Introduction
The species investigated in this study belong to two different and very distant taxonomic groups (dinoflagellates and tunicates) but they have some characteristics in common: (i) high feeding efficiency, (ii) low prey selectivity (with preference for phytoplankton) and (iii) population outbursts under favourable conditions. The opportunistic omnivorous dinoflagellate species Noctiluca scintillans (Macartney) Kofoid and Swezy 1921 (syn. milaris Suriray 1836) is one of the most prominent ‘red tide’ organisms. This large-sized dinoflagellate can be categorized in the same size range with micro- to mesozooplankton communities (Elbrächter & Qi, Reference Elbrächter, Qi and Anderson1998). Measurements of Noctiluca scintillans in the northern Adriatic indicated a maximal cell diameter of up to 1000 µm (Mikaelyan et al., Reference Mikaelyan, Malej, Shiganova, Turk, Sivkovitch, Musaeva, Kogovšek, Taisia and Lukasheva2014). Noctiluca scintillans is broadly distributed and mainly found in temperate and sub-tropical coastal waters. Red-tide forming Noctiluca is a neritic species and oceanic occurrences are rare (Steidinger & Tangen, Reference Steidinger, Tangen and Tomas1996; Harrison et al., Reference Harrison, Furuya, Glibert, Xu, Liu, Yin, Lee, Anderson, Gowen, Al-Azri and Ho2011 and references therein). The ability of the species to reproduce rapidly, together with its polyphagous feeding behaviour, enables population outbursts under favourable conditions, frequently dominating the >200 µm fraction of the plankton (Uhlig & Sahling, Reference Uhlig and Sahling1990; Shanks & Walters, Reference Shanks and Walters1996; Elbrächter & Qi, Reference Elbrächter, Qi and Anderson1998). The diet of the species consists of a broad spectrum of prey, including phytoplankton, nauplii and eggs of zooplankton, organic detritus and bacteria (Schaumann et al., Reference Schaumann, Gerdes and Hesse1988; Kirchner et al., Reference Kirchner, Sahling, Uhlig, Gunkel and Klings1996; Elbrächter & Qi, Reference Elbrächter, Qi and Anderson1998; Quevedo et al., Reference Quevedo, Gonzales-Quiros and Anadon1999), thus the species may prey on organisms theoretically higher up in the trophic web. This feeding can lead to competition with other grazers, such as copepods that are reliant on the same food source (Elbrächter & Qi, Reference Elbrächter, Qi and Anderson1998). Noctiluca has also been implicated in the decline of fisheries in a variety of locations (Huang & Qi, Reference Huang and Qi1997; Smayda, Reference Smayda1997; Thangaraja et al., Reference Thangaraja, Al-Aisry and Al-Kharusi2007).
Salps are filter-feeders, distributed widely in the world ocean, and often recognized as conspicuous members of zooplankton assemblages. They are characterized by high feeding efficiency (Madin & Kremer, Reference Madin and Kremer1995; Madin & Deibel, Reference Madin, Deibel and Bone1998), rapid growth (Heron, Reference Heron1972; Heron & Benham, Reference Heron and Benham1985; Andersen & Nival, Reference Andersen and Nival1986; Madin & Deibel, Reference Madin, Deibel and Bone1998) and unusual life history with alternation of asexual and sexual reproduction (Alldredge & Madin, Reference Alldredge and Madin1982). These features enable them to form extensive swarms (Kremer, Reference Kremer2002) with hundreds of specimens per cubic metre (Deevey, Reference Deevey1971). These can affect the trophodynamics in surface waters hampering the development of other zooplankton (Berner, Reference Berner1967; Alldredge & Madin, Reference Alldredge and Madin1982) by the reduction of phytoplankton (Dubischar & Bathman, Reference Dubischar and Bathmann1997; Madin & Deibel, Reference Madin, Deibel and Bone1998) and by their capability of exploiting small particles over a wide size range, from bacteria to large diatoms and microzooplankton (Kremer & Madin, Reference Kremer and Madin1992). Salps are significant contributors to oceanic carbon flux with very fast growth rates and through their fast-sinking, resistant faecal pellets (Henschke et al., Reference Henschke, Bowden, Everett, Holmes, Kloser, Lee and Suthers2013; Smith et al., Reference Smith, Sherman, Huffard, McGill, Henthorn, Von Thun, Ruhl, Kahru and Ohman2014).
