Dye-contaminated water is a widespread environmental problem that limits sustainable development. Several methods, such as adsorption, biological treatment, coagulation/flocculation, membrane filtration, degradation and dialysis (Du et al., Reference Du, Yuan, Sun, Peng, Jiang and Qiu2011; Ali et al., Reference Ali, Wang, Boukherroub, Lei and Xia2019; Zhou et al., Reference Zhou, Xiao, He, Wu, Shi and Zhong2021), have been developed for the treatment of wastewaters contaminated with dyes. Among them, adsorption technology has some remarkable advantages, such as its high efficiency, economic feasibility and ease of operation (Qin et al., Reference Qin, Wang, Zhao, Chen, Ma and Yang2016; Wu et al., Reference Wu, Ge, Wang, Chen, Li, Xuan and Li2018; Manera et al., Reference Manera, Tonello, Perondi and Godinho2019). There are many types of materials with large specific surface areas and pore volumes, such as clays, zeolites, activated carbon and polymeric materials, which are used as adsorbents to remove contaminants (Frost et al., Reference Frost, Xi and He2010; Ma et al., Reference Ma, Zhang and Li2010; Mahi et al., Reference Mahi, Khaldi, Belardja, Belmokhtar and Benyoucef2021). Clay minerals are low-cost and have superior physical and chemical properties either by themselves or after modification (Chen et al., Reference Chen, Zhong, Wu, Zhao and Yan2012; Dali Youcef et al., Reference Dali Youcef, Belaroui and López-Galindo2019; Boutaleb et al., Reference Boutaleb, Chouli, Benyoucef, Zeggai and Bachari2021). Montmorillonite (Mnt; (Na,Ca)0.33(Al1.67,Mg0.33)2[Si4O10](OH)2⋅nH2O; (Warr, Reference Warr2020), a typical clay mineral, is a phyllosilicate with a nanolayered structure. Its layered structure (~1 nm thick) is composed of two O–Si–O tetrahedral sheets sandwiching one O–Al(Mg)–O octahedral sheet (~100 nm × 100 nm, width × length), with a negative charge on the surface that has adsorption capacity for dyes (Almeida et al., Reference Almeida, Debacher, Downs, Cottet and Mello2009; Kang et al., Reference Kang, Zhao, Wang, Zhang, Chen and Yi2018) and heavy metal ions (Ahmed et al., Reference Ahmed, Forster, Clowes, Bradshaw, Myers and Zhang2013; Zhu et al., 2018). The organic modification of Mnt is an effective method for changing its surface polarity and reducing the surface energy, which improve its adsorption properties (Bertuoli et al., Reference Bertuoli, Piazza, Scienza and Zattera2014).
Metal-organic frameworks (MOFs) are typical porous functional materials (Huang & Xu, Reference Huang and Xu2019) made up of organic linkers and metal ions (or clusters). They might be used in adsorption owing to their ultra-high porosity, adjustable aperture/shape, large surface area and ease of functionalization (Xiao et al., Reference Xiao, Deng, Xue and Gao2021). Recently, MIL-53(Fe), an Fe-based MOF with significant chemical stability, chemical versatility and specific surface area and eco-friendly character (Yilmaz et al., Reference Yılmaz, Sert and Atalay2016), has been applied in catalysis, adsorption, electrochemical processes and lubrication. Modification methods, including hybridization (Jiang & Li, Reference Jiang and Li2016), loading (Jabbari et al., Reference Jabbari, Veleta, Zarei-Chaleshtori, Gardea-Torresdey and Villagrán2016) and calcination (Derakhshani et al., Reference Derakhshani, Hashamzadeh and Amini2018), were used to improve the activity of MIL-53(Fe). However, there are few reports on the preparation of clay-based MOF composites to be used as adsorbents for dye removal.
Low-cost clay minerals with superior adsorption activities might be used to prepare eco-friendly and low-priced clay-based adsorbents for the removal of harmful substances from aqueous solutions, such as dyes, heavy metal ions and surfactants (He et al., Reference He, Zhang, Wang, Li and Wang2012; Jian et al., Reference Jian, Puerto, Wehowsky, Dong, Johnston, Hirasaki and Biswal2016; Aziz et al., Reference Aziz, Salh Shwan and Kaufhold2021). In this study, based on the structural characteristics of Mnt and MIL-53(Fe), a novel composite adsorbent material (MIL-53(Fe)@Mnt) was prepared using a simple one-step solvothermal reaction in which MIL-53(Fe) was grown on the surface of Mnt particles in situ. The MIL-53(Fe)@Mnt composite obtained was used to remove methylene blue (MB), a cationic dye, from aqueous solution. The adsorption thermodynamics and kinetics were also investigated.
Materials and methods
Montmorillonite was purchased from Zhejiang Fenghong New Material Co., Ltd (P.R. China). FeCl3⋅6H2O was obtained from Tianjin Baishi Chemical Co., Ltd and 1,4-benzenedicarboxylic acid (H2BDC) was purchased from Shanghai Aladdin Reagent Co. Methylene blue of analytical grade was purchased from Tianjin Kaixin Chemical Industry Co., Ltd. Other reagents, such as N,N'-dimethylformamide (DMF) and ethanol (EtOH), were obtained commercially. All reagents were used without further purification. Distilled water was used in all experiments.
Preparation of MIL-53(Fe)
MIL-53(Fe), an Fe-based MOF, was synthesized according to Feng et al. (Reference Feng, Chen and Jiang2017). Firstly, FeCl3⋅6H2O, H2BDC and DMF in a molar ratio of 1:1:280 were stirred at room temperature until a uniform solution was formed. The solution was transferred to a Teflon-lined steel autoclave and heated at 150°C for 15 h. The product was collected by centrifugation at 8000 rpm and washed with DMF and ethanol, and the resulting yellow powder was dried under vacuum at 100°C for 10 h.
Preparation of MIL-53(Fe)@Mnt
A total of 2.00 g of Mnt was dispersed in 56 mL of DMF under stirring. Then, 0.674 g of FeCl3⋅6H2O and 0.415 g of H2BDC were added and the mixture was stirred for 1 h at room temperature. Subsequently, the mixture was transferred into a Teflon-lined stainless-steel reactor, heated at 150°C for 15 h and then cooled to room temperature. The products were collected by centrifugation at 8000 rpm and washed with DMF and ethanol. The resulting pale yellow powder was finally filtered and dried in vacuum for 10 h at 100°C, yielding MIL-53(Fe)@Mnt.
Characterization methods
The products were analysed using X-ray diffraction (XRD) with Cu-Kα radiation at 40 kV, 40 mA and a step size of 0.02° in the range of ~3–70°. Fourier-transform infrared (FTIR) spectra were recorded between 4000 and 400 cm−1 using the KBr method with an FTS-3000 spectrophotometer. The micromorphology and particle size were measured by scanning electron microscopy (SEM; ZEISS ULTRA Plus Scanning Electron Microscope, Germany). The specific surface area was determined from the adsorption isotherm using the multipoint Brunner–Emmet–Teller (BET) method and the pore-size distribution was determined from the desorption isotherm using the density functional theory (DFT) method. Thermogravimetric analysis (TGA) was performed with a Pyris Diamond thermal analyser (PerkinElmer, USA) under a nitrogen atmosphere from room temperature to 600°C at a heating rate of 10°C min−1.
Adsorption behaviour
The adsorption behaviour was studied using MB as a typical cationic dye. In 50 mL of MB solution, an amount of adsorbent was added and the mixture was shaken at 150 rpm, recording the temperature and time. Subsequently, the solutions were separated using 0.22 μm membrane filters and the concentration of MB was determined using ultraviolet–visible (UV–Vis) spectroscopy at 664 nm. The removal rate and adsorption capacity of MB were calculated from the following equations:
where C 0 and C e are the initial and time t concentration of dye (mg L−1) in the solution, respectively, V is the volume of dye solution (L) and m is the mass of MIL-53(Fe)@Mnt (g).
