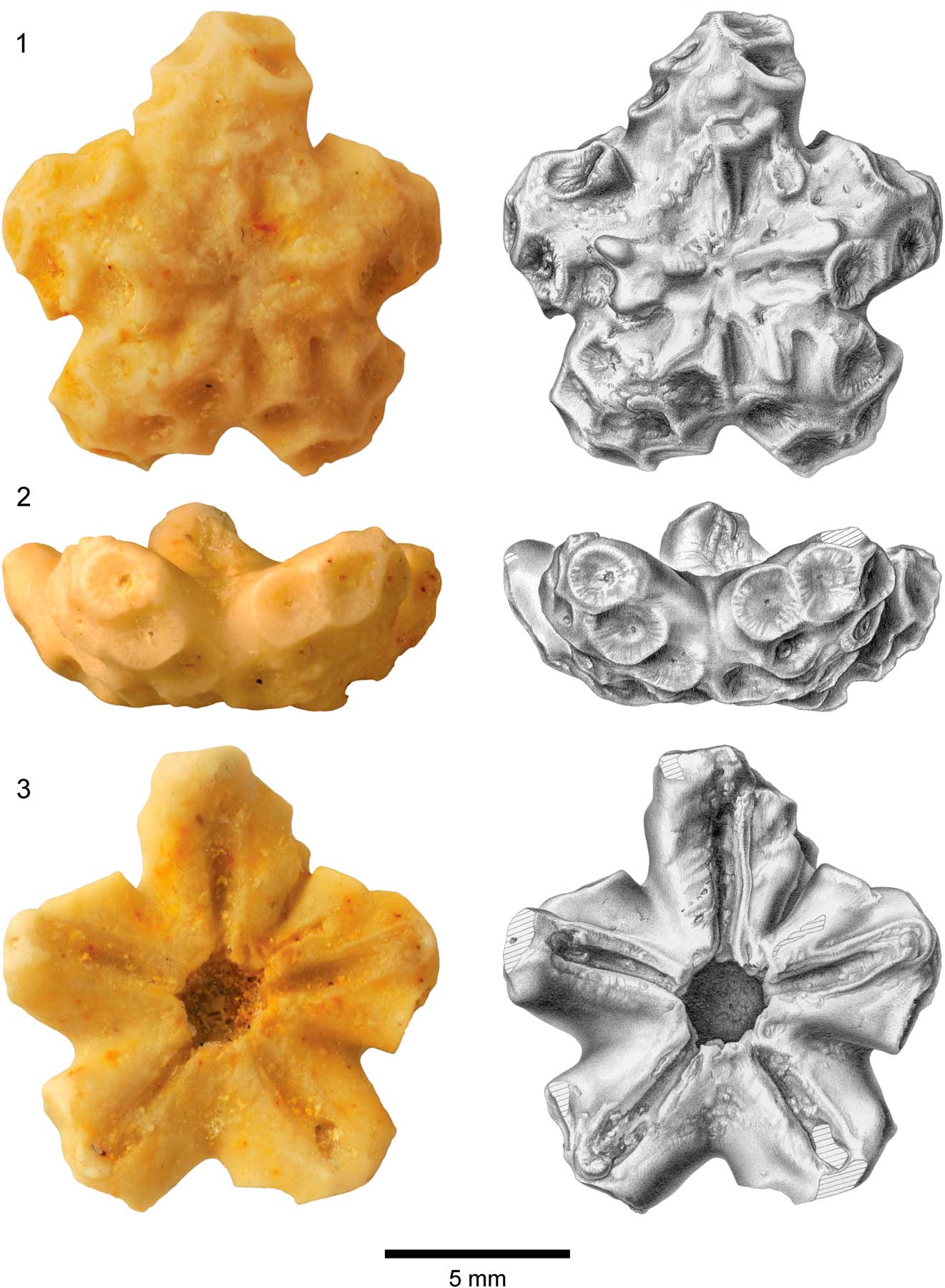
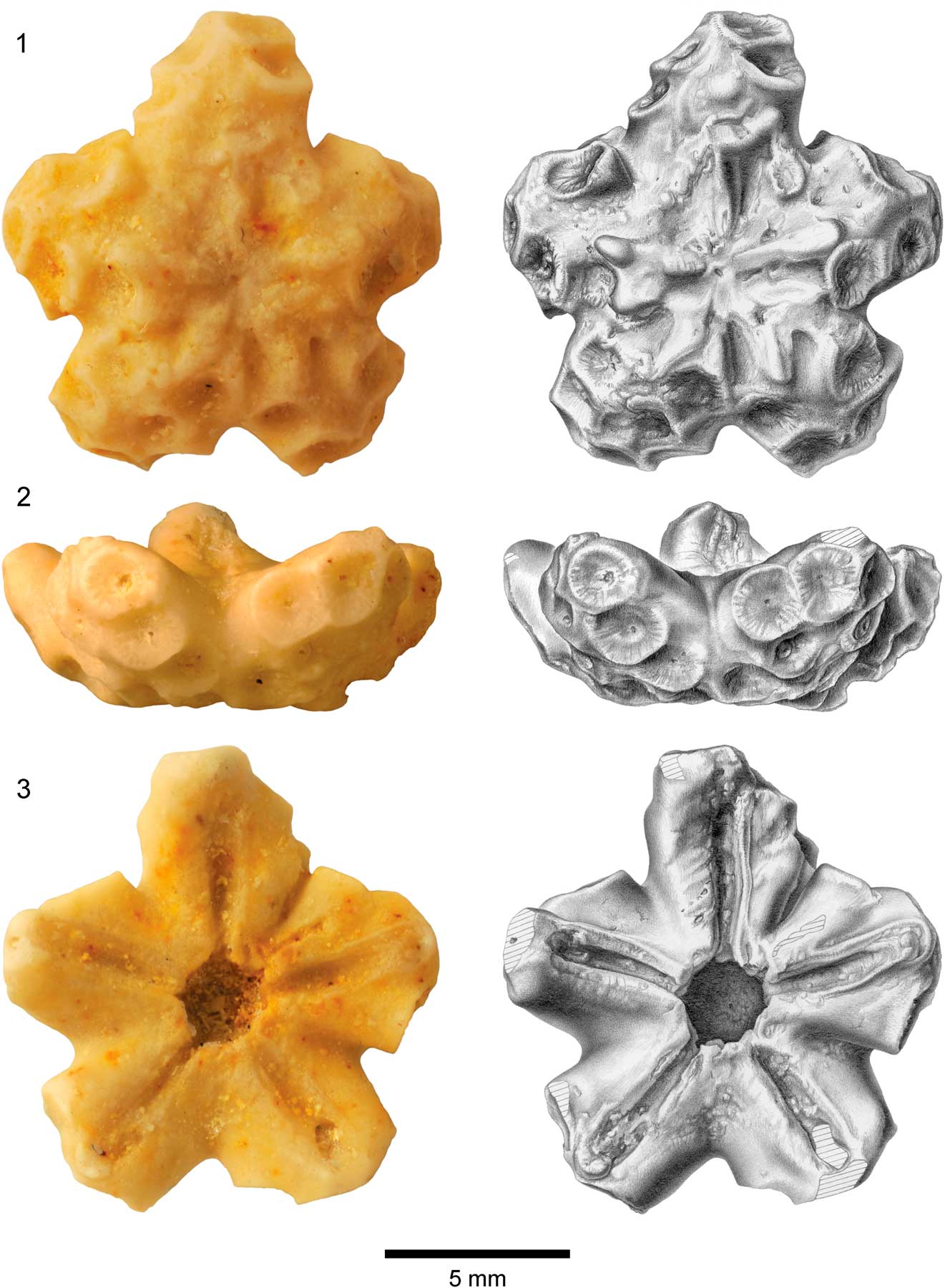
Figure 2 Centrodorsal of Rautangaroa aotearoa n. gen. n. comb. from the Otekaike Limestone, Oligocene, Haughs’ Quarry, South Canterbury, New Zealand, (OU44147). (1) Aboral, (2) lateral, and (3) adoral views; photos in left column and corresponding drawings in right column. Hatching indicates broken surface. Scale as indicated.

Figure 3 Lateral view of Rautangaroa aotearoa n. gen. n. comb. from the Otekaike Limestone, Oligocene, ‘Waipati,’ North Otago, New Zealand, (OU46680). Micro-CT slices and a 3D rendering of the specimen are provided in the Supplemental Data 2 and 3. Scale bar as indicated.
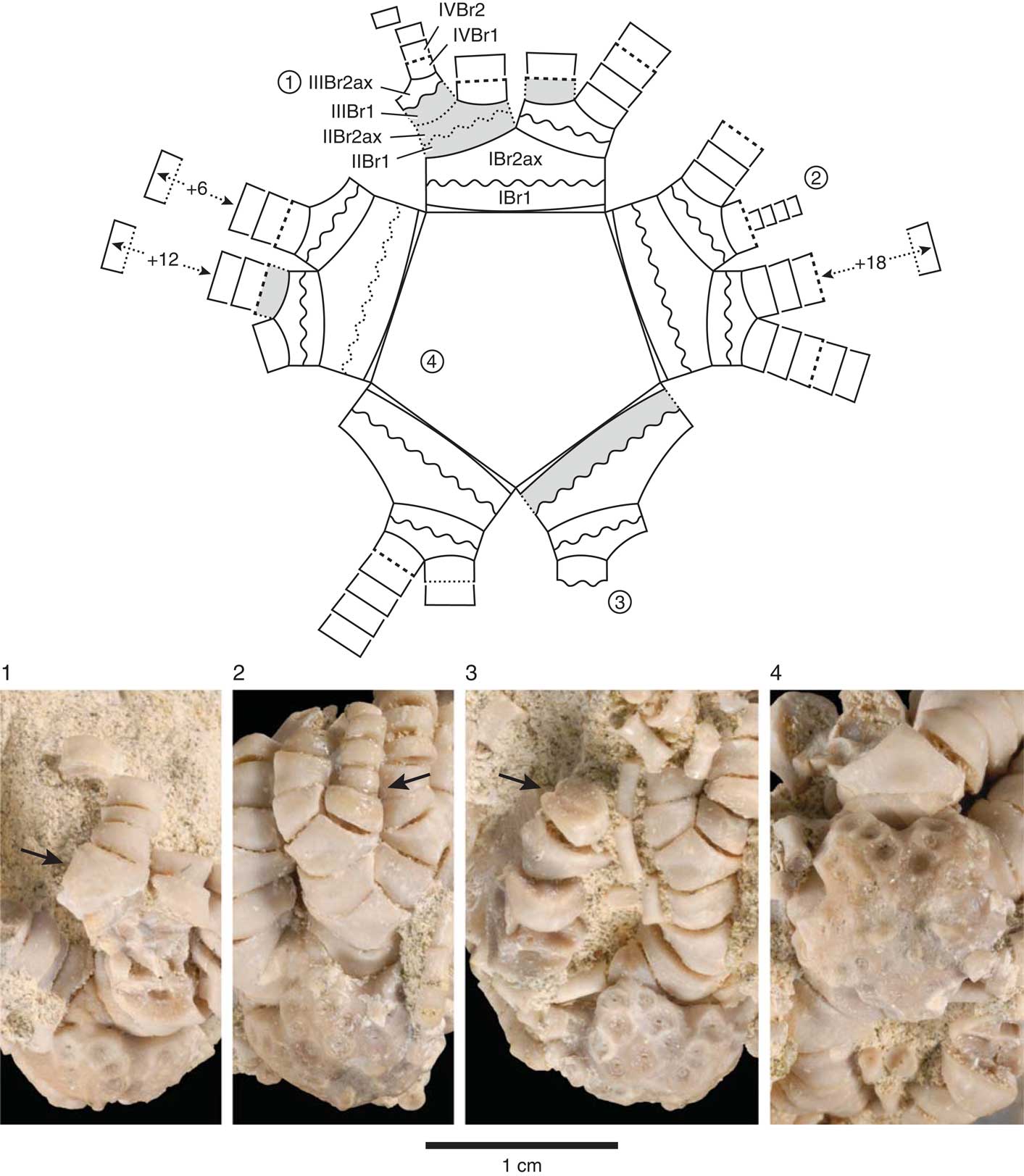
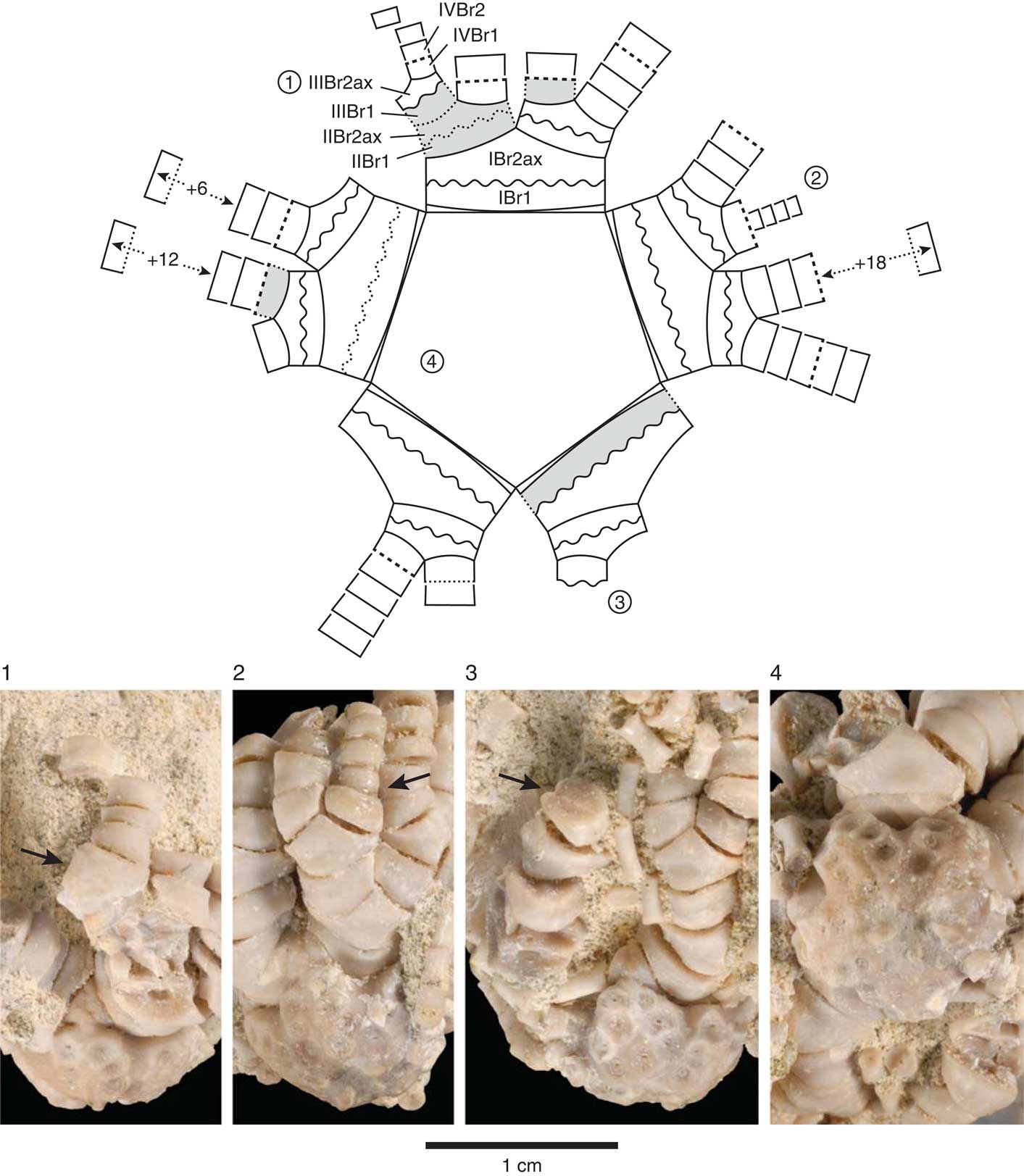
Figure 4 Schematic line drawing of centrodorsal and arms in aboral view (top) with four corresponding photographic views of Rautangaroa aotearoa n. gen. n. comb. from the Otekaike Limestone, Oligocene, ‘Waipati,’ North Otago, New Zealand, (OU46680). The schematic drawing (not to scale) illustrates the preserved and missing brachials, the arm-branching pattern, and type of brachial articulations observed on the intact specimen. The