In this study we investigated the occurrence of blooms of Noctiluca scintillans and salps in 2009 in the open southern Adriatic (OSA) waters which are usually considered as oligotrophic (Cerino et al., Reference Cerino, Bernardi A, Coppola, La Ferla, Maimone, Socal and Totti2012 and references therein). The southern Adriatic is the deepest part of the Adriatic (reaching a maximum depth of 1242 m), characterized by winter convection events and dense water formation for the eastern Mediterranean deep circulation cell. Winter convection injects nutrients into the euphotic zone, thereby stimulating phytoplankton development in the otherwise oligotrophic OSA (Gačić et al., Reference Gačić, Civitarese, Cardin, Crise, Miserocchi and Mauri2002; Batistić et al., Reference Batistić, Jasprica, Carić, Čalić, Kovačević, Garić, Njire, Mikuš and Bobanović-Ćolić2012; Ljubimir et al., Reference Ljubimir, Jasprica, Čalić, Hrustić, Dupčić Radić and Car2017). In addition, the southern Adriatic is the entry point for advection of water masses of different thermohaline properties and nutrient load originating from the Ionian Sea (IS). The exchange of the southern Adriatic and Ionian Sea are intimately linked by means of the mechanism of the Bimodal Oscillating System (BiOS) that changes the circulation of the North Ionian Gyre (NIG) from cyclonic to anticyclonic, and vice versa, on a decadal time scale, during which advection of Atlantic Water (AW) or Levantine Intermediate Water (LIW), respectively, to the Adriatic prevails (Gačić et al., Reference Gačić, Eusebi Borzelli, Civitarese, Cardin and Yari2010).
Blooms of Noctiluca scintillans have not previously been recorded in the OSA. In contrast, in the northern Adriatic, N. scintillans blooms are regular events which usually occur in the spring-summer period (Fonda-Umani et al., Reference Fonda-Umani, Beran, Parlato, Virgilio, Zollet, De Olazabal, Lazzarini and Cabrini2004; Mikaelyan et al., Reference Mikaelyan, Malej, Shiganova, Turk, Sivkovitch, Musaeva, Kogovšek, Taisia and Lukasheva2014). Salp blooms have been rarely documented in the area and they have been attributed to either Salpa maxima (Babnik, Reference Babnik1948; Boero et al., Reference Boero, Belmonte, Bracale, Fraschetti, Piraino and Zampardi2013) or S. fusiformis (Kršinić, Reference Kršinić1998). However, OSA waters have not regularly been sampled for plankton in the past so these blooms which are usually of short duration and patchy distribution might have been overlooked by standard monitoring.
The aim of this paper was to investigate the occurrence of blooms of three species from two distinct taxonomic groups in OSA waters, usually considered as oligotrophic. Our intention was to understand the physical and biological context in which these blooms occur. This would give us improved understanding for prediction of the occurrence of such events in the future. In addition, we reviewed the potential of these species to serve as possible indicators of changes of trophic status of the area in some parts of the year.
Materials and methods
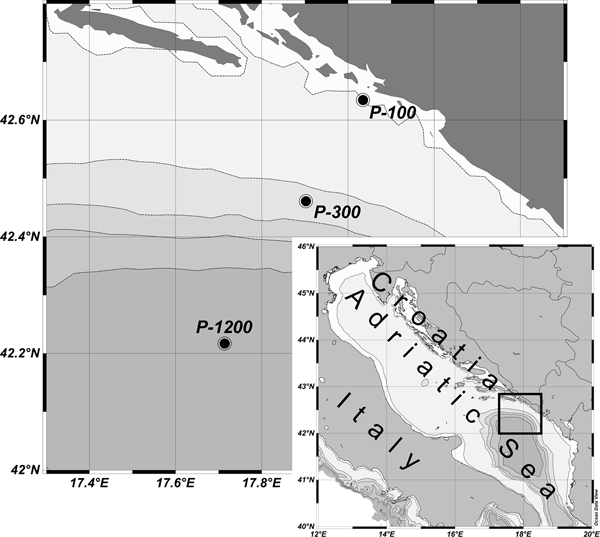
This study was conducted at three stations in the South Adriatic: nearshore station P-100 (100 m depth) and open sea stations P-300 (300 m depth) and P-1200 (1200 m depth) on 23 February, 23 April and 15 June 2009 along the central transect of the South Adriatic (Figure 1) with RV ‘Naše More’.