Results and discussion
Adsorption is a process of enrichment of chemical species from a fluid phase on the surface of a solid. In water treatment, molecules or ions are removed from the aqueous solution by adsorption onto solid surfaces. Therefore, the specific surface area and pore size are very important properties of the adsorbent. In order to increase the adsorption capacity, a novel eco-friendly clay-based adsorbent (MIL-53(Fe)@Mnt) with a large specific surface area and pore size was prepared (Scheme 1). Using Mnt as the carrier and terephthalic acid as the ligand, MIL-53(Fe) particles were synthesized and grown on the surface of the Mnt. The MIL-53(Fe)@Mnt produced not only has the advantages of Mnt (low cost, layer structure), but also has the characteristics of MIL-53(Fe) particles (environmentally friendly, large specific surface area, easy to use).
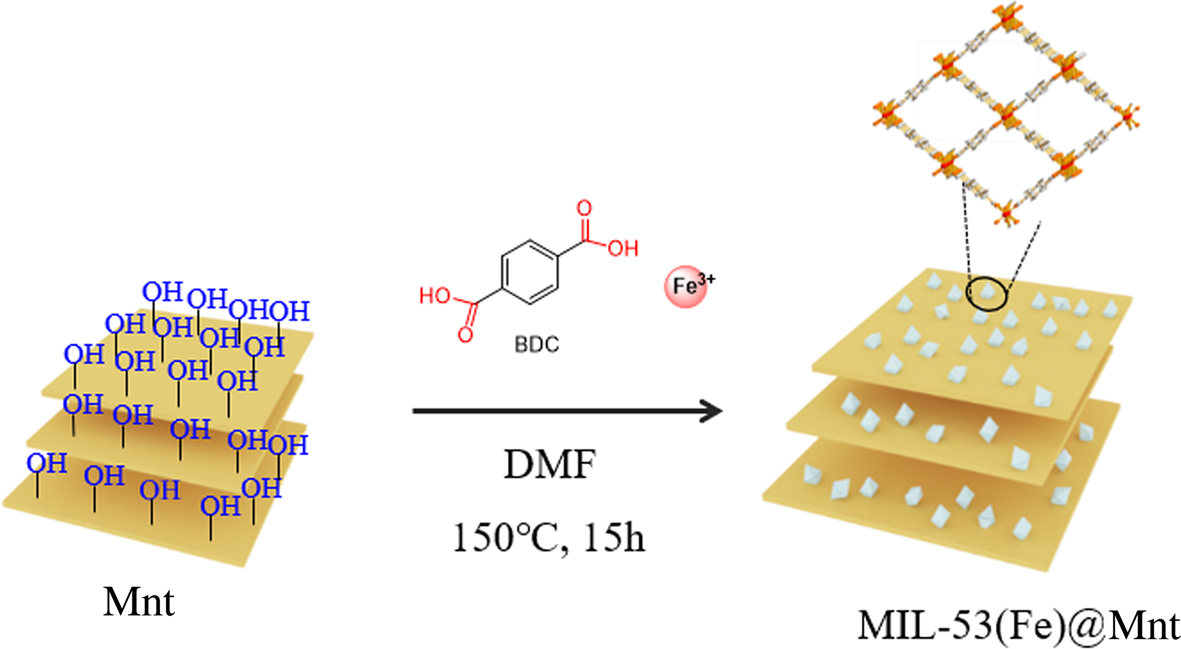
Scheme 1. Synthesis of MIL-53(Fe)@Mnt.
The micro-topography of MIL-53(Fe)@Mnt was observed using SEM and was compared with Mnt and MIL-53(Fe). As is shown in Fig. 1, Mnt presented a typical layered structure, whereas the MIL-53(Fe) formed mostly octahedral particles 400–900 nm in size. In MIL-53(Fe)@Mnt, the tiny particles of MIL-53(Fe) were successfully loaded onto Mnt using the in situ growth method. Compared with pure MIL-53(Fe), the particle size of MIL-53(Fe) in MIL-53(Fe)@Mnt was smaller and more uniform (~300 nm). Hence, Mnt significantly reduced the agglomeration of MIL-53(Fe), yielding a larger specific surface area and improving the adsorption performance. This was probably due to the hydroxyl groups (–OH) on Mnt, which were conducive to the growth of MIL-53(Fe) particles.
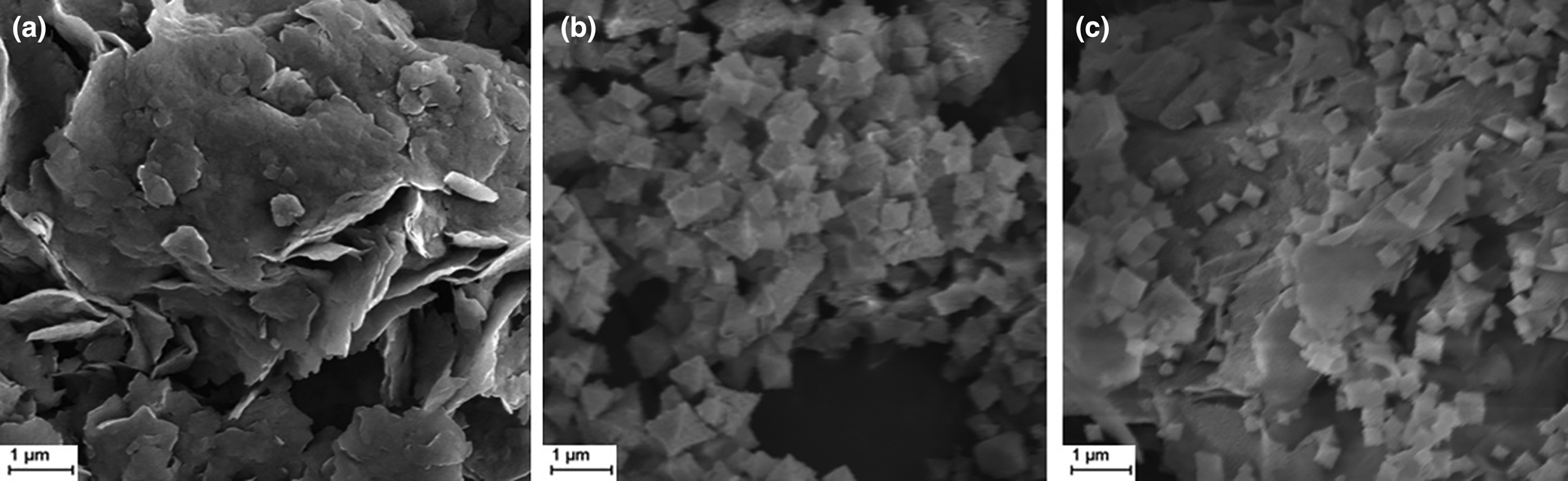
Fig. 1. SEM images of (a) Mnt, (b) MIL-53(Fe) and (c) MIL-53(Fe)@Mnt.
The FTIR spectra of the MIL-53(Fe)@Mnt, Mnt and MIL-53(Fe) are shown in Fig. 2. Characteristic bands of Fe–O (538 cm−1), C–H (745 cm−1), C–O (1380 and 1560 cm−1) and C=O (1620 cm−1) were observed in MIL-53(Fe). In Mnt, the absorption band at ~3603 cm−1 is attributed to O–H stretching, and the bands at 2914 and 2846 cm−1 are attributed to C–H. The band at 1016 cm−1 is assigned to asymmetric vibration of Si–O, and the bands at 513 and 455 cm−1 are ascribed to Al–O–Si and Si–O–Si vibrations (Wiśniewska et al., Reference Wiśniewska, Fijałkowska and Szewczuk-Karpisz2018; Umer et al., Reference Umer, Tahir, Azam and Jaffar2019). In MIL-53(Fe)@Mnt, the typical absorption bands of Mnt and MIL-53(Fe) were present. Hence, the bands at 1380 and 745 cm−1 are attributed to the carboxyl group in MIL-53(Fe) (Yang et al., Reference Yang, Xu, Liang, Lei, Wei and He2016), suggesting that MIL-53(Fe) was successfully loaded onto Mnt using the in situ growth method.
