Introduction
Protein kinase C (PKC) is a multigene family of Ser/Thr kinases that is central to many signal transduction pathways (Hug & Sarre, Reference Hug and Sarre1993). The family is composed of 11 different isoforms that are subdivided into three groups based on sequence homology, as well as on activator and cofactor requirements. These groups include the conventional (PKCα, βI, βII and γ), novel (PKCδ, ϵ, θ, μ and η), and atypical (PKCλ/τ and ζ) isoforms (Knopf et al., Reference Knopf, Lee, Sultzman, Kriz, Loomis, Hewick and Bell1986; Osada et al., Reference Osada, Mizuno, Saido, Akita, Suzuki, Kuroki and Ohno1990; Selbie et al., Reference Selbie, Schmitz-Peiffer, Sheng and Biden1993). Several studies have shown that mammalian oocytes are well equipped for PKC signalling and isoforms of all three subfamilies of PKCs have been identified at protein level in mouse (Luria et al., Reference Luria, Tennenbaum, Sun, Rubinstein and Breitbart2000; Quan et al., Reference Quan, Fan, Meng, Huo, Chen, Schatten, Yang and Sun2003), rat (Raz et al., Reference Raz, Eliyahu, Yesodi and Shalgi1998), pig (Fan et al., Reference Fan, Tong, Li, Lian, Chen, Schatten and Sun2002) and human (Wu et al., Reference Wu, Zhang, Li, Cheng, Kuai, Wang and Guo2006) oocytes. PKC isoforms were found to exist in mouse oocytes and early embryos, and their subcellular localization was in a stage-dependent fashion during oocyte maturation, activation and early embryonic mitosis (Gangeswaran & Jones, Reference Gangeswaran and Jones1997; Luria et al. Reference Luria, Tennenbaum, Sun, Rubinstein and Breitbart2000; Pauken & Capco, Reference Pauken and Capco2000; Dehghani & Hahnel, Reference Dehghani and Hahnel2005). Numerous investigators have proposed that PKC is involved in many biological processes during mouse oocyte meiosis, fertilization and early embryonic mitosis, including spindle organization and stabilization, polar-body extrusion, cortical granule exocytosis, oocyte activation, completion of the second meiosis and initiation of the first mitosis, nuclear remodeling, embryo compaction, and blastocyst formation as well (Luria et al., Reference Luria, Tennenbaum, Sun, Rubinstein and Breitbart2000; Viveiros et al., Reference Viveiros, Hirao and Eppig2001, Reference Viveiros, O'Brien and Eppig2004; Fan et al., Reference Fan, Tong, Li, Lian, Chen, Schatten and Sun2002; Avazeri et al., Reference Avazeri, Courtot and Lefevre2004; Page Baluch et al., Reference Page Baluch, Koeneman, Hatch, McGaughey and Capco2004; Dehghani & Hahnel, Reference Dehghani and Hahnel2005).
Advanced maternal age in mammals is associated with reduced fertility (Hassold & Chiu, Reference Hassold and Chiu1985). The cause of this decline of fertility in older mammals has been the subject of many studies. Maternal age has been shown to affect oocyte quality and early development (Navot et al., 1991; Battaglia et al., Reference Battaglia, Goodwin, Klein and Soules1996). Our previous study suggested that oocytes from 12-month-old mice showed a significantly higher rate of chromosome misalignment and premature chromatids than that from 6–8-week-old mice (Cui et al., Reference Cui, Huang and Sun2005a). However, it is unknown whether PKC signalling is affected during ageing. Nuclear transfer is a useful technique for studying nuclear–cytoplasmic interaction in mammalian oocytes during meiotic maturation (Sun & Moor, Reference Sun and Moor1991). It has been proposed that transplanting a germinal vesicle (GV) from an aged woman's oocyte into a younger ooplasm might be a way to reduce the incidence of oocyte aneuploidy (Takeuchi et al., Reference Takeuchi, Ergün, Huang, Rosenwaks and Palermo1999; Zhang et al., Reference Zhang, Wang, Krey, Liu, Meng, Blaszczyk, Adler and Grifo1999; Palermo et al., Reference Palermo, Takeuchi and Rosenwaks2002). By GV transfer, we have found that the ooplasm from young mice could not rescue ageing-associated chromosome misalignment in meiosis of GV from aged mice (Cui et al., Reference Cui, Huang and Sun2005a). However, it is not clear whether GV transfer impairs PKC signal transduction process, and PKC in GV or the cytoplasm from young and aged oocytes affects maturation and development of the reconstructed oocytes. The nucleus and the cytoplasm have complementary roles in determining outcome of mammalian oocyte maturation and embryonic development (Fulka et al., Reference Fulka, First and Moor1998). Kárníková et al. (Reference Kárníková, Urban, Moor and Fulka1998) found that the decrease of cytoplasmic volume influenced the time course of GV breakdown (GVBD) and the ability of oocytes to extrude the first polar body. Our previous results suggested that nucleocytoplasmic ratio is essential for normal meiotic spindle formation, chromosome alignment and development to 2-cell stage (Cui et al., Reference Cui, Huang and Sun2005b). However, little is known about the effect of modified nucleocytoplasmic ratio of GV oocytes on PKC activity during oocyte maturation and subsequent developments.