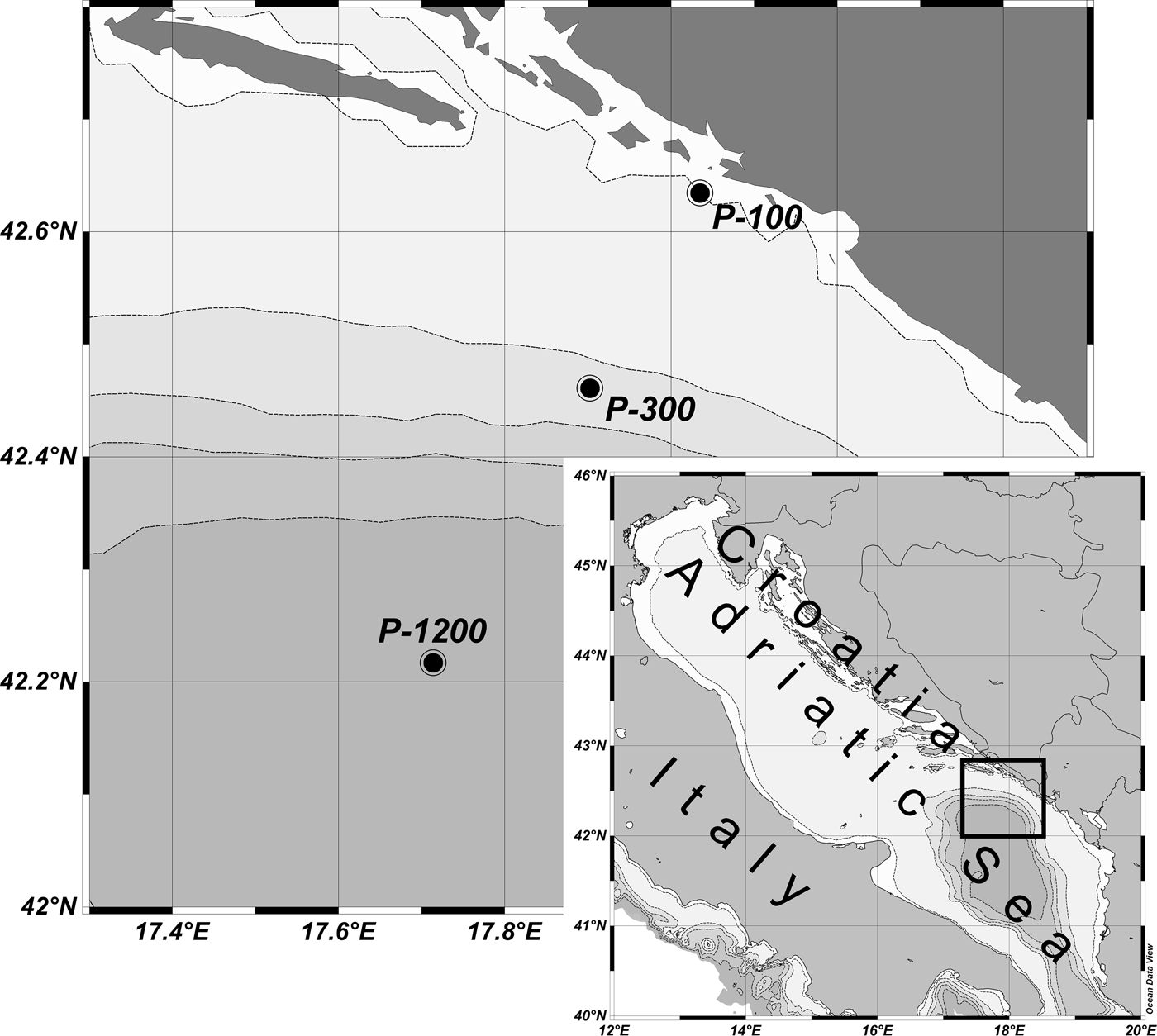
Fig. 1. Sampling stations.
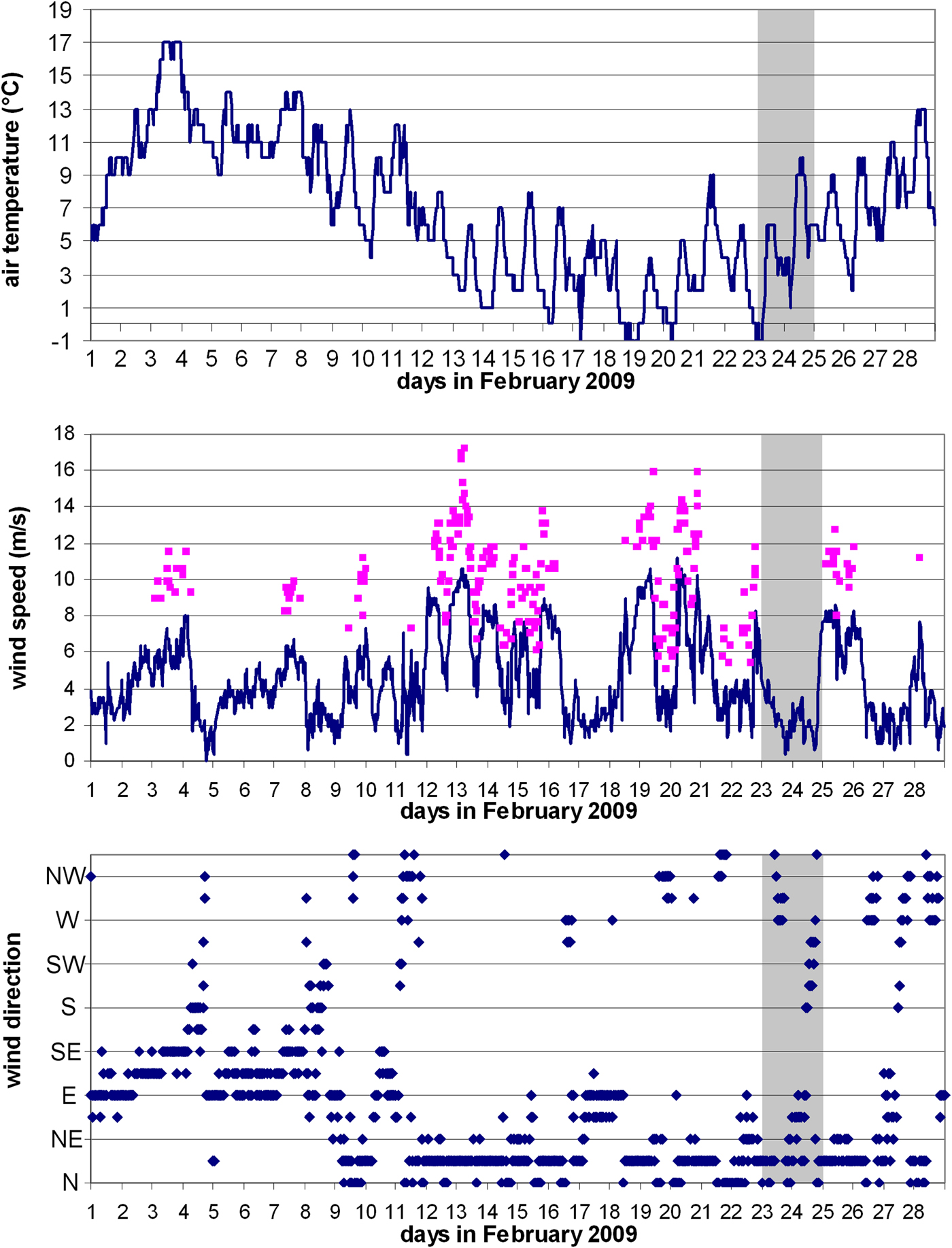
Meteorological parameters such as air temperature and wind speed, were extracted from monthly climatological reports issued by the Croatian Meteorological and Hydrological Service (DHMZ) for the Dubrovnik station. Meteorological data for Dubrovnik were representative for the area given the fact that bura wind develops when dense, cold and dry continental air surges towards the sea down the mountain slopes. The wind blows from the eastern Adriatic coast across the Adriatic, towards Italy, and causes basin-scale effects. The data were analysed for February 2009 in order to determine if meteorological conditions were favourable for inducing vertical mixing in the open sea.
Temperature and salinity profiles (0–1200 m, averaged over 1-m intervals) were taken with a CTD multiparametric sonde (SEA Bird Electronics Inc., USA).
Water samples for phytoplankton were taken with 5-litre Niskin bottles at 0, 5, 10, 20, 50, 75 and 100 m. Phytoplankton samples were fixed with neutralized formaldehyde (2% final concentration). Only the phytoplankton fraction larger than 2 µm, recognizable under the light microscope were taken into account. A subsample of 100 ml was settled in an Utermöhl chamber (Utermöhl, Reference Utermöhl1958). Phytoplankton abundance was determined with an inverted microscope (Olympus IX 71) equipped with phase contrast. Microphytoplankton taxa (cells >20 µm) were counted at a magnification of 200× in 2–8 transects of the central part of the chamber and at 100× in transects along the rest of the base-plate. Nanophytoplankton (cells 2–20 µm) were counted in 50 randomly selected fields along the settling chamber base-plate at 600×. Results are expressed as number of cells per litre (abundance). Cells were counted as nanophytoplankton or microphytoplankton size classes according to their maximum cellular linear dimension. In the case of the chain-forming diatom taxa (e.g. Chaetoceros), chain length was considered, instead of single cell size, which was smaller than 20 µm and species were attributed to the larger size class. The microphytoplankton fraction was divided into several groups: diatoms (Heterokontophyta, Bacillariophyceae); dinoflagellates (Dinophyta, Dinophyceae); coccolithophorids (Prymnesiophyta, Prymnesiophyceae); silicoflagellates (Heterokontophyta, Dictyochophyceae); and other groups with taxa found only sporadically – chlorophytes (Chlorophyta, Prasinophyceae); euglenophytes (Euglenophyceae) and cryptophytes (Cryptophyta). Nanophytoplankton cells, in general, were not determined to species rank, except in cases when recognition of some taxa (e.g. Emiliania huxleyi) were possible. Small (2–10 µm), more or less spherical mono- or biflagellate specimens, which could not be identified, were included in a group named Unidentified phytoflagellates.
For each station and date, the mean for the 0–100 m water column was calculated using the phytoplankton abundance data for the seven sampling depths (0, 5, 10, 20, 50, 75 and 100 m). The standard deviation (±SD) for the average abundance was also calculated.
Satellite surface chlorophyll maps were taken from the site of the Copernicus (Marine environment monitoring service; http://marine.copernicus.eu/).
