Introduction
Necrotising otitis externa is an invasive infectious process of the external auditory canal that may extend into the skull base.Reference Peled, El-Seid, Bahat-Dinur, Tzvi-Ran, Kraus and Kaplan1–Reference Peled, Kraus and Kaplan3 Skull base osteomyelitis is a rare but serious and life-threatening complication that is mostly secondary to necrotising otitis externa. The mortality rate of skull base osteomyelitis is 33 per cent, reaching up to 80 per cent in patients with cranial nerve involvement.Reference Hatch, Bauschard, Nguyen, Lambert, Meyer and McRackan4–Reference Kornilenko, Rocka, Balseris and Arechvo8 The management for patients with skull base osteomyelitis is different from that for patients with necrotising otitis externa without skull base involvement. Surgery may be used in the treatment of skull base osteomyelitis, especially when there is cranial nerve involvement.Reference Le Clerc, Verillaud, Duet, Guichard, Herman and Kania7–Reference Chen, Yeh, Shiao and Tu9
Imaging is used for the assessment of skull base osteomyelitis, but the results overlapping with imaging findings for necrotising otitis externa.Reference van Kroonenburgh, van der Meer, Bothof, van Tilburg, van Tongeren and Postma10–Reference Razek and Huang12 Routine magnetic resonance imaging (MRI) and isotope studies are used for the prediction of skull base osteomyelitis, but their results are non-specific.Reference Goh, Karandikar, Loke and Tan13–Reference Rozenblum-Beddok, Verillaud, Paycha, Vironneau, Abulizi and Benada15 Diffusion-weighted imaging offers better characterisation of the soft tissues and their physiological processes because it reflects the random motion of the water protons, which is disturbed by the intracellular organelles and macromolecules located within the tissues.Reference Abdel Razek and Kamal16,Reference Abdel Razek, Mossad and Ghonim17 Diffusion-weighted imaging is commonly used for the assessment of head and neck cancer,Reference Abdel Razek, Mossad and Ghonim17,Reference Connolly and Srinivasan18 soft tissue infection,Reference Abd-El Khalek Abd-ALRazek and Fahmy19,Reference Schmid-Tannwald, Schmid-Tannwald, Morelli, Albert, Braunagel and Trumm20 and bone infection with osteomyelitis.Reference Ozgen, Oguz and Cila21–Reference Abdel Razek and Samir23 It is commonly used in the assessment of middle-ear cholesteatoma and recently in the characterisation of external-ear masses.Reference Lingam and Bassett24,Reference Razek25
Only one study has discussed the role of diffusion-weighted imaging in the follow up of patients with necrotising otitis externa.Reference Cherko, Nash, Singh and Lingam26 To our knowledge, no previous study in the English literature has investigated the role of diffusion-weighted imaging for predicting skull base osteomyelitis in necrotising otitis externa patients. This work aimed to predict skull base osteomyelitis in patients with necrotising otitis externa using diffusion-weighted imaging.
Materials and methods
Patients
This study was approved by the ethics board; the need for patients’ informed consent was waived given that this is a retrospective study. A retrospective analysis was conducted of the magnetic resonance images of patients with necrotising otitis externa. The inclusion criteria were patients with necrotising otitis externa who underwent diffusion-weighted imaging. We excluded only one patient from the study, because of motion artefacts.
A total of 24 patients were included in this study (18 males and 6 females), with a mean age of 53 years (age range, 44–62 years). These patients presented with: external-ear swelling (n = 24), headache (n = 24), otalgia (n = 24), otorrhoea (n = 19), trismus (n = 18) and cranial nerve palsy (n = 8). All patients were diabetic and the final diagnosis was confirmed via biopsy. Patients were classified according to skull base involvement and spilt into two groups accordingly: necrotising otitis externa without skull base involvement (n = 14), or necrotising otitis externa with skull base osteomyelitis (n = 10).