circled numbers, 1–4, on the schematic drawing correspond to numbers associated with the photographic views. Missing brachials colored grey. Brachial articulations coded in schematic drawing as follows: solid line=muscular; wavy line=synarthrial; dashed line=syzygial; dotted lines represent inferred brachial articulation. Brachials identified with symbols as in text following Hess and Messing (Reference Hess and Messing2011): IBr1=first primibrachial; IBr2ax=second primibrachial, axillary; IIBr1=first secundibrachial, etc. Number of articulated distal tetribrachials indicated by + symbol. Scale as indicated. (1) IIIBr2ax with arrow pointing at the muscular articular facet. (2) Regenerating arm consisting of four tetribrachs, with arrow pointing at syzygy at IIIBr1-2. (3) Synarthrial articulation (arrow), distal facet of IIIBr1. (4) Adoral, slightly oblique radial view of centrodorsal showing IBr1 and IBr2ax.
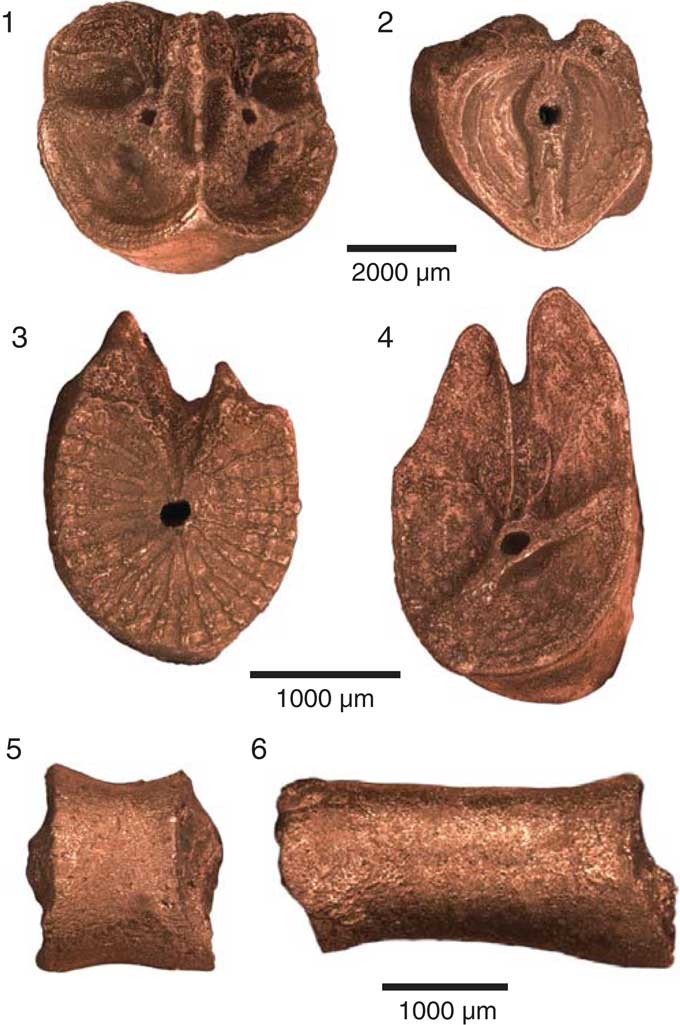
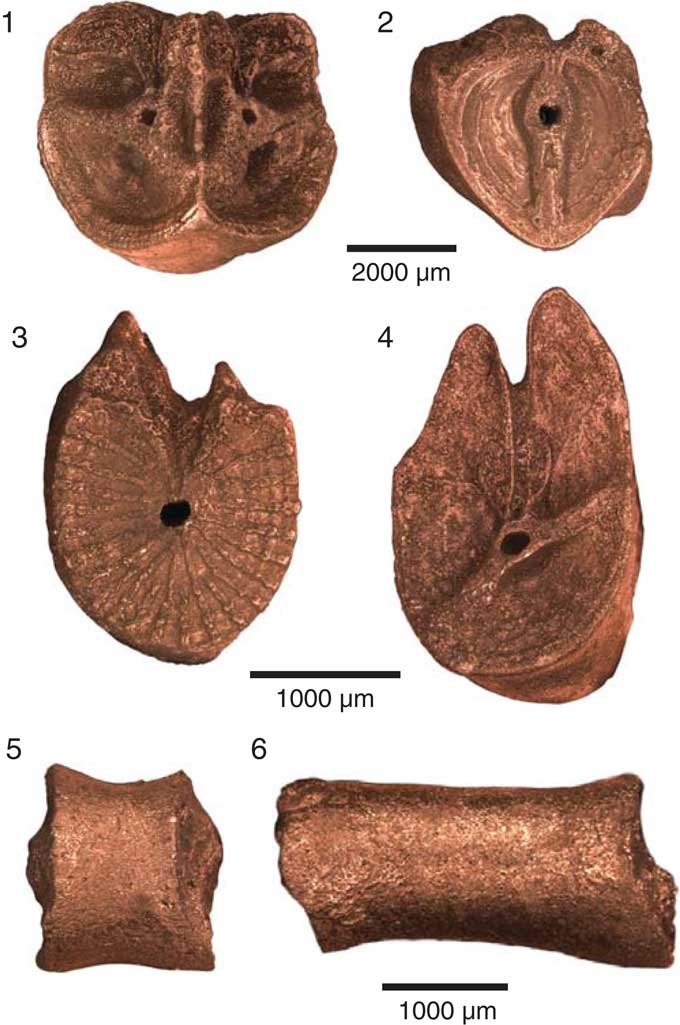
Figure 5 Brachials and cirrals of Rautangaroa aotearoa n. gen. n. comb. from the Otekaike Limestone, Oligocene, ‘Waipati,’ North Otago, New Zealand, (OU46680). Scale as indicated. (1) Straight