Zooplankton were sampled with vertical hauls of a Nansen opening–closing net (manufactured by the Institute for Marine and Coastal Research, University of Dubrovnik) with a 250-μm mesh (114-cm diameter, 380 cm length) in two layers, surface (0–50 m) and subsurface (50–100 m). This mesh also provided a representative sample for the dinoflagellate Noctiluca scintillans because of its size (200–2000 µm in diameter). Average hauling speed was 1 m s−1. Samples were preserved in a 2.5% formalin–seawater solution buffered with CaCO3.
Mesozooplankton and N. scintillans were counted and identified with an Olympus stereomicroscope SZX9 at 25× and 40×. For calanoid copepods and appendicularians, sub-samples up to 1/32 of the original sample volume were counted depending on zooplankton abundance. Aliquots were determined in such a way that at least 200 individuals were counted in total. The entire catch was examined for salps. The abundance of all groups is presented as the number of specimens per cubic metre (ind. m−3). Additionally, during zooplankton sampling, the sea surface was observed (visual census) for gelatinous zooplankton blooms. Large salps such as Salpa maxima are hard to quantify using plankton nets due to their patchy distribution and large chains of aggregate zooids. For such large gelatinous animals, a visual census is the most appropriate method (Boero et al., Reference Boero, Belmonte, Bracale, Fraschetti, Piraino and Zampardi2013). We estimated their abundance as number of zooids per square metre (m2).
Results
Meteorological conditions
Meteorological conditions were analysed for February 2009. Temperature, wind direction and speed are important in creating preconditions for winter vertical mixing which is important for the enrichment of upper layers by nutrients and development of phytoplankton in winter and spring in the open sea of the southern Adriatic. Meteorological conditions in February 2009 were characterized by several episodes of the bura, the region's cold, dry north-easterly wind (Figure 2). Between 13 and 20 February, wind speed was between 7 and 10 m s−1. This was accompanied by a marked drop in air temperature from 16 to 20 February, which fell briefly below 0°C. These conditions naturally favour cooling of surface water and can be expected to have triggered vertical convection in the South Adriatic Pit.
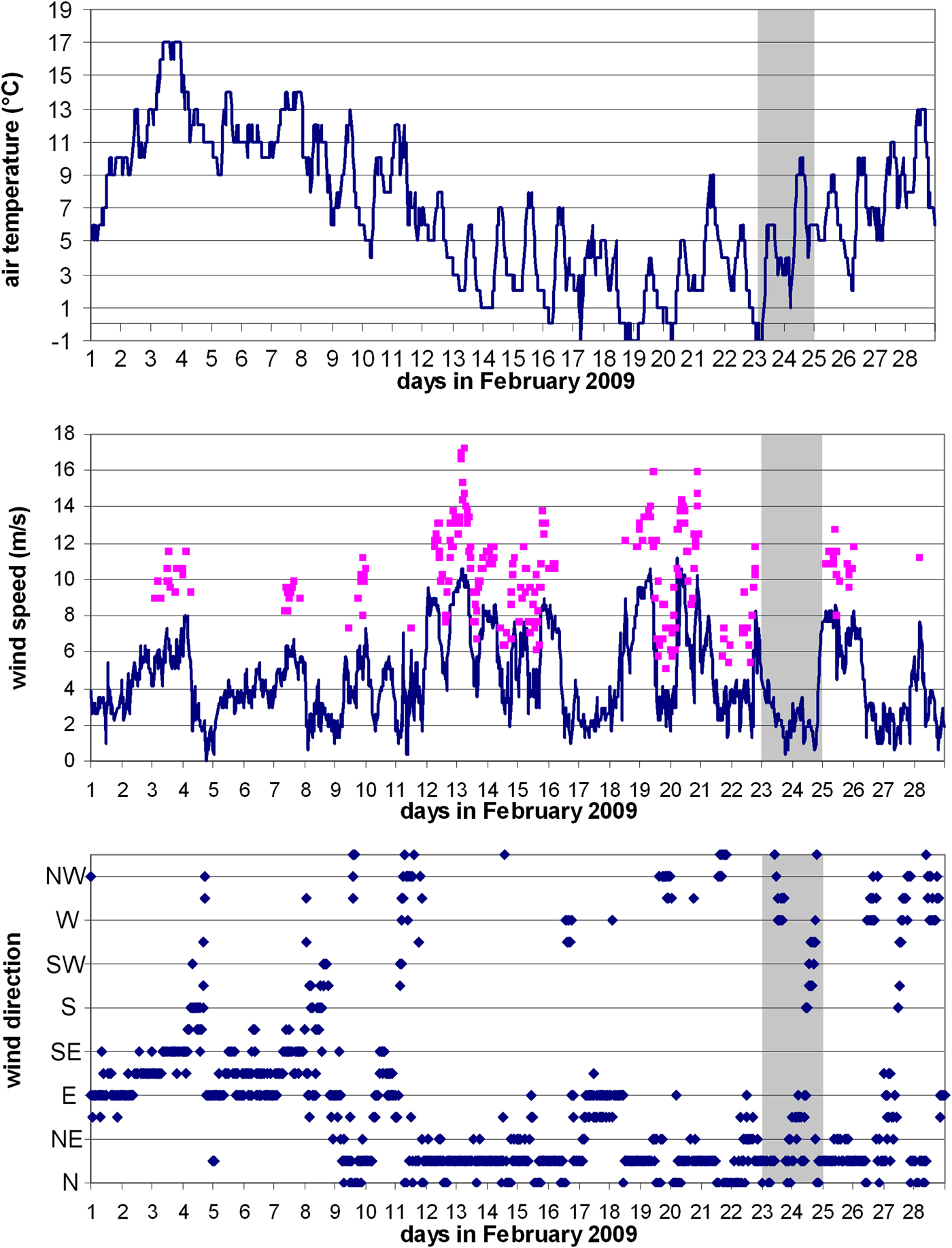
Fig. 2. Air temperature, wind speed and direction (clockwise, from north) at Dubrovnik meteorological station. Squares on the wind speed chart denote wind gusts. Shaded portion indicates the cruise dates.