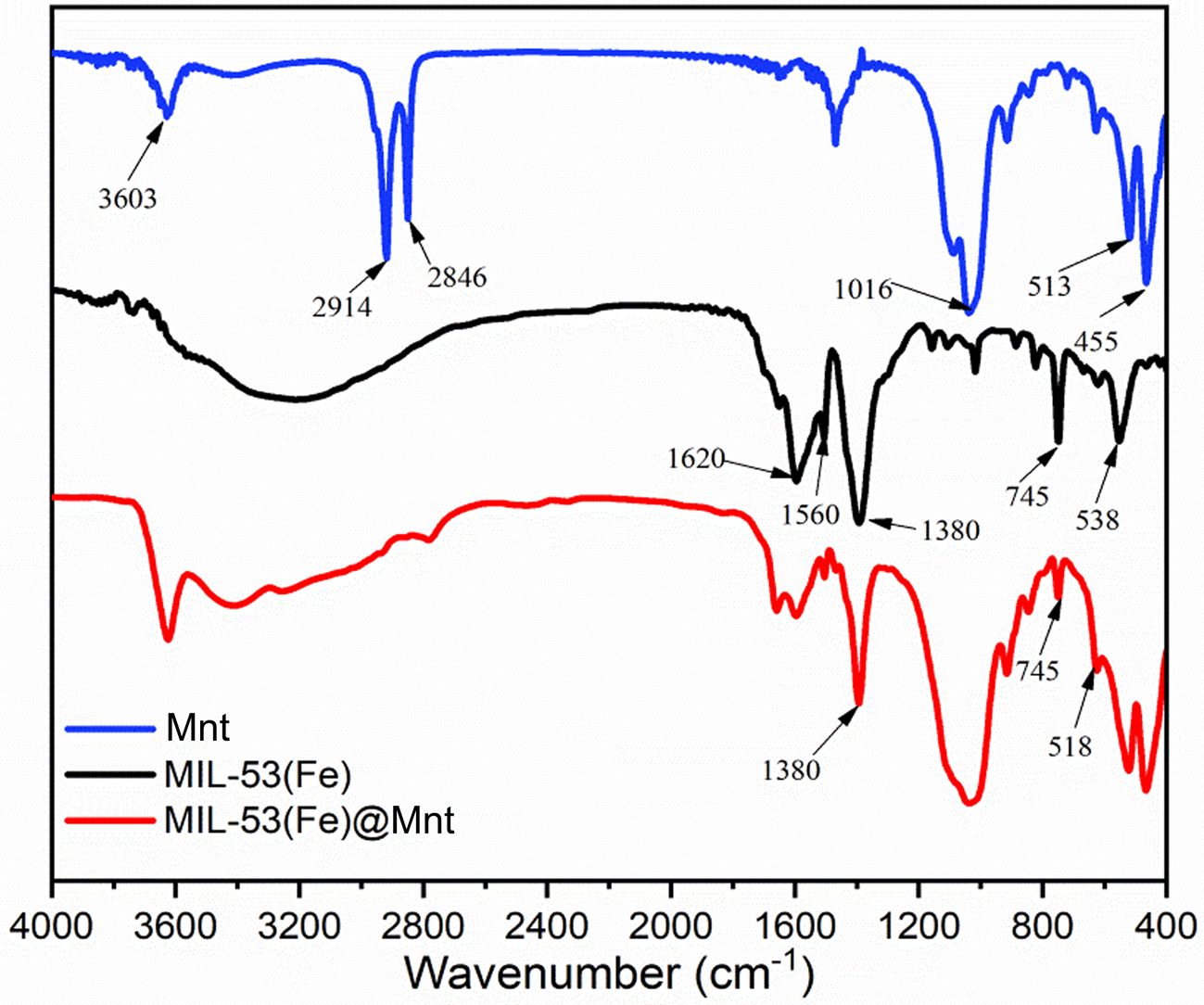
Fig. 2. FTIR spectra of Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt.
The XRD traces of MIL-53(Fe), Mnt and MIL-53(Fe)@Mnt are shown in Fig. 3. The XRD trace of the synthesized MIL-53(Fe) does not contain impurities, indicating that the synthesized MIL-53(Fe) was in a single phase (Li et al., Reference Li, Xiong, Zhang and Wu2015). The diffraction peaks of the Mnt appeared at 17.2° and 19.7°, which correspond to the (003) and (020) planes, respectively (Tong et al., Reference Tong, Wu, Adebajo, Jin, Yu, Ji and Zhou2018). The XRD trace of the synthesized MIL-53(Fe)@Mnt consisted of the diffraction peaks of the Mnt and MIL-53(Fe) structures. The introduction of Mnt weakened the diffraction peak of MIL-53(Fe). Hence, Mnt does not hinder the formation and crystallization of MIL-53(Fe).
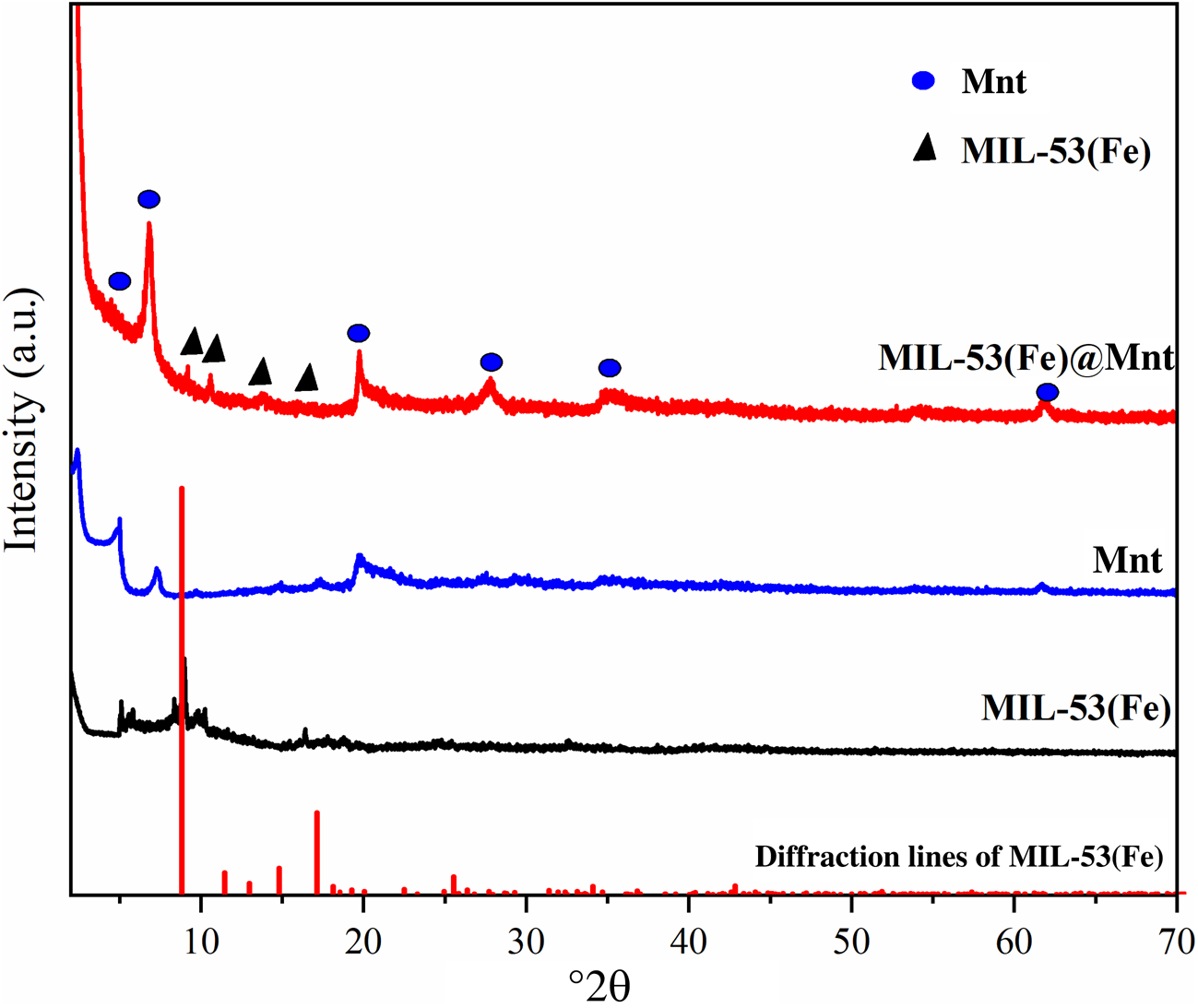
Fig. 3. XRD traces of Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt.