muscular articulations on the distal facet of an axillary (IIBr2ax). (2) Synarthrial articulation on the proximal facet of an axillary in (1) (IIBr2ax). (3) Syzygial articulation on a proximal brachial. (4) Oblique muscular articulation on a proximal brachial. (5) Proximal cirral in lateral view. Proximal articular facet is to the right. (6) Medial cirral in lateral view. Proximal articular facet is to the right.
2007 Cypelometra aotearoa; Reference EagleEagle, p. 94, figs. 10–12.
Types
Holotype AU19053 (E868), Ardlogie, South Canterbury, New Zealand.
Paratypes
Ardlogie, South Canterbury, New Zealand: AK7331, GS 11338, AU19053 (E869), AK73312, GS11156.49, GS11156.69, GS11156.67, GS11156.71, GS11156.18; Haughs’ Quarry, South Canterbury, New Zealand: AU 19054 (E870). Type locality: Otekaike Limestone, Ardlogie, South Canterbury, New Zealand.
Occurrence
As for generic occurrence.
Description
Centrodorsal large, truncated conical; maximum diameter ~1 cm. Aboral pole flat to slightly concave, cirrus-free, and rugose or granulated, occupying ~50% of basal diameter (Fig. 2). Cirri arranged in 10 more or less distinct vertical columns of two to four sockets, separated by naked midradial