Routine magnetic resonance imaging
Magnetic resonance imaging of the petrous bone was performed on a 1.5 Tesla Symphony scanner (Siemens, Munich, Germany) using a head coil with a circular polarised surface. All patients underwent axial T1-weighted imaging (repetition time = 800 ms, echo time = 15 ms) and axial T2-weighted imaging (repetition time = 800 ms, echo time = 20 ms), with section thickness of 3 mm, an interslice gap of 1–2 mm, and field of view of 25 × 20 cm. Axial, contrast-enhanced, T1-weighted images (repetition time = 800 ms, echo time = 15 ms) were obtained after an intravenous bolus injection of gadopentetate gadolinium (0.1 ml/kg of body weight).
Diffusion-weighted imaging
A single-shot echo-planar imaging diffusion-weighted imaging (‘EPI-DWI’) sequence was used prior to contrast medium injection with b-values of 0, 500 and 1000 s/mm2. The imaging parameters were: repetition time = 1000 ms, echo time = 108 ms, field of view = 25 × 20 cm and section thickness = 5 mm, with an interslice gap of 1–2 mm. The apparent diffusion coefficient map was automatically constructed.
Image analysis
Image analysis was performed by two radiologists (AA and WM) with 30 and 11 years’ respective experience in head and neck imaging. They independently calculated the apparent diffusion coefficient of the skull base lesion; the radiologists were blinded to the patient data and the final diagnosis. A region of interest was drawn on the apparent diffusion coefficient map using an electronic cursor at the skull base lesion, and the apparent diffusion coefficient value of the lesion was calculated.
Statistical analysis
The data were described in terms of mean and standard deviation values. The data were analysed to determine statistically significant differences. The student's t-test was used to compare the two groups (necrotising otitis externa patients with or without skull base involvement). The p-value was considered significant if 0.05 or lower. Receiver operating characteristic curves were used to determine the optimal threshold apparent diffusion coefficient for predicting skull base osteomyelitis in patients with necrotising otitis externa. The data were statistically analysed using SPSS software version 22 (SPSS, Chicago, Illinois, USA).
Results
All patients with suspected skull base osteomyelitis presented with otalgia and otorrhoea; 34 per cent had cranial nerve palsies and 17 per cent had hearing loss. Culture was positive for Pseudomonas aeruginosa in 12 patients (50 per cent). Staphylococcus aureus was the causative organism in 8 patients (34 per cent). Fungal infection was found in two patients. The causative organism was difficult to detect in two patients in light of antibiotics given prior to MRI.
The mean apparent diffusion coefficient of the skull base in patients with skull base osteomyelitis (n = 10) (Figure 1) was 0.851 ± 0.15 ×10-3mm2/s (range, 0.72–1.23 ×10-3mm2/s) for the first reviewer and 0.841 ± 0.14 ×10-3mm2/s (range, 0.74–1.20) for the second reviewer (Table 1). The mean apparent diffusion coefficient of the skull base in patients with necrotising otitis externa without skull base involvement (n = 14) (Figure 2) was 1.065 ± 0.19 ×10-3mm2/s (range, 0.73–1.32 ×10-3mm2/s) for the first reviewer and 1.045 ± 0.20 ×10-3mm2/s (range, 0.73–1.30 ×10-3mm2/s) for the second reviewer. There was a significant difference in the apparent diffusion coefficient of the skull base between necrotising otitis externa patients with and without skull base osteomyelitis for the first reviewer (p = 0.008) and for the second reviewer (p = 0.012). Only one patient with skull base osteomyelitis was determined to have a high apparent diffusion coefficient of the skull base by both reviewers who was misdiagnosed as having an uninvolved skull base.