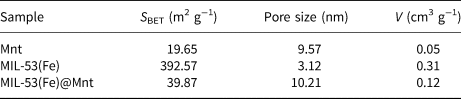
The pore-size distribution, pore volume and specific surface area of MIL-53(Fe)@Mnt and its materials (Mnt, MIL-53(Fe)) are shown in Fig. 4 and Table 1. The adsorption isotherms of MIL-53(Fe) and MIL-53(Fe)@Mnt are type IV isotherms with H3-type hysteresis loops, indicating the presence of mesoporous materials. When compared with the Mnt, the specific surface area, pore size and pore volume of MIL-53(Fe)@Mnt increased significantly. The specific surface area of MIL-53(Fe)@Mnt was 39.9 m2 g−1, which was substantially larger than that of Mnt (19.7 m2 g−1). The pore volume increased from 0.047 to 0.115 cm3 g−1 and the pore diameter increased from 9.570 to 10.210 nm. This mesoporous structure with large specific surface areas was more favourable for the adsorption of dyes.
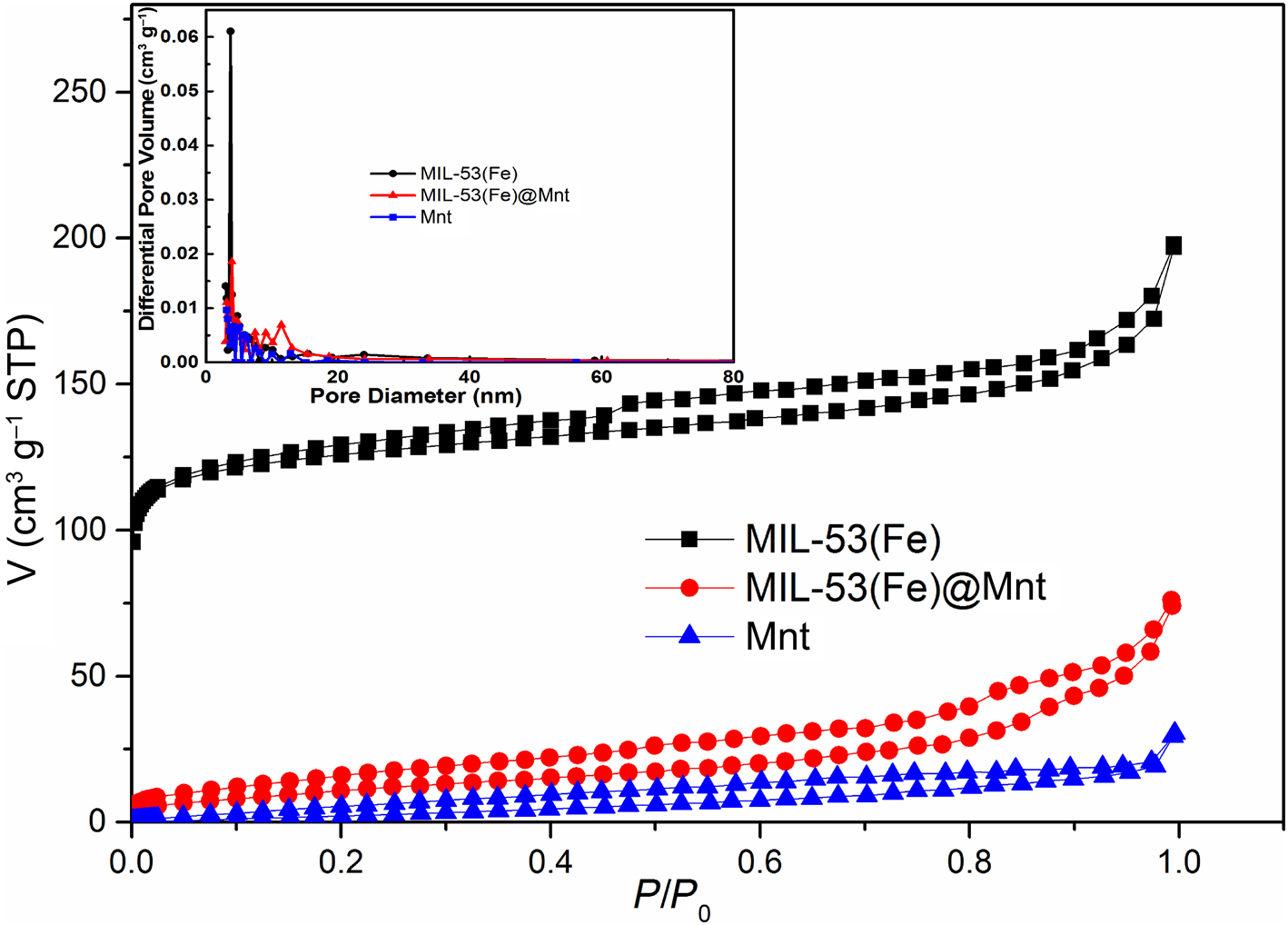
Fig. 4. N2 adsorption–desorption isotherms and pore-size distribution (inset) of Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt. STP = standard temperature and pressure.
Table 1. Specific surface area, average pore size and pore volume of the adsorbents.
The TGA curves of MIL-53(Fe), Mnt and MIL-53(Fe)@Mnt are shown in Fig. 5. MIL-53(Fe) displayed initial weight loss (16.24%) at <374°C, attributed to the loss of water and solvent molecules from the MIL-53(Fe). The second weight loss event in the 374–500°C range was due to the removal of structural organic ligands from their frameworks (Zhang et al., Reference Zhang, Wu, Wang, Li, Wu, Liang and Yang2019). At 600°C, the weight loss of MIL-53(Fe) was 58.0%, while that of MIL-53(Fe)@Mnt was 21.6%. This difference is attributed to the fact that the MIL-53(Fe)@Mnt composite was formed on a Mnt substrate, and the presence of hydroxyls on Mnt promoted the growth of the composite. It is concluded that MIL-53(Fe)@Mnt had greater thermal stability than MIL-53(Fe), mainly due to the introduction of Mnt as a substrate.