space; sockets concave, moderately deep, and bordered with radiating crenelae. Axial canal at center of each socket; oval to slightly bilobate. Peripherally, one to three sockets may converge interradially forming weak to moderate ridge where socket margins touch or overlap. Dorsal star lacking.
In profile, interradial corners very prominent with deep incision of adoral edge of centrodorsal below radials.
Adoral outline of centrodorsal pentastellate, with deep interradial notches and triangular to blunt rectangular interradial projections. Adoral surface slightly concave, with five distinct near-rectangular furrows (~0.6 mm long and ~0.1 mm wide); furrows slightly narrower where they open into central cavity and taper slightly at interradial corner. No radial pits. Centrodorsal cavity about one-third of centrodorsal diameter.
Skeletal elements other than the centrodorsal known with certainty only from the single intact Waipati specimen of R. aotearoa n. comb., which was examined with a binocular microscope as well as micro-CT; many individual elements free of the matrix were studied with SEM (Fig. 3, Supplemental Data 1, 2, 3). In this specimen, basals are not visible. The radials are short, smooth, with the free surface visible only as an extremely narrow band beyond the centrodorsal margin, though more exposed in interradial angles where radials meet above the basals; their distal margin is concave (Fig. 4.2, 4.4). Subradial cleft is present. No radial articular facets are exposed.
All arms are divided at primibrachial 2, secundibrachial 2; a single articulated tertibrachial 2 indicates that there were at least 21 free arms. In first and second division series, Br1–2 synarthrial. Brachitaxes aborally convex, with weak midaboral synarthrial swellings. Brachials smooth, wider than high (Figs. 3, 4).
IBr1 short, proximal margin U-shaped, distal margin nearly straight, taller radially than interradially with straight lateral margins; ratio of width to length ~2.5. IBr1–2 synarthrial. IBr2ax pentagonal with short diverging lateral margins, width-to-length ratio ~1.4–1.6 (Fig 4.4).
IIBr1 with slightly U-shaped proximal and distal margins and subequal, slightly diverging lateral margins touching interiorly, width-to-length ratio ~2.0. IIBr1–2 synarthrial. IIBr2ax pentagonal with short diverging lateral margins, width-to-length ratio ~1.3–1.6 (Fig. 4.2).