Hydrographic parameters and chlorophyll a
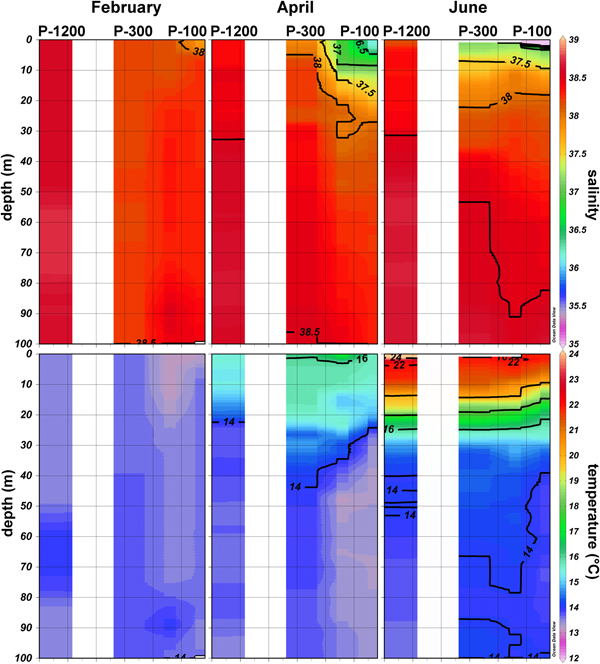
Physical properties of the east South Adriatic (SA) along the 57-km cross-shore section (stations P-100, P-300 and P-1200) had noteworthy features. In February, due to cold air conditions, an inverse stratification, with slightly cooler water on the surface and warmer water below the surface, was observed in investigated stations. The vertical distribution of thermal properties in February in the upper 100 m (Figure 3) showed slightly warmer water at P-300 (13.59°C–13.72°C). At P-1200 and at P-100 the temperature maximum in the upper layer did not exceed 13.59°C. Salinity gradually increased in the offshore direction, P-100 (37.79–38.42), P-300 (38.18–38.22), P-1200 (38.54–38.67). In April, due to heating, stratification started to form in a surface layer with the thermocline positioned between 25 and 30 m depth. The temperature maximum was in the upper 5 m, 16.07°C at P-300, 15.65°C at P-100 and 15.45°C at P-1200. Below 30 m, relatively uniform thermal conditions (>13.3°C and <14.5°C) were present. Salinity increased in the offshore direction: P-100 (36.15–38.29), P-300 (37.89–38.51), P-1200 (38.35–38.65). The vertical salinity distribution indicated that major fluctuations occurred in the upper 30 m while down to 100 m, salinity was rather uniform (Figure 3). In June thermal stratification formed, with the thermocline positioned between 20 and 30 m depth. A maximum temperature of 24.81°C was recorded at the surface at P-1200 while for the other two stations maximum temperature did not exceed 22°C. Below 30 m quite uniform thermal conditions were present, 13.7–14.6°C. Salinity increased in an offshore direction and ranged between 31.97–38.61 at P-100, 37.18–38.65 at P-300 and 38.15–38.68 at P-1200. A halocline was formed at all three stations. It was shallowest at station P-100 and deeper at stations P-300 and P-1200.