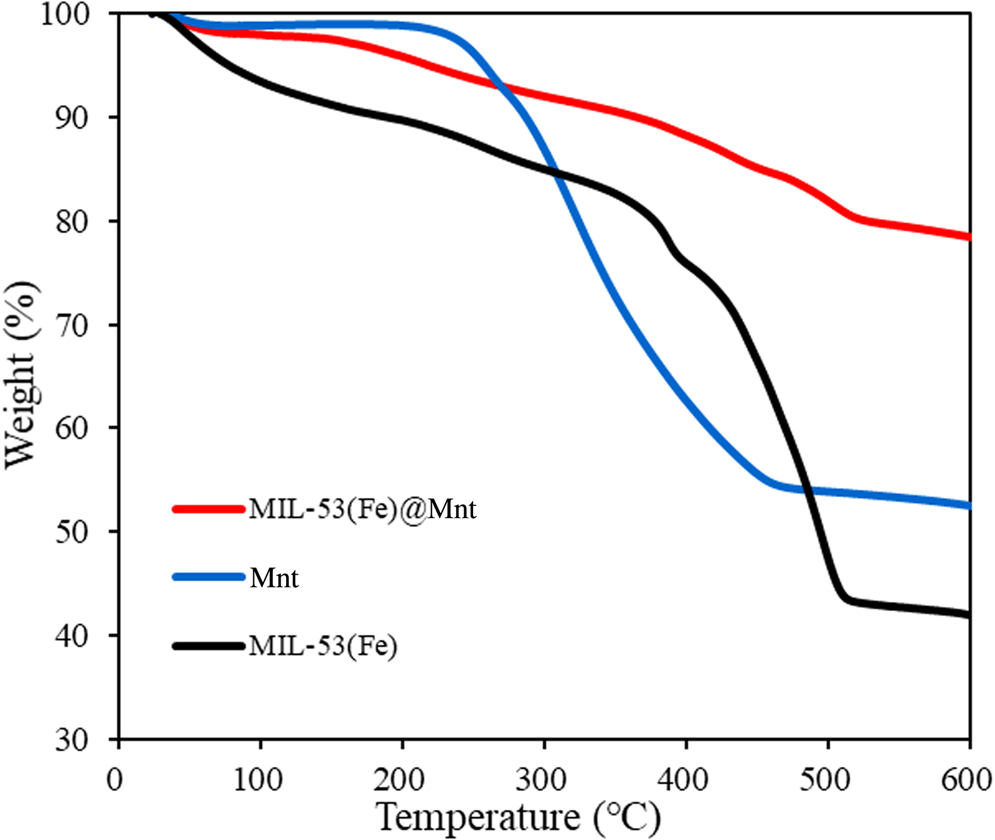
Fig. 5. TGA curves of Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt.
Adsorption behaviour of MIL-53(Fe)@Mnt
The adsorption properties of MIL-53(Fe)@Mnt were measured through the removal of some typical dyes, such as MB, malachite green (MG), crystal violet (CV) and rhodamine B (RhB). The results are shown in Fig. 6. The MIL-53(Fe)@Mnt adsorbed the dyes effectively, showing removal rates of >66.7%, with the removal rate of MB reaching 99.4%. Hence, MIL-53(Fe)@Mnt displayed an excellent adsorption capacity for MB, a cationic dye, which reached 165.7 mg g−1. Therefore, in the subsequent experiments, using MB as a model, the optimal conditions were investigated. The effects of adsorbent dosage, initial concentration of MB and pH of the MB solution on the removal of MB were investigated.
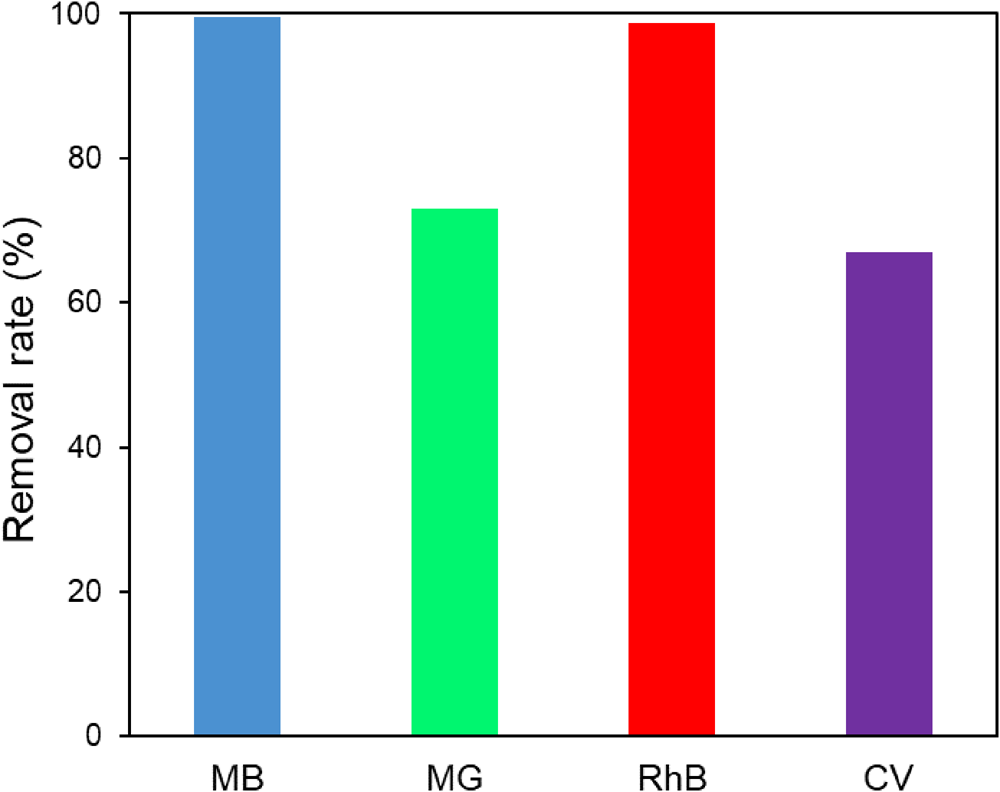
Fig. 6. Removal rate of MB, MG, RhB and CV dyes by MIL-53(Fe)@Mnt at 25°C. Conditions: adsorbent = 0.03 g; [dye] = 100 mg L−1.
The adsorption capacity of MIL-53(Fe)@Mnt was compared with that of its constituent materials (Mnt, MIL-53(Fe)), and the results are shown in Fig. 7a. The MIL-53(Fe)@Mnt displayed significantly enhanced adsorption capacity for MB compared to its constituent components. The removal of MB increased to 99.1% in 50 min, and the adsorption capacity for MB was 3.02 and 3.54 times greater than that of pure Mnt and MIL-53(Fe), respectively. The UV–Vis spectra of the adsorption of MB by MIL-53(Fe)@Mnt over time at 298 K are also given in Fig. 7b (the colour change in the aqueous MB solution is also shown Fig. 7b). The absorption intensity at λmax = 664 nm decreased with increasing contact time. The colour change showed that the residual MB in the aqueous solution was gradually reduced.