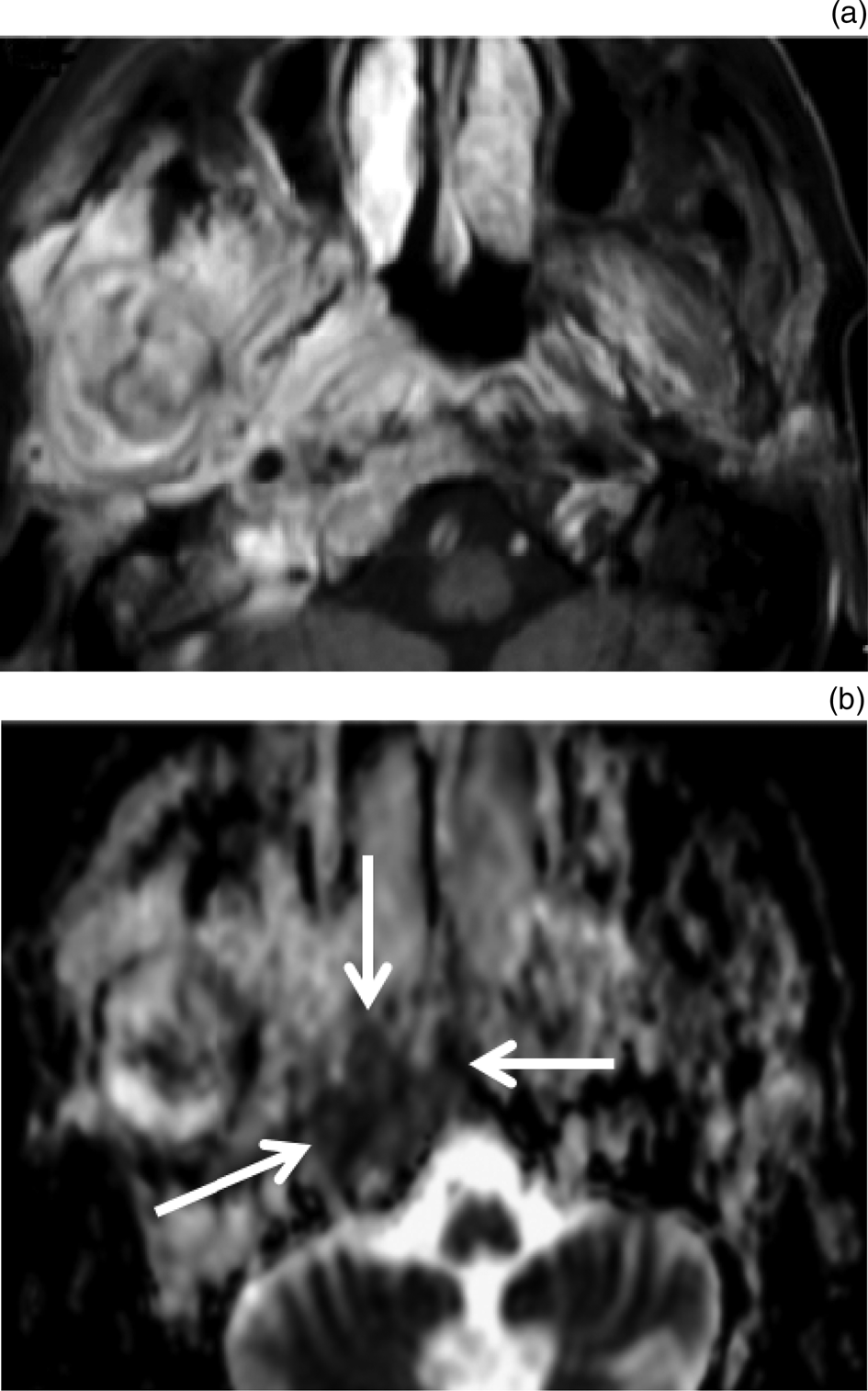
Fig. 1. Necrotising otitis externa with skull base osteomyelitis. (a) Axial, contrast-enhanced magnetic resonance imaging scan shows diffuse enhancing lesion infiltrating the right masticator space in a patient with necrotising otitis externa. The lesion extends into the skull base bone marrow on the right side. Note abnormal marrow signal intensity of the ramus of the right mandible, denoting osteomyelitis of the mandible. (b) Apparent diffusion coefficient map shows low apparent diffusion coefficient values for the bony lesion of the skull base (arrows), with calculated apparent diffusion coefficient values of 0.83 and 0.79 ×10-3mm2/s for each reviewer respectively.