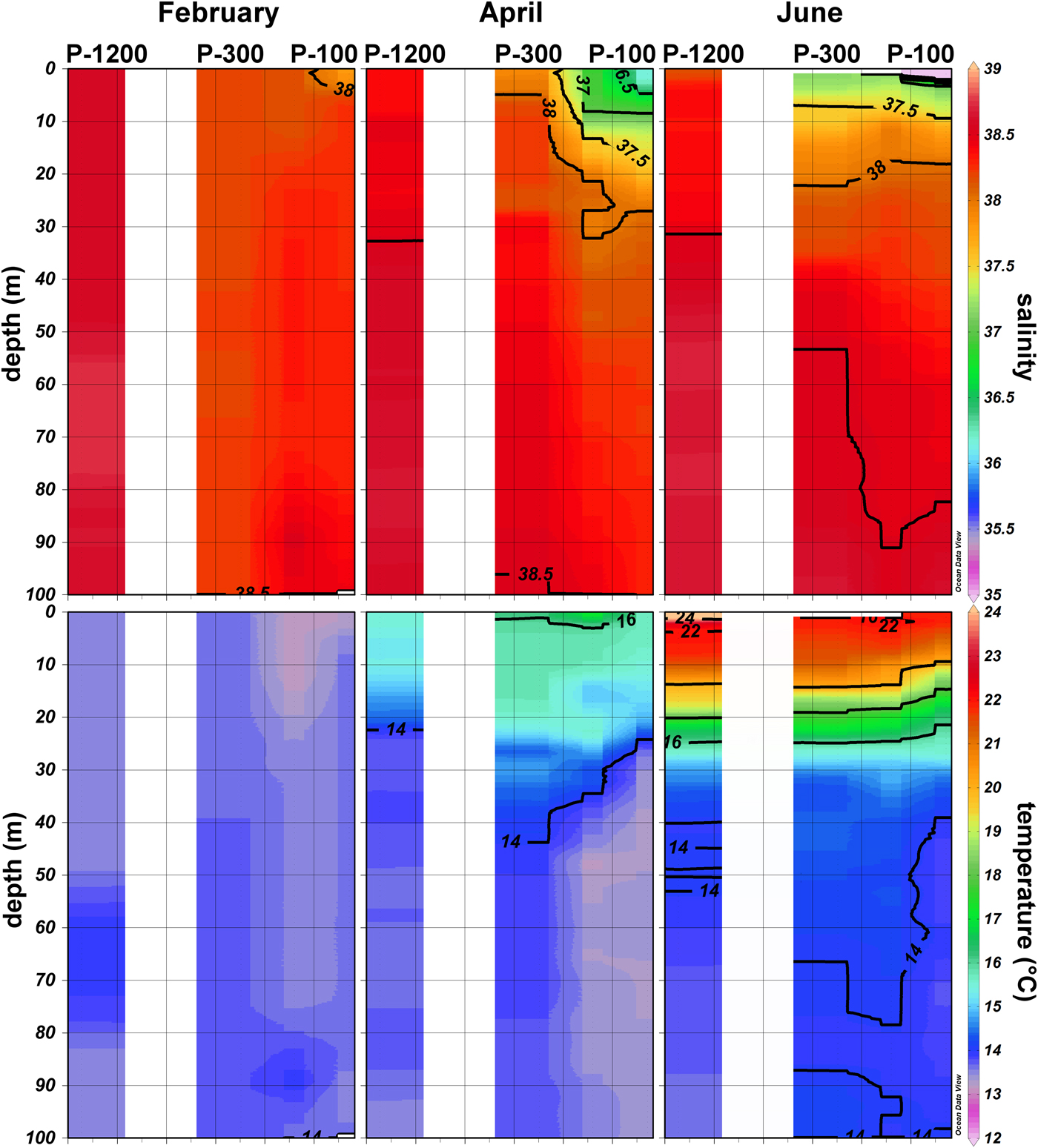
Fig. 3. Vertical profiles of temperature (°C) and salinity along the investigated transect in the southern Adriatic (February, April and June 2009).
Satellite surface chlorophyll maps for the southern Adriatic, provided by the Copernicus marine environment monitoring service (Figure 4), indicated medium-high (around 0.3 to 0.5 mg m−3) chlorophyll concentration (chl-a) prior to our sampling (from the beginning of February until mid-February), which then started to decrease towards the end of month. In March surface chl-a started to increase and reached from 0.5 to 0.7 mg m−3 until the beginning of April 2009. Chl-a values declined slightly towards the end of April. From the beginning of June, until mid-June, the surface chl-a signature was low.