IIIBr1 rhomboid, lateral margins touching interiorly, width-to-length ratio ~1.9–2.3. IIIBr1–2 muscular, syzygial, or rarely synarthrial. Shape of IIIBr2 variable: when IIIBr1–2 muscular, IIIBr2 width-to-length ratio ~1.7-2.0; when IIIBr1–2 syzygial, IIIBr2 width-to-length ratio ~3.5; when IIIBr1–2 synarthrial, IIBr2 width-to-length ratio ~1.7 (Fig. 4.3).
Syzygies at IIIBr1–2 (6/10) or IIIBr3–4 (4/10) and, where tetribrachials present, at IVBr1–2. Remaining undivided arms with no additional syzygies, none complete, the longest consisting of 21 tetribrachials.
Cirri XXVIII to ~XXXI, moderately stout; smooth; largest intact cirrus of seven cirrals (16 mm long), but 553 cirrals recovered from the matrix suggest cirri may consist of ~20 cirrals and be ~40–50 mm long. Proximal cirrals cylindrical and stout: first cirral (c1) short (0.8 mm); following cirrals increasing in length to c7 (2.3 mm); length-to-width ratio of first seven cirrals increases from ~0.5 to 2.2; longest cirrals (probably c8–c9) ~3.2 mm long and 1.4 mm wide (length-to-width ratio=2.3); following four cirrals gradually shorter and slightly compressed, reaching length-to-width ratio of 1.6 by about c12; no cirri intact beyond about c12; numerous disarticulated cirri ranging from cylindrical (0.3 mm long by 0.3 mm wide; length-to-width ratio=1) to long, compressed, hour-glass shaped; no spines, no swellings or ridges; claw not recovered (Fig. 5).
Remarks
Assignment of R. aotearoa n. comb. to the Conometridae is based primarily on the centrodorsal morphology and arrangement, size, and shape of cirrus sockets. Given that the Waipati specimen of R. aotearoa n. comb. is largely intact, it may seem surprising that other skeletal elements were not useful in this regard, but this is in part a consequence of the highly incomplete preservation of the other six genera currently assigned to this family (Hess and Messing, Reference Hess and Messing2011). Furthermore, the radials, which are often taxonomically important, proved of limited use in this case because whereas they are known in the other conometrid genera, and are highly variable, in the Waipati specimen of R. aotearoa n. comb., they are articulated to the primibrachials, and disappointingly, their facets remain concealed. Nevertheless, in its shape, arrangement of cirrus sockets, and sculpturing, Rautangaroa n. gen. most closely resembles other conometrids.
Discussion
Taphonomy
The crinoids that have been recovered from the Otekaike Limestone occur in two states of preservation: most, including Eagle’s (Reference Eagle2007, Reference Eagle2008) material, consist of dissociated elements that include brachials, pinnules, cirri, centrodorsals, and only in a few instances, centrodorsals with a radial circlet attached; the specimen of R. aotearoa n. comb. collected near Waipati described herein is remarkably complete and articulated, consisting of a centrodorsal, many cirri of several cirrals (none complete), with a radial circlet, radiating arms of as many as 21 brachials (none complete), and numerous pinnulars (Fig. 3, Supplemental Data 1, 2, 3). This dichotomous nature of preservation may indicate that different taphonomic processes were responsible: in the first instance, a period of postmortem decay, disarticulation, and burial, and in the second, rapid burial of an intact, perhaps live individual.
For fossil feather stars, the high degree of disarticulation and incompleteness is by far the most common mode of preservation. This is perhaps not surprising given that their multiplated skeletons are not particularly resistant to postmortem processes and disarticulate easily (Meyer and Meyer, Reference Meyer and Meyer1986). Most crinoid skeletal elements are only loosely held together by slender fibers of soft tissue (ligament and muscle) that decay quickly, whereas those articulations that are more tightly bound (e.g., syzygies) tend to be specialized for autotomy and fail even more quickly either due to the active response of the organism to the death-related stress or because they are not resistant to decay. The more frequent recovery of fossil centrodorsals or centrodorsals with a basal and radial ring is consistent with the fact that the centrodorsal is the largest element, and the articulation between it and the radials is nonmoveable, tightly sutured, and not specialized for autotomy. Experiments on extant feather stars confirm that postmortem disarticulation may be rapid, with high potential for differential sorting of elements soon after death even with minimal transport (Meyer, Reference Meyer1971; Liddell, Reference Liddell1975; Meyer and Meyer, Reference Meyer and Meyer1986; Baumiller, Reference Baumiller2003). The Otekaike feather stars described by Eagle (Reference Eagle2007, Reference Eagle2008) had to experience some period of decay prior to burial given that they are highly disarticulated: centrodorsals were recovered either as single elements or, in a few instances, with articulated radials, but with no other elements attached. Yet the material appears autochthonous, given the absence of abrasion and the fact that other dissociated elements (brachials, pinnules, cirri) were also recovered. Eagle (Reference Eagle2007) noted the presence of epibionts on some echinoid fragments at these localities, which is also consistent with rather slow sedimentation rates and the potential for exposure on the sediment–water interface of some duration prior to burial.