In this study, reconstructed GV oocytes were created by micromanipulation and electrofusion; GV oocytes with modified nucleoplasmic ratio were created by removing different amount of cytoplasm. We then analyzed and compared the subcellular distribution of PKCα in MII oocytes, pronuclear and 2-cell embryos matured and developed from the oocytes of young and old mice, from the reconstructed GV oocytes and from the oocytes with modified nucleoplasmic ratio. Our experiments showed that age, GV transfer and modified nucleocytoplasmic ratio did not affect distribution of PKCα during mouse oocyte maturation, activation, and early embryonic mitosis.
Materials and methods
Animals
Kunming (KM) mice at 6–8 weeks of age were purchased from the Institute of Laboratory Animal Science, Chinese Academy of Medical Science of PLA, and breed up to 12 months. The mice were maintained under a 12-h light (06:00–18:00 h) and 12-h dark photoperiod with room temperature between 21°C and 23°C, relative humidity of 50 ± 5% and free access to water and food. Animal care and handling were conducted in accordance with policies on the care and use of animals promulgated by the ethical committee of the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences.
Collection of GV oocytes
Female KM mice of various ages were superovulated by a single i.p. injection with 5 IU pregnant mare serum gonadotrophin (PMSG; Sigma). Immature GV oocytes were collected by puncturing the ovarian follicles at 44–48 h post injection and attached cumulus cells were dissociated by repeated pipetting. Germinal vesicle oocytes were cultured in human tubule fluid (HTF) medium (Irvine Scientific) supplemented with 10% fetal calf serum (FCS; HyClone) and 50 μg/ml 3-isobutyl-1-methylxanthine (IBMX; sigma) for 2 h to prevent spontaneous GVBD and to develop a perivitelline space.
All in vitro manipulations were carried out at 36–37°C on a heated stage.
Micromanipulation: preparation of karyoplasts and cytoplasts for GV transfer
Preparation of karyoplasts and cytoplasts for GV transfer was conducted as described by Takeuchi et al. (Reference Takeuchi, Ergün, Huang, Rosenwaks and Palermo1999) and Liu et al. (1999). Briefly, GV oocytes were exposed to modified HTF medium (Irvine Scientific) supplemented with 10% FCS, 50 μg/ml IBMX and 7.5 μg/ml cytochalasin B (Sigma) for 30 min at room temperature before micromanipulation. Following lancing of the zona pellucida with a sharp-tripped pipette, the GV was gently aspirated into a cylindrical micropipette with an inner diameter of 20 μm. Each GV was surrounded by a small amount of cytoplasm (karyoplasts), and appeared to be encapsulated by a membrane. Cytoplasts were obtained by enucleating GV oocytes with the same procedure. Karyoplasts were transferred individually into the perivitelline space of the previously prepared cytoplasts by microinjection, and the obtained GV-cytoplast complexes were incubated for 30 min in M2 medium (Sigma) at 37°C prior to electrofusion.
Electrofusion of GV-cytoplast complexes
An Electro Cell Manipulator (BTX 200, BTX Inc.) was used for the fusion. Each GV-cytoplast complex was placed in M2 medium (fusion medium) between two platinum electrodes of a fusion chamber. The complex was manually aligned, and then fused with a direct current (DC) electrical pulse of 160 V/cm for 90 μs. The incorporation of GV into the cytoplast was monitored 30 min later.