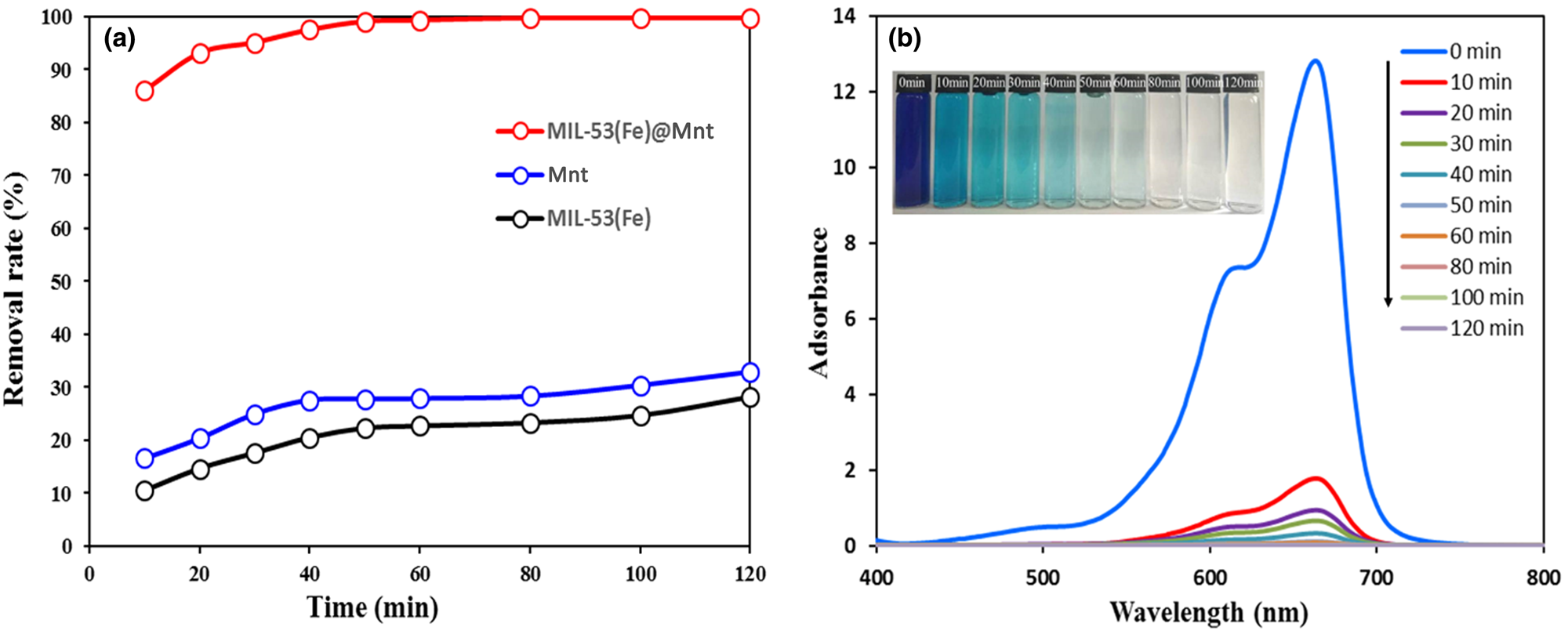
Fig. 7. (a) Removal rate of MB by Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt. (b) Time-dependent UV–Vis spectra of MB in MIL-53(Fe)@Mnt at 25°C.
In order to study the effects of Mnt content on the adsorption of MB by MIL-53(Fe)@Mnt, a series of MIL-53(Fe)@Mnt composites was synthesized by changing the dosage of Mnt from 0.5 to 3.0 g. The adsorption capacity of this series was also investigated, and the results are shown in Fig. 8. With increasing Mnt content, the adsorption first increased and then decreased. The optimum dosage of Mnt in MIL-53(Fe)@Mnt was 2.0 g.
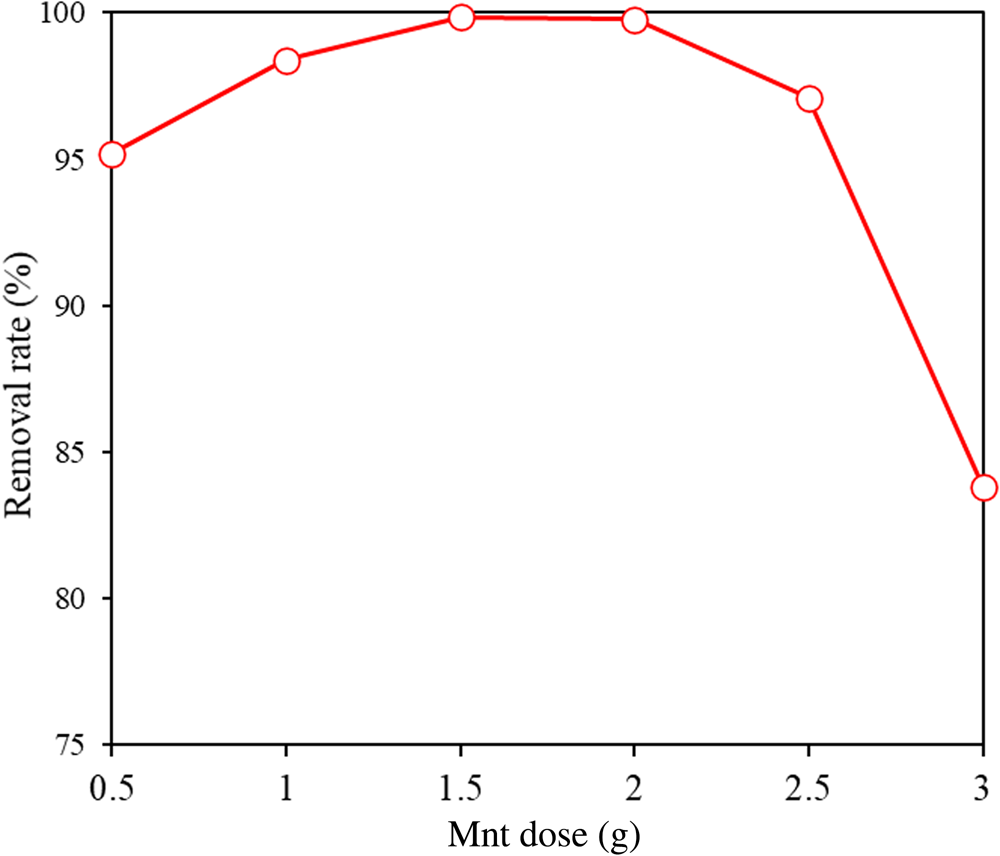
Fig. 8. Effect of Mnt content on the MB removal rate in MIL-53(Fe)@Mnt at 25°C. Conditions: adsorbent = 0.035 g; [MB] = 200 mg L−1.
The effects of initial MB concentration on the adsorption of MB are shown in Fig. 9a. The adsorption capacity of MIL-53(Fe)@Mnt for MB was enhanced with increasing dye concentrations. In addition, the removal of MB first increased and then decreased with increasing initial MB concentration because most of the adsorption sites on the surface of MIL-53(Fe)@Mnt were occupied by MB molecules, hence some MB molecules remained in the aqueous phase. The effect of adsorbent dosage on the adsorption of MB of MIL-53(Fe)@Mnt is displayed in Fig. 9b. With the increase of MIL-53(Fe)@Mnt dosage from 10 to 100 mg, the removal of MB increased from 43.3% to 99.7%. As the amount of adsorbent dose increased, more active sites became available (Zhang et al., Reference Zhang, Wu, Wang, Li, Wu, Liang and Yang2019). The effect of pH on the adsorption of MB by MIL-53(Fe)@Mnt was examined at 200 mg L−1 and 298 K, and the results are shown in Fig. 9c. A change in pH had little effect on the adsorption of MB, suggesting that MIL-53(Fe)@Mnt might be used for MB adsorption regardless of pH.
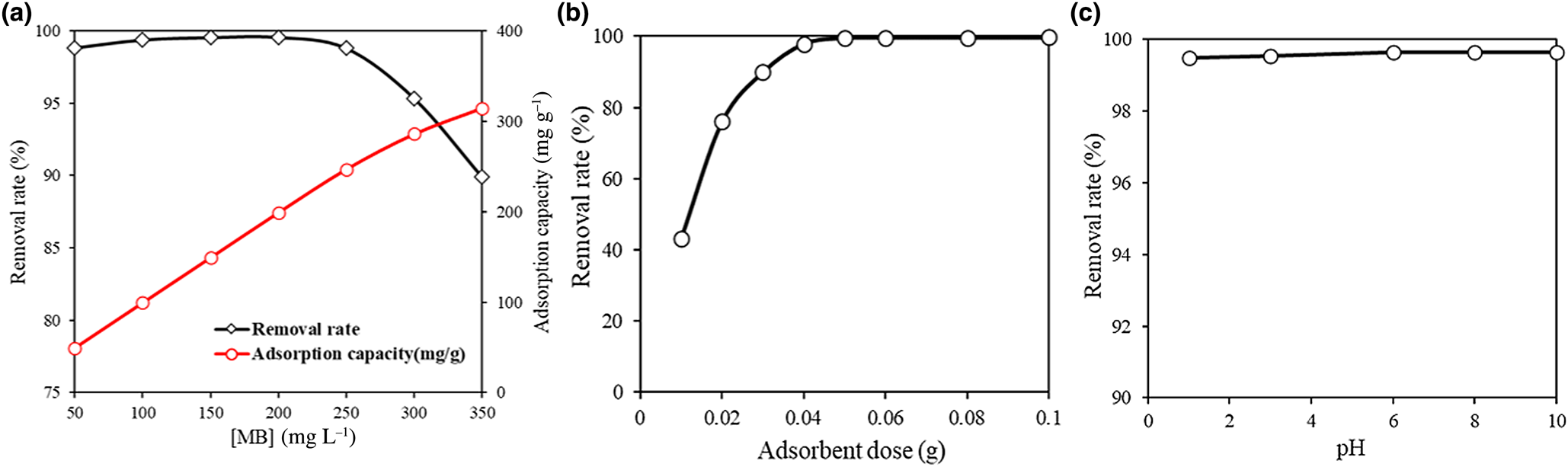
Fig. 9. Optimum conditions for the adsorption of MB by MIL-53(Fe)@Mnt. (a) Effect of initial MB concentration using 0.05 g of adsorbent at 25°C. (b) Effect of adsorbent dosage at 25°C: [MB] = 200 mg L−1, time = 20 h. (c) Effect of pH at 25°C: adsorbent = 0.05 g; [MB] = 200 mg L−1, time = 80 min.