The preservation of the highly articulated Waipati specimen of R. aotearoa n. comb. indicates that this specimen was buried while still intact, perhaps while still alive, and that the decay of soft tissues occurred after burial. It has been generally assumed that this type of preservation implies little transport, but experiments suggest that live crinoids or their fresh (nondecayed) carcasses can remain fully intact even when moved by turbulent, sediment-laden flows over substantial distances (Baumiller, Reference Baumiller2003; Baumiller et al., Reference Baumiller, Gahn, Hess and Messing2008a). The Waipati specimen of R. aotearoa n. comb. is preserved with the long axes of the arms aligned parallel to the oral–aboral axis, in what has been referred to as a ‘shaving brush’ posture (Fig. 3, Supplemental Data 1, 2, 3). This may well represent feather stars’ response to trauma, as this posture has also been recognized in experiments with live specimens tumbled for over one hour in sediment-laden water (Baumiller et al., Reference Baumiller, Gahn, Hess and Messing2008a, fig. 1.1). Counterintuitively, the intact R. aotearoa n. comb. specimen may have experienced transport prior to burial, whereas the highly disarticulated feather stars described by Eagle (Reference Eagle2007, Reference Eagle2008) may have been buried in situ, though following a longer period of decay on the sediment–water interface. The existence of such a range of hydrodynamic conditions is consistent with the interpretation of the Otekaike sediments as representing an upper to mid-shelf environment occasionally subjected to currents/storms capable of producing concentrations of aligned penatulaceans (Eagle, Reference Eagle2007).
An unusual aspect of the pristinely preserved Waipati specimen of R. aotearoa n. comb. is its close association with fragments of calcitic bivalves and echinoid tests. Whereas the presence of both larger and smaller fragments entombed within the matrix between the arms indicates a lack of sorting, the long axes of these fragments are aligned parallel to the long axes of the crinoid’s arms, suggesting some process of preferential alignment. In addition, the shell fragments have sharp, angular edges, show no evidence of encrustation, and although most are ~1 cm in their largest dimension, several are larger than 3 cm (Supplemental Data 3). The production of such angular fragments might generally be interpreted as the result of in situ compaction (Zuschin et al., Reference Zuschin, Stachowitsch and Stanton2003; Zatoń and Salamon, Reference Zatoń and Salamon2008), but that seems unlikely in this instance because: (1) most fossils show no structural deformation, and (2) judging from the shape, size, and surface detail of the angular fragments, it is evident that they represent multiple individuals with only parts of their skeletons present. It is plausible that biological agents may have been involved in producing these fragments (Oji et al., Reference Oji, Ogaya and Sato2003; Cintra-Buenrostro, Reference Cintra-Buenrostro2007; Stafford et al., Reference Stafford, Chojnacki, Tyler, Schneider and Leighton2012; Salamon et al., Reference Salamon, Gorzelak, Niedźwiedzki, Trzęsiok and Baumiller2014).
Ecological and evolutionary implications
Several features of the Otekaike crinoids are worthy of note. First, the described fauna from a single locality, Ardlogie, consists of nine feather star species (Eagle Reference Eagle2007, Reference Eagle2008; this study). This is a slightly lower diversity than noted by Messing et al. (Reference Messing, Meyer, Siebeck, Jermiin, Vaney and Rouse2006), who found 12 species of feather stars living on sandy substrates between 10 and 20 m depth at Lizard Island, Great Barrier Reef, Australia. However, the latter are part of an extremely rich fauna of Lizard Island consisting of over 50 species (Hoggett and Vail cited in Messing et al., Reference Messing, Meyer, Siebeck, Jermiin, Vaney and Rouse2006) dominated by reef-dwelling feather stars, and five of the 12 Lizard Island species observed on soft substrates are also known to occur on reefs. Given that feather stars from Ardlogie likely lived in deeper water and not in vicinity of reefs, the diversity of this Oligocene locality must be considered strikingly similar to that at a comparable modern setting described by Messing et al. (Reference Messing, Meyer, Siebeck, Jermiin, Vaney and Rouse2006). This suggests that alpha diversity in this type of an environment has not changed dramatically since the Oligocene. Admittedly, this is but a single comparison from one habitat, but it does shed some light on the history of feather stars, hinting that their past diversity may be greatly underrepresented by their known fossil record.