Micromanipulation and electrofusion were used to create the following three groups of reconstructed oocytes: (i) GV from oocytes of 6–8-week-old mice – cytoplast from oocytes of 6–8-week-old mice (6W GV–6W cytoplast); (ii) GV from oocytes of 6–8-week-old mice – cytoplast from oocytes of 12-month-old mice (6W GV–12M cytoplast); and (iii) GV from oocytes of 12-month-old mice – cytoplast from oocytes of 6–8-week-old mice (12M GV–6W cytoplast).
Micromanipulation: preparation of GV oocytes with modified nucleocytoplasmic ratio
Germinal vesicle oocytes from 6–8-week-old mice were incubated in modified HTF medium supplemented with 10% FCS, 50 μg/ml IBMX and 7.5 μg/ml cytochalasin B for 30 min at room temperature before micromanipulation. Following lancing of the zona pellucida with a sharp-tripped pipette, one-half, or two-thirds of the cytoplasm was removed by a cylindrical micropipette with an inner diameter of 20 μm to prepare GV oocytes with one-half or one-third of the original oocyte volume (Cui et al., Reference Cui, Huang and Sun2005b). The oocytes with modified nucleocytoplasmic ratio were washed five times in modified HTF medium and then cultured in HTF medium with 10% FCS at 37°C 5% CO2.
In vitro maturation, artificial activation and in vitro fertilization (IVF)
Maturation of the GV oocytes, the GV transferred oocytes and the GV oocytes with modified nucleocytoplasmic ratio was evaluated after 16–18 h culture in vitro in HTF medium with 10% FCS at 37°C, 5% CO2. The oocytes displaying a polar body were selected for further experiments.
Matured oocytes were activated artificially as described by Hagemann et al. (1995). The oocytes were placed in phosphate-buffered saline (PBS) containing 3 μm A23187 (Sigma) for 5 min at room temperature, washed three times in modified HTF medium, and then cultured in HTF medium supplemented with 10% FCS and 7 μg/ml cycloheximide (Sigma) for 6–7 h. The oocytes were then cultured in vitro in HTF medium with 10% FCS at 37°C, 5% CO2, and monitored 4 h later for activation as indicated by the presence of a female pronucleus and 24 h later for 2-cell embryos.
Matured oocytes were fertilized in vitro as described by Hogan et al. (1986). Spermatozoa were collected from the cauda epididymides of male mice and capacitated in IVF medium containing 15 mg/ml BSA (Sigma) for 1.5 h. The oocytes were incubated with the spermatozoa in IVF medium with 15 mg/ml BSA for 6 h, and then transferred to HTF medium with 10% FCS at 37°C, 5% CO2 for in vitro culture. The activation was identified by the presence of pronuclei and the 2-cell embryos were monitored after 6 h and 24 h culture, respectively.
Immunocytochemistry
Germinal vesicle oocytes, MII oocytes, pronuclear embryos and 2-cell embryos were selected for the immunocytochemistry study of PKCα. The oocytes and the embryos were fixed in 3.7% paraformaldehyde (Sigma) in PBS for 40 min, and then permeabilized in PBS containing 0.1% Triton X-100 (Sigma) for 30 min at room temperature. They were subsequently washed for 1 h in PBS containing 5% BSA. Afterwards, the oocytes and the embryos were incubated with rabbit polyclonal antibody against PKCα (1:150; Santa Cruz) overnight at 4°C, washed, and then incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (1:200; Sigma) at room temperature for 2 h. During one of the final washing steps, 5 μg/ml Hoechst 33258 (Sigma) in PBS was added to localize chromosome or nucleus.
Laser scanning confocal microscope was used to obtain the FITC localization patterns using a Nikon Labphot Microscope coupled to a Bio-Rad confocal laser. Hoechst 33258 fluorescence was obtained simultaneously, and optical sections were collected and reproduced on a SPARC workstation. Paired images were digitally reproduced to examine the co-localization of PKCα and chromosome or nucleus.