Adsorption isotherms and kinetics
In order to investigate the interaction between the MIL-53(Fe)@Mnt adsorbent and the adsorbate (MB), four adsorption isotherm models, namely Langmuir (Equation 3), Freundlich (Equation 4), Langmuir–Freundlich (L–F) (Equation 5) and Toth (Equation 6), were used to analyse the adsorption data. The Langmuir model assumes a uniform adsorbent surface and is used to describe a single layer of adsorption. The Freundlich model is an empirical model used to describe heterogeneous adsorption systems and assumes an exponential distribution of active sites on heterogeneous adsorbents and the energy of the adsorbate (Zhang et al., Reference Zhang, Wu, Wang, Li, Wu, Liang and Yang2019). The L–F equation is a semi-empirical equation that introduces the Langmuir equation into the Freundlich equation in exponential form to describe the surface heterogeneity of the adsorbent (Batebi et al., Reference Batebi, Abedini and Mosayebi2021). To make up for the deficiency of the Freundlich equation, the semi-empirical Toth equation was also tested (Kumar et al., Reference Kumar, Gadipelli, Howard, Kwapinski and Brett2021). These equations are as follows:
where q e (mg g−1) is the amount of adsorption at equilibrium, q m (mg g−1) is maximum adsorption capacity, C e (mg L−1) is the concentration of adsorbate at equilibrium and K (L mg−1) and n are constants related to the adsorption equilibrium.
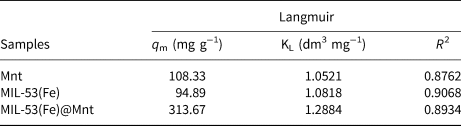
The Langmuir, Freundlich, L–F and Toth models were used to fit the adsorption isotherm data of MIL-53(Fe)@Mnt, and the results are shown in Fig. 10a and Table 2. The equilibrium data using the Langmuir model fit better the isotherm data, and the coefficient of determination (R 2 = 0.8934) was the highest. Using the L–F equation, the maximum adsorption capacity (335.68 mg g−1) was the highest among the models tested. However, its coefficient of determination (R 2 = 0.8649) was lower. Hence, the adsorption on the MIL-53(Fe)@Mnt surface was mainly controlled by homogeneous monolayer adsorption. Furthermore, the adsorption equilibrium data of the constituent materials (Mnt, MIL-53(Fe)) was also fitted by the Langmuir equation. The results are shown in Fig. 10b and Table 3. The maximum adsorption capacity of MIL-53(Fe)@Mnt (313.7 mg g−1) was greater than those of Mnt (108.33 mg g−1) and MIL-53(Fe) (94.89 mg g−1).
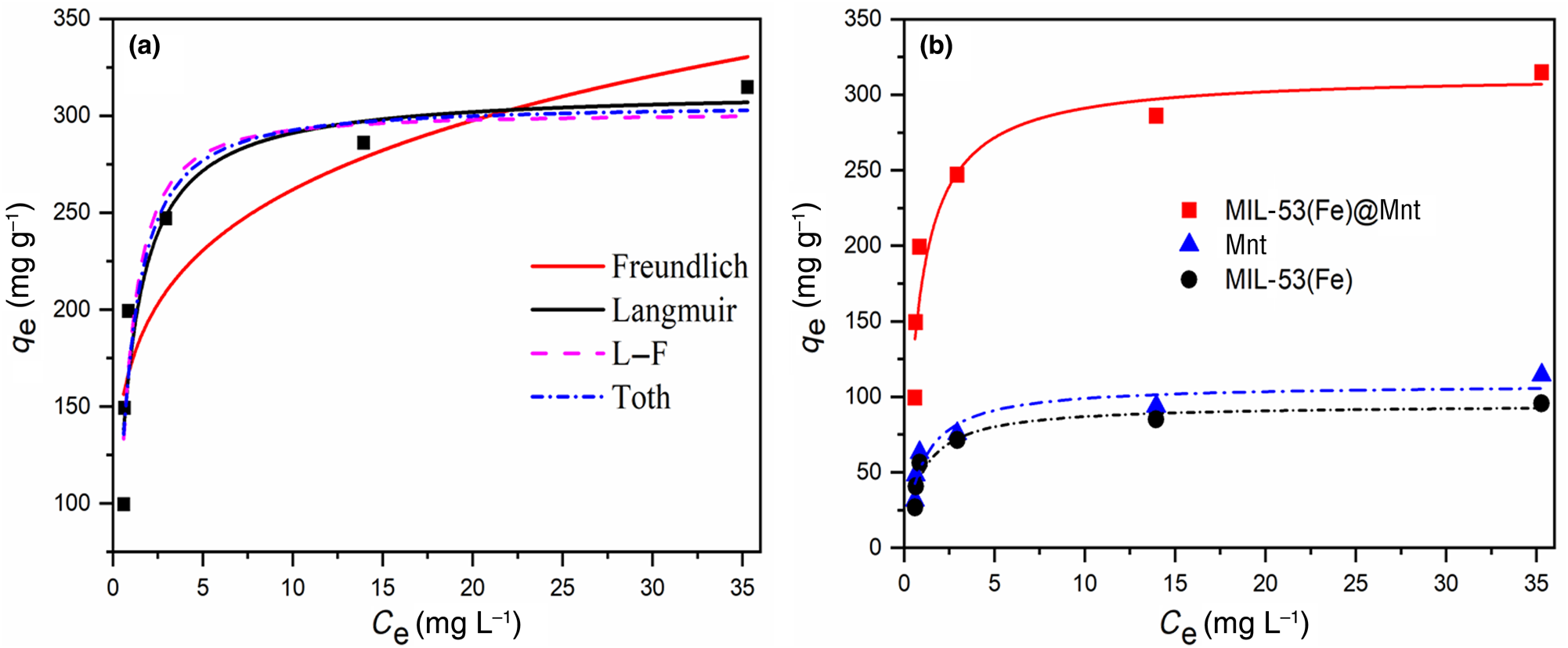
Fig. 10. (a) Adsorption isotherms and fitting curves for the adsorption of MB on MIL-53(Fe)@Mnt. (b) Adsorption isotherms of Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt using the Langmuir equation and their fitting.
Table 2. Models used to fit the adsorption data of MB and their characteristic parameters.
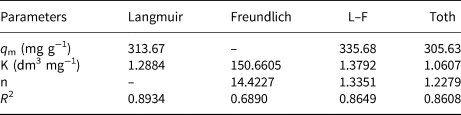
Table 3. Langmuir isotherm parameters for the adsorption of MB onto Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt.