A second noteworthy aspect of the Otekaike feather stars relates to the presence of a regenerating arm in the Waipati specimen of R. aotearoa n. comb. (Fig. 4.2). The extraordinary ability to regenerate is a characteristic of all echinoderms, and in crinoids regenerating body parts are commonly encountered in extant specimens and have been reported in numerous fossil stalked crinoids (see Oji, Reference Oji2001; Gahn and Baumiller, Reference Gahn and Baumiller2010 for reviews). However, regeneration has not previously been recorded in a fossil feather star. This may seem surprising given that observations of living populations reveal extremely high regeneration frequencies, sometimes with all individuals regenerating one or more arms (Mladenov, Reference Mladenov1983; Meyer, Reference Meyer1985; Schneider, Reference Schneider1988; Nichols, Reference Nichols1994; Oji, Reference Oji1996; Baumiller, Reference Baumiller2013a). However, as was previously discussed, fossils of intact feather stars are so rare that opportunities for recognizing regeneration are very few. Yet data on regeneration are crucial for evaluating hypotheses related to functional morphology and evolutionary history of crinoids. For example, today’s success of feather stars relative to stalked crinoids in terms of their bathymetric distribution, abundance, and diversity has often been linked to the various ways in which they have been able to cope with interactions with other organisms (Meyer and Macurda, Reference Meyer and Macurda1977; Messing, Reference Messing1997; Gorzelak et al., Reference Gorzelak, Salamon and Baumiller2012). Several in situ observations have confirmed that such interactions do occur in the Recent (Magnus, Reference Magnus1963; Fishelson, Reference Fishelson1972, Reference Fishelson1974; Conan et al., Reference Conan, Roux and Sibuet1981; Meyer and Ausich, Reference Meyer and Ausich1983; Meyer et al., Reference Meyer, LaHaye, Holland, Arenson and Strickler1984; Vail, Reference Vail1987; Messing et al., Reference Messing, RoseSmyth, Mailer and Miller1988; Baumiller et al., Reference Baumiller, LaBarbera and Woodley1991; Nichols, Reference Nichols1994; Baumiller et al., Reference Baumiller, Mooi and Messing2008b; Bowden et al., Reference Bowden, Schiaparelli, Clark and Rickard2011; Stevenson et al., Reference Stevenson, Gahn, Baumiller and Sevastopulo2017), often involving sublethal damage to the crinoid, including injuries to arms and/or pinnules. Consequently, regenerating arms have been assumed to represent repair of injuries and used as a proxy for antagonistic interactions (Meyer, Reference Meyer1985; Schneider, Reference Schneider1988; Oji, Reference Oji1996; Aronson et al., Reference Aronson, Blake and Oji1997; Baumiller and Gahn, Reference Baumiller and Gahn2003, Reference Baumiller and Gahn2004, Reference Baumiller and Gahn2013; Gahn and Baumiller, Reference Gahn and Baumiller2005; Baumiller, Reference Baumiller2013b). Using this logic, the regenerating arm in the Waipati specimen of R. aotearoa n. comb. not only provides the first fossil example of this phenomenon in a feather star, but supports the claim that they suffered sublethal predation in the geologic past.
Another interesting feature of the intact specimen of R. aotearoa n. comb. from Waipati relates to the position of the regenerating arm, more specifically to the articulation between the distal brachial of the remaining portion of the original arm (IIIBr1) and the proximal brachial of the regenerating portion (IIIBr2). This articulation, IIIBr1–2, is a syzygy, an articulation type that is generally thought to facilitate the shedding (autotomy) and regrowth of arms in some crinoids, including feather stars (Wilkie, Reference Wilkie2001). In extant crinoids, autotomy at syzygies can occur in response to mechanical stimulation; in addition, syzygies are almost always the site of arm regeneration (Holland and Grimmer, Reference Holland and Grimmer1981; Mladenov, Reference Mladenov1983; Oji and Okamoto, Reference Oji and Okamoto1994). Drawing on these observations, Oji and Okamoto (Reference Oji and Okamoto1994) presented a compelling argument that the function of syzygies is to reduce damage and mortality from predators, and presence and specific placement of syzygies have been linked to the success of feather stars in handling predation pressure (Oji and Okamoto, Reference Oji and Okamoto1994; Baumiller, Reference Baumiller1997, Reference Baumiller2008). So whereas syzygies have been documented in fossil feather stars because of their distinct morphology, data on their placement, which is taxon specific and highly predictable (Hess and Messing, Reference Hess and Messing2011), requires intact specimens, and confirming the autotomy function necessitates a regenerating arm. In this regard, the intact specimen of R. aotearoa n. comb. is of special importance as it: (1) provides data on the position of syzygies in Conometridae, a family with no extant representatives, and (2) confirms syzygial autotomy function in a Paleogene feather star.
Acknowledgments
We are grateful to the following for their assistance with museum specimens: N. Hudson, University of Auckland; C. del Rio, Museo Argentino de Ciencias Naturales; J. Simes and M. Terezow, Geological and Nuclear Sciences; the late A. Grebneff, University of Otago. We thank the following for help with Māori names: M. Brunton, K. Cassidy, and the University of Otago Office of Māori Affairs, and T. King and R. Donaldson, manager of Waihao Marae. Foveran Station and the Harvey family are thanked for access to localities. We thank M. Eleaume for help with translating de Loriol, M. Griffin for help in locating specimens of Cypelometra inhering, M. Lynch, A. Rountrey, J. Pang, J. Saulsbury for assistance with CT scan and 3D rendering, and two anonymous reviewers for comments and suggestions. Figures by C. Abraczinskas. Figure 4 by C. Abraczinskas and B. Miljour. T.K.B. was supported in part by the US and New Zealand Fulbright during his sabbatical at the University of Otago. This work was partially funded by grants to T.K.B. from the National Science Foundation (EAR 0824793; DEB 1036260) and the National Geographic Society (NGS 8505-08), and to R.E.F. from the National Geographic Society (NGS 4846-92).
Accessibility of supplemental data
Data available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.58j041n