Fig. 2. Necrotising otitis externa without skull base involvement. (a) Axial, contrast-enhanced magnetic resonance imaging scan shows diffuse enhancing lesion infiltrating the left masticator space in a patient with necrotising otitis externa. The lesion extends into the skull base bone marrow on the left side. (b) Apparent diffusion coefficient map shows high apparent diffusion coefficient values for the bony lesion of the skull base (arrows), with calculated apparent diffusion coefficient values of 1.21 and 1.17 ×10-3mm2/s for each reviewer respectively.
Table 1. Mean apparent diffusion coefficients of skull base in necrotising otitis externa patients with and without skull base osteomyelitis

Data represent mean (± standard deviation) apparent diffusion coefficients (10-3mm2/s), with ranges in parentheses, unless indicated otherwise. *n = 10; †n = 14
The apparent diffusion coefficients of 0.945 and 0.915 ×10-3mm2/s were optimal threshold values for predicting skull base osteomyelitis in necrotising otitis externa patients, with area under the curve values of 0.825 and 0.800, accuracy of 87.5 per cent and 83.3 per cent, sensitivity of 85.7 per cent and 90.0 per cent, specificity of 90.0 per cent and 78.6 per cent, positive predictive values of 92.3 per cent and 75.0 per cent, and negative predictive values of 81.8 per cent and 91.7 per cent, for each reviewer respectively (Table 2 and Figure 3).

Fig. 3. Receiver operating characteristic curve. The optimal threshold apparent diffusion coefficient (ADC) used to predict skull base osteomyelitis in patients with necrotising otitis externa was 0.945 ×10-3mm2/s and 0.915 ×10-3mm2/s, with an area under the curve of 0.825 and 0.800, accuracy of 87.5 per cent and 83.3 per cent, sensitivity of 85.7 per cent and 90.0 per cent, and specificity of 90.0 per cent and 78.6 per cent for each reviewer respectively.
Table 2. Receiver operating characteristic curve results of apparent diffusion coefficients for both reviewers

AUC = area under the curve; PPV = positive predictive value; NPV = negative predictive value
Discussion
The main finding in this study is that the apparent diffusion coefficient of the skull base can predict skull base osteomyelitis in patients with necrotising otitis externa. There is restricted diffusion with a low apparent diffusion coefficient of the skull base lesion in patients with skull base osteomyelitis, and unrestricted diffusion with a high apparent diffusion coefficient of the skull base in patients with necrotising otitis externa without skull base involvement, with excellent inter-observer agreement of the apparent diffusion coefficients by both reviewers.
Previous studies using computed tomography and routine MRI reported that there is a thin line of demarcation between osteitis and osteomyelitis. Differentiation between both entities is crucial because they involve different treatment methods and planning.Reference Razek and Huang12–Reference Rozenblum-Beddok, Verillaud, Paycha, Vironneau, Abulizi and Benada15 The treatment of osteitis involves antibiotics alone, whereas osteomyelitis must be treated surgically with debridement and sequestrectomy.Reference Prasad, Prasad, Kumar, Thada, Rao and Chalasani27
Previous studies applied diffusion-weighted imaging in the assessment of infection of the spine, masticator space and diabetic foot.Reference Daghighi, Poureisa, Safarpour, Behzadmehr, Fouladi and Meshkini22,Reference Abdel Razek and Samir23,Reference Abdel Razek and Nada28 Diffusion-weighted imaging has the ability to distinguish between acute and chronic inflammatory changes. Only one study investigated the role of diffusion-weighted imaging for monitoring patients with necrotising otitis externa after therapy.Reference Cherko, Nash, Singh and Lingam26 That study reported a decrease in the apparent diffusion coefficient value for necrotising otitis externa after a response to the therapy. The apparent diffusion coefficient value facilitated quantitative assessment of the disease activity; hence, this parameter can be helpful for assessing the therapeutic response in subsequent follow-up examinations.Reference Cherko, Nash, Singh and Lingam26