Adsorption kinetics play an essential role in the explanation of the adsorption mechanism and adsorption efficiency. Two widely used kinetic models are the pseudo-first order (Equation 7) and pseudo-second order (Equation 8) models shown below (Lin & Wang, Reference Lin and Wang2009):
where t (min) is the contact time, qt (mg g−1) and q e (mg g−1) are the amounts of MB adsorbed at time t and equilibrium, respectively, and K1 (min−1) and K2 (g mg−1 min−1) are the equilibrium rate constants for the pseudo-first order and pseudo-second order models, respectively.
The adsorption data and fitting values of the pseudo-first order and pseudo-second order models are shown in Fig. 11 and Table 4. The coefficients of determination (R 2) of pseudo-second order reaction rates for Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt were all greater than those of the pseudo-first order rates. It is concluded that Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt were compatible with pseudo-second order dynamics. The K2 values of Mnt, MIL-53(Fe) and MIL-53(Fe)@Mnt were 0.0013, 0.0007 and 0.0027 g mg−1 min−1, respectively. Hence, MIL-53(Fe)@Mnt had a greater absorption capacity for MB (K2 = 0.0027 g mg−1 min−1), which further demonstrated that the composite formed by Mnt and MIL-53(Fe) improved the adsorption capacity for MB.
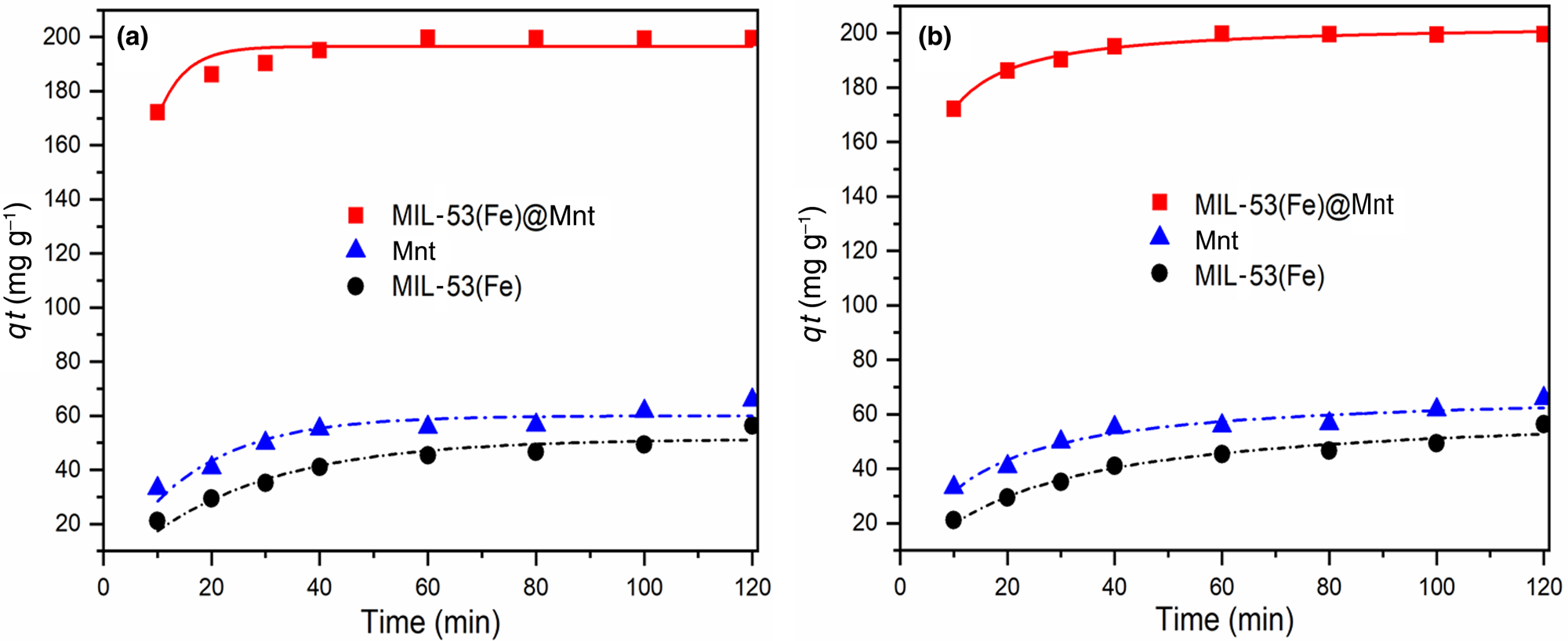
Fig. 11. Kinetics of adsorption of MB onto Mnt, MIL 53(Fe) and MIL-53(Fe)@Mnt: (a) pseudo-first order kinetics; (b) pseudo-second order kinetics.
Table 4. Parameters of the kinetic models used to fit the adsorption of MB onto MIL-53(Fe)@Mnt.
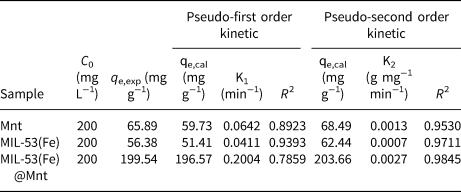
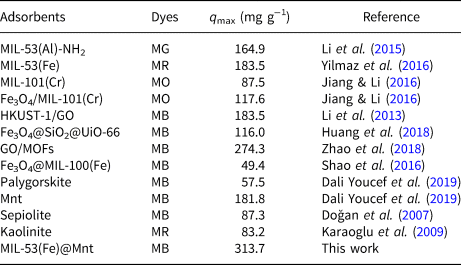
Table 5 summarizes the reported results regarding the adsorption of dyes on various MOF materials, such as MIL-53(Al)-NH2 (Li et al., Reference Li, Xiong, Zhang and Wu2015), MIL-53(Fe) (Yilmaz et al., Reference Yılmaz, Sert and Atalay2016), MIL-101(Cr) and Fe3O4/MIL-101(Cr) (Jiang & Li, Reference Jiang and Li2016), HKUST-1/GO (Li et al., Reference Li, Liu, Geng, Hu, Song and Xu2013), Fe3O4@SiO2@UiO-66 (Huang et al., Reference Huang, He, Chen and Hu2018), graphene oxide (GO)/MOFs (Zhao et al., Reference Zhao, Chen, Wei, Chen, Liang and Luo2018) and Fe3O4@MIL-100(Fe) (Shao et al., Reference Shao, Zhou, Bao, Ma, Liu and Wang2016). MB, MG, methyl red (MR) and methyl orange (MO) were used as adsorbates. When compared with other reported MOF materials, MIL-53(Fe)@Mnt presented excellent adsorption activity. In addition, when compared with other clays, such as palygorskite, Algerian Mnt (Dali Youcef et al., Reference Dali Youcef, Belaroui and López-Galindo2019), sepiolite (Doğan et al., Reference Doğan, Özdemir and Alkan2007) and kaolinite (Karaoglu et al., Reference Karaoglu, Dogan and Alkan2009), the Mnt composites obtained are low-cost, eco-friendly materials with a multilayer structure.
Table 5. Reported results for the adsorption capacities of various adsorbents for various dyes.
Conclusion
MIL-53(Fe)@Mnt, a novel, eco-friendly, clay-based adsorbent, was synthesized successfully through an in situ growth technique in which MIL-53(Fe) particles were grown on the surface of Mnt. When comparing with raw materials and various dyes, the MIL-53(Fe)@Mnt composite exhibited an excellent adsorption capacity for MB and a significant removal rate. The equilibrium adsorption isotherm was fitted with the Langmuir model, suggesting monolayer adsorption of MB on MIL-53(Fe)@Mnt. Adsorption followed pseudo-second order kinetics. Therefore, the adsorbent obtained – a low-cost and eco-friendly material – is a candidate for the removal of dyes from aqueous solutions in water treatment.
Financial support
This project was supported by the National Natural Science Foundation of China (21865030).



