Results
Distribution of PKCα in oocytes and early embryos from 6–8-week-old and 12-month-old mice
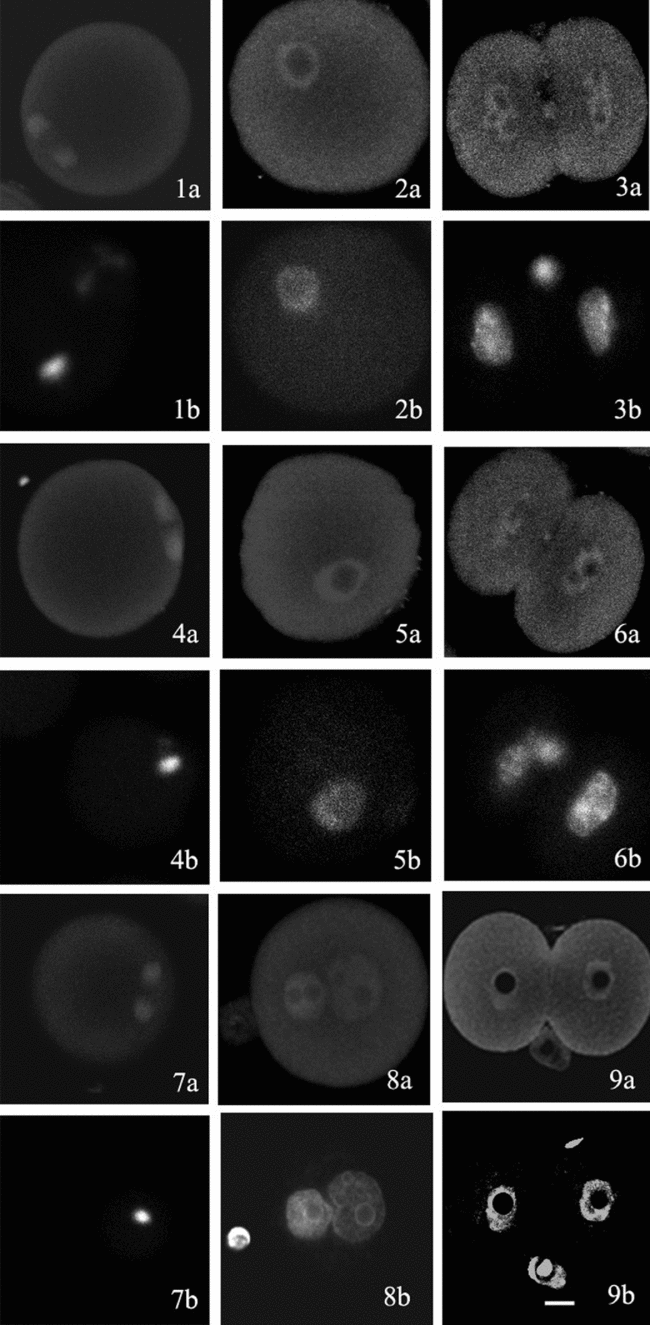
In order to monitor possible age-dependent effects on PKCα, we compared distribution of this kinase in young and old mice. Immunocytochemistry analysed by laser scanning confocal microscopy demonstrated that distribution of PKCα in oocytes and early embryos from 6–8-week-old mice is similar to that from 12-month-old mice. In the GV oocytes, PKCα was present throughout the cytoplasm and at slightly lower levels in the GV but not the nucleoli (Fig. 1.1, 1.5). In the matured oocytes, PKCα was co-located mainly with MII spindle (Fig. 1.2, 1.6). In the pronuclear embryos developed from matured oocytes after artificial activation or IVF, PKCα was concentrated in the pronuclei except for the nucleolar region, with weak staining in the cytoplasm (Fig. 1.3, 1.7). The distribution of PKCα in nuclei continued in the 2-cell embryos (Fig. 1.4, 1.8).

Figure 1 Distribution of PKCα in oocytes and early embryos from 6–8-week-old and 12-month-old mice. Figure 1.1–1.4 shows GV oocyte, MII oocyte, pronuclear embryo, and 2-cell embryo from 6–8 week-old mice, respectively. Figure 1.5–1.8 shows GV oocyte, MII oocyte, pronuclear embryo, and 2-cell embryo from 12-month-old mice, respectively. (Figure 1.1a–1.8a: PKCα stained by anti-PKCα and FITC-conjugated second antibody; Figure 1.1b–1.8b: chromosome or nucleus stained by Hoechst 33258. Bar = 20 μm.)