In this study, the mean apparent diffusion coefficient value for the skull base bone marrow in nine patients with skull base osteomyelitis was lower than that in necrotising otitis externa patients without skull base involvement, as determined by two reviewers. The difference in the apparent diffusion coefficient between both entities may be attributed to the different pathological nature of the two conditions. Skull base osteomyelitis is characterised by high cellularity (inflammation and pus cells, dead organisms), decreased diffusion space for the water protons, and, hence, subsequent restricted diffusion and lower apparent diffusion coefficient values. The lower skull base bone marrow diffusion-weighted imaging signal intensity in necrotising otitis externa may be attributed to lower cellularity and consequently higher apparent diffusion coefficient values. These pathological criteria are responsible for the significant difference in the apparent diffusion coefficient values between both entities.Reference Abd-El Khalek Abd-ALRazek and Fahmy19,Reference Abdel Razek and Samir23,Reference Abdel Razek and Nada28,Reference Razek, Sieza and Maha29
In this study, only one patient with skull base osteomyelitis was determined to have a high apparent diffusion coefficient value for the skull base that was misdiagnosed as non-involvement of the skull base. This may be attributed to the early skull base infection with bone marrow oedema of the skull base, which is associated with more fluid and unrestricted diffusion, with a high apparent diffusion coefficient value for the skull base.Reference Daghighi, Poureisa, Safarpour, Behzadmehr, Fouladi and Meshkini22,Reference Abdel Razek and Samir23
Diffusion-weighted imaging can be conducted using either echo-planar imaging or non-echo-planar imaging diffusion-weighted imaging sequences. Echo-planar imaging diffusion-weighted imaging involves scanning a large volume within a short period (1 minute) and has an adequate signal-to-noise ratio; however, it is associated with susceptibility and chemical shift artefacts, especially at air–tissue interfaces, although parallel imaging can decrease susceptibility artefacts and image blurring. Non-echo-planar imaging diffusion-weighted imaging has a long echo time, high signal-to-noise ratio and is without susceptibility artefacts or image distortion at air–tissue interfaces such as the skull base; however, it is associated with ghosting artefacts and is more sensitive to motion artefacts.Reference Razek30–Reference Soni, Gupta, Kumar, Mangla and Mangla38 Diffusion-weighted imaging of the skull base has been conducted using echo-planar imaging diffusion-weighted imagingReference Abdel Razek, Mossad and Ghonim17,Reference Guler, Ozgen, Mut, Soylemezoglu and Oguz35 and non-echo-planar imaging diffusion-weighted imaging.Reference Soni, Gupta, Kumar, Mangla and Mangla38 In our study, we employed echo-planar imaging diffusion-weighted imaging using a shorter echo time and a large receiver bandwidth, to reduce the time of dephasing and minimise subsequent signal loss.
This study has a few limitations. First, it was a retrospective study with a small number of patients. Further studies with larger numbers of patients are recommended. Second, this study applied diffusion-weighted imaging; further studies using diffusion tensor imaging combined with dynamic contrast-enhanced MRI and proton magnetic resonance spectroscopy, on a 3 Tesla scanner,Reference Razek and Nada39–Reference Allam, Abdel Razek, Ashraf and Khaled46 will improve the results. Third, this study employed echo-planar imaging diffusion-weighted imaging of the skull base, which is associated with susceptibility changes at air–tissue interfaces that lead to image distortion and artefacts. Further studies that use non-echo-planar imaging diffusion-weighted imaging are recommended, with the application of parallel imaging to decrease the artefacts of diffusion-weighted imaging at the skull base.
• The apparent diffusion coefficient of the skull base is lower in necrotising otitis externa patients with skull base osteomyelitis than in those without skull base involvement
• Apparent diffusion coefficient can be used for predicting skull base osteomyelitis in necrotising otitis externa patients
• The optimal threshold apparent diffusion coefficient values of two reviewers used to predict skull base osteomyelitis in necrotising otitis externa patients were 0.945 and 0.915 ×10-3mm2/s
Conclusion
Apparent diffusion coefficient is a non-invasive imaging parameter that can be used to predict skull base osteomyelitis in patients with necrotising otitis externa.
Competing interests
None declared