Distribution of PKCα in oocytes and early embryos from the GV reconstructed oocytes
In order to determine whether GV transfer affected PKCα, we evaluated its distribution in MII oocytes and early embryos from the GV reconstructed oocytes. Using micromanipulation and electrofusion, we created three groups of GV reconstructed oocytes: (i) 6W GV–6W cytoplast; (ii) 6W GV–12M cytoplast; and (iii) 12M GV–6W cytoplast. Immunocytochemistry showed that, in spite of differences in the groups of GV-reconstructed oocytes, the distribution of PKCα in the MII oocytes and in early embryos matured and developed from these reconstructed oocytes was similar to the control from 6–8-week-old mice. PKCα was detected mainly in the spindle, nuclei, and nuclei in the MII oocytes, the pronuclear embryos, and the 2-cell embryos, respectively (Fig. 2.1–2.9).

Figure 2 Distribution of PKCα in oocytes and early embryos from the GV reconstructed oocytes. Figure 2.1–2.3 shows MII oocyte, pronuclear embryo, and 2-cell embryo matured and developed from 6W GV–6W cytoplast reconstructed oocytes, respectively. Figure 2.4–2.6 shows MII oocyte, pronuclear embryo, and 2-cell embryo matured and developed from 6W GV–12M cytoplast reconstructed oocytes, respectively. Figure 2.7–2.9 shows MII oocyte, pronuclear embryo, and 2-cell embryo matured and developed from 12M GV–6W cytoplast reconstructed oocytes, respectively. (Figure 2.1a–2.9a: PKCα stained by anti-PKCα and FITC-conjugated second antibody; Figure 2.1b–2.9b: chromosome or nucleus stained by Hoechst 33258. Bar = 20 μm.)
Distribution of PKCα in oocytes and early embryos from the GV oocytes with modified nucleoplasmic ratio
In order to determine whether reduction of cytoplasmic volume affected PKCα, we evaluated its distribution in MII oocytes and early embryos from the GV oocytes with modified nucleoplasmic ratio. After the GV oocytes with one-half or one-third of the original oocyte volume created by micromanipulation were cultured in vitro for 16–18 h, the matured oocytes that displayed a polar body were selected, some of these then developed to pronuclear and 2-cell embryos after artificial activation or IVF. As in the MII oocytes and early embryos matured and developed from the intact GV oocytes, PKCα was co-located mainly with the spindle in the MII oocytes matured from the GV oocytes with one-half or one-third of the original oocyte volume (Fig. 3.1, 3.4), and high levels of PKCα were detected in the nuclei with notably lower levels of the protein in the cytoplasm in either pronuclear or 2-cell embryos developed from the oocytes with one-half or one-third of the original oocyte volume (Fig. 3.2, 3.3, 3.5 and 3.6).

Figure 3 Distribution of PKCα in oocytes and early embryos from the GV oocytes with modified nucleoplasmic ratio. Figure 3.1–3.3 shows MII oocyte, pronuclear embryo, and 2-cell embryo matured and developed from the GV oocytes with one-half of the original oocyte volume, respectively. Figure 3.4–3.6 shows MII oocyte, pronuclear embryo, and 2-cell embryo matured and developed from the GV oocytes with one-third of the original oocyte volume, respectively. (Figure 3.1a–3.6a: PKCα stained by anti-PKCα and FITC-conjugated second antibody; Figure 3.1b–3.6b: chromosome or nucleus stained by Hoechst 33258. Bar = 20 μm.)
Discussion
Mammalian oocytes contain several different isoforms of PKC in distinct spatial patterns (Raz et al., Reference Raz, Eliyahu, Yesodi and Shalgi1998; Pauken & Capco, Reference Pauken and Capco2000). Expression of individual PKC isoforms depends on developmental stage of the cells. Different isotypes of PKC are differentially activated and are involved in different events during oocyte maturation and embryonic development (Luria et al., Reference Luria, Tennenbaum, Sun, Rubinstein and Breitbart2000; Fan et al., Reference Fan, Tong, Li, Lian, Chen, Schatten and Sun2002; Dehghani & Hahnel, Reference Dehghani and Hahnel2005).
In our experiments, PKCα was detected in GV oocytes, MII oocytes, pronuclear embryos and 2-cell embryos, and had unique distribution patterns in the nucleus and the cytoplasm. PKCα was found in the cytoplasm of GV oocytes and was localized to the meiotic spindle in MII oocytes. It was enriched in the nuclei of pronuclear and 2-cell embryos developed from the matured oocytes after artificial activation or IVF. Luria et al. (Reference Luria, Tennenbaum, Sun, Rubinstein and Breitbart2000) and Quan et al. (Reference Quan, Fan, Meng, Huo, Chen, Schatten, Yang and Sun2003) have shown PKCα distribution similar to ours in GV oocytes, pronuclear and 2-cell embryos. However, it was reported that PKCα was present only in the cytoplasm and never in the spindle of mouse MII oocytes (Luria et al., Reference Luria, Tennenbaum, Sun, Rubinstein and Breitbart2000; Quan et al., Reference Quan, Fan, Meng, Huo, Chen, Schatten, Yang and Sun2003), a finding that appeared to contradict our data. Moreover, PKCα was found to be concentrated in GV at the GV stage in human and pig (Fan et al., Reference Fan, Tong, Li, Lian, Chen, Schatten and Sun2002; Wu et al., Reference Wu, Zhang, Li, Cheng, Kuai, Wang and Guo2006). Our experiments on GV oocytes were performed at only one time point, immediately after oocyte recovery from the follicle. Avazeri et al. (Reference Avazeri, Courtot and Lefevre2004) found that at the beginning of meiosis reinitiation, PKCα was mainly distributed throughout the cytoplasm, and became progressively more concentrated in the nucleus during the progression of the oocytes to the GVBD stage of meiosis. By microinjection of isozyme-specific antibodies into GV or the cytoplasm of oocytes, Avazeri et al. (Reference Avazeri, Courtot and Lefevre2004) hypothesized that, at the beginning, the cytoplasmic cPKCs were involved in meiotic arrest, whereas later on, before GVBD, the nuclear cPKCs were responsible for meiosis resumption. Page Baluch et al. (Reference Page Baluch, Koeneman, Hatch, McGaughey and Capco2004) indicated that many isoforms of PKC were enriched around the meiotic spindle. In different mitotic cells, various PKC isoforms were found to associate with the mitotic apparatus and colocalize with β-tubulin in spindle microtubules (Battistella-Patterson et al., Reference Battistella-Patterson, Fultz, Li, Geng, Norton and Wright2000; Chen et al., Reference Chen, Purohit, Halilovic, Doxsey and Newton2004). In our study, PKCα was present in the spindle at the MII oocyte, suggesting a functional role for PKCα in spindle organization and stabilization during mouse oocyte meiosis. It has been shown that PKCs are regulators of cell proliferation and differentiation in various cell types (Wagner et al., Reference Wagner and Takemoto2001) and they exist in the nuclei of somatic cells (Garcia et al., Reference Garcia, Edwards, Brennan and Harlan2000). Thus, the localization of PKCα in nuclei of pronuclear and 2-cell embryos suggests that PKCα may be involved in regulation of nuclear organization and function in the early mouse embryos.
We focus on PKCα isoform only in this experiment, because the enzyme is one of the conventional isoforms of PKC and is probably the best characterized of PKC isoforms as some results have suggested a possible involvement of PKCα in the mechanism of mouse oocyte maturation and activation (Luria et al., Reference Luria, Tennenbaum, Sun, Rubinstein and Breitbart2000; Quan et al., Reference Quan, Fan, Meng, Huo, Chen, Schatten, Yang and Sun2003). As the subcellular localization of PKCα in mouse oocytes and embryos is developmental-stage associated, we can evaluate effect of some factors on PKCα distribution during mouse oocyte meiosis, activation, and early embryonic mitosis.
The subcellular distribution of PKCα was observed in GV oocytes, MII oocytes, pronuclear embryos and 2-cell embryos with no difference between the two groups of age. Our results about distribution of PKCα in young and old oocytes are consistent with earlier observations, in which calcium imaging showed that the two groups of oocytes exhibited a similar pattern of calcium oscillations upon stimulation with bovine sperm extracts (Cui et al., Reference Cui, Huang and Sun2005a). Maturation rate, fertilization rate and developmental capacity to 2-cell embryos were also similar in the two groups of age. However, ageing caused indeed a significantly higher rate of chromosome misalignment and premature chromatids than that of the young MII oocytes (Cui et al., Reference Cui, Huang and Sun2005a), and pronuclear formation was delayed in oocytes of old females as compared with young ones (unpublished data). Thus, we could not exclude the possibility that other PKC isoforms were impacted in female aging. Carbone & Tatone (Reference Carbone and Tatone2009) provided evidence that aging affects the correct storage and activation of some PKCs, and functional components of the machinery. There is increasing evidence that physiological and pathological aging target PKC signalling transduction pathways in somatic cells (Battaini & Pascale, Reference Battaini and Pascale2005). Age may also influence PKC by affecting anchoring proteins (Corsini et al., Reference Corsini, Racchi, Sinforiani, Lucchi, Viviani, Rovati, Govoni, Galli and Marinovich2005).
In this experiment, we created three groups of GV reconstructed oocytes between young and young or young and aged oocytes by micromanipulation and electrofusion, and observed distribution of PKCα in the MII oocytes and the early embryos matured and developed from these reconstructed oocytes. The MII oocytes and the embryos showed a similar distribution pattern to controls, suggesting that there was no effect of GV transfer on PKCα distribution, and GV or the cytoplasm from aged oocytes did not affect the distribution. We have previously demonstrated that in three groups of GV reconstructed oocytes, they showed similar a maturation rate, fertilization rate and developmental capacity to 2-cell embryos and exhibited a similar pattern of calcium oscillations upon stimulation with bovine sperm extracts (Cui et al., Reference Cui, Huang and Sun2005a).
According to Halet (Reference Halet2004), the maternal pool of PKCs is most likely synthesized in the cytoplasm during oogenesis. In this experiment, we wanted to verify whether reduction of cytoplasmic volume in the fully grown oocyte affect the distribution of PKCα. In the MII oocytes and early embryos matured and developed from the GV oocytes with one-half or one-third of the original oocyte volume, PKCα showed a similar distribution pattern as in that from the intact GV oocytes. The results suggest that reduction of cytoplasmic volume does not affect distribution of PKCα. It is well known that oocyte activation is driven by sperm-induced Ca2+ oscillations (Halet et al., Reference Halet, Tunwell, Parkinson and Carroll2004). Parthenogenetic agents, such as Sr2+, are also able to mimic sperm penetration to trigger oocyte activation and embryonic development by increasing intracellular free Ca2+ level in the oocytes (Tang et al., 1998). We have previously demonstrated that, although the oocyte volume was reduced even to a fourth of normal volume, the Sr2+-induced Ca2+ oscillation pattern was not affected compared with that of the intact oocytes and the oocytes could be activated to form pronuclei (Cui et al., Reference Cui, Huang and Sun2005b). The observation that oocytes of different sizes produced a similar Ca2+ oscillation pattern may also explain why the modified nucleocytoplasmic ratio does not affect distribution of PKCα, which can be activated by Ca2+. However, when more than half of GV oocyte cytoplasm was removed, the time course of GVBD was delayed, maturation rate and development to 2-cell stage decreased, and rate of abnormal chromosome segregation increased significantly (Cui et al., Reference Cui, Huang and Sun2005b).
Taken together, we show that age, GV transfer and modified nucleocytoplasmic ratio does not affect distribution of PKCα during mouse oocyte maturation, activation, and early embryonic mitosis. However, further investigations are necessary to identify distribution and activity of other PKC isoforms and temporal and spatial correlation of the isoforms with the specific processes to better understand their functions during mammalian meiosis and early embryonic development.
Acknowledgement
This work was supported partly by grants from the State key Basic Research Program of China (Grant No. 2007CB948101 and 2006CB944004), Knowledge Innovation Program of the Chinese Academy of Sciences (Grant No. KSCX2-YW-N-017), the National Natural Science Foundation of China (Grant No. 30430390) and the Natural Science Foundation of Shandong Province in China (Grant No. YD2008D30).