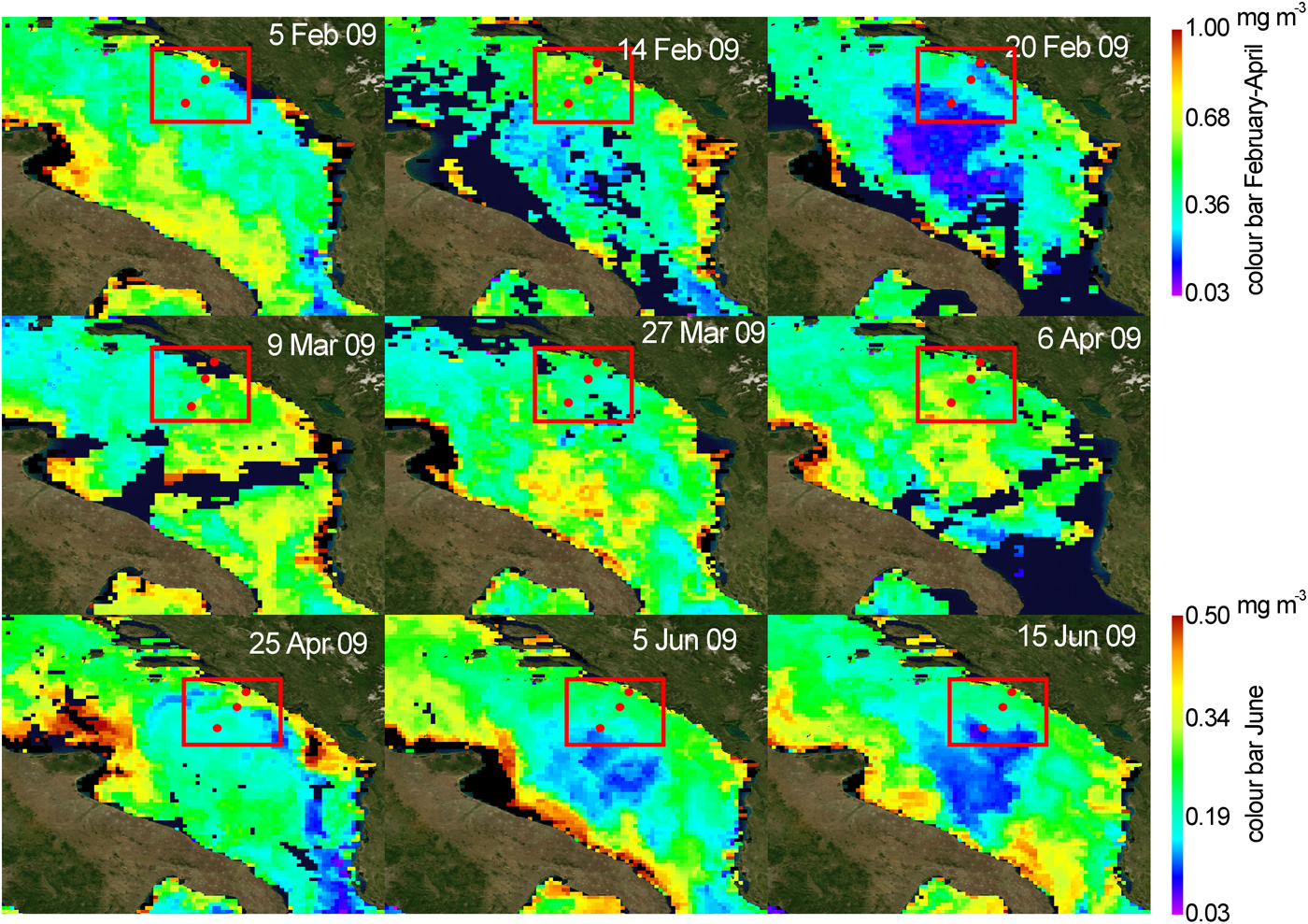
Fig. 4. Chlorophyll a satellite images during the investigated period.
Abundance of phytoplankton
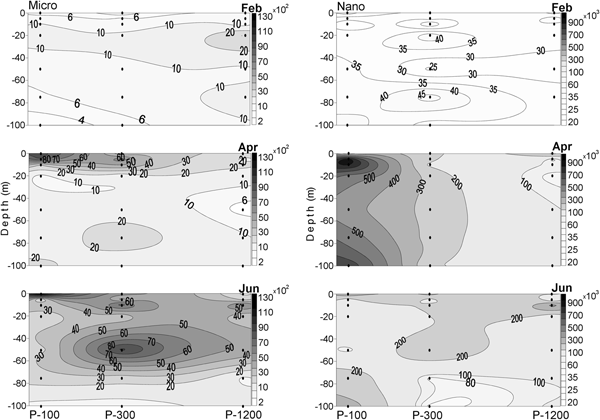
In February 2009, microphytoplankton abundance was low. There was a slight offshore increase with highest abundance (1.2–3.0 × 103 cells l−1) in the 20–75 m layer at P-1200. Microphytoplankton was composed mostly of coccolithophorids (with percentage contribution of 44–60%) and dinoflagellates (32–39%) at stations P-100 and P-300, while diatoms dominated (46%) at station P-1200. In general, there was no difference in nanophytoplankton abundance among stations (averages for the 0–100 layer were from 3.5, 3.3 and 3.2 × 104 cells l−1 at P-100, P-300 and P-1200 with a standard deviation (SD) of 5.6, 9.6 and 3.8 × 103 cells l−1, respectively).
In April 2009, phytoplankton abundance decreased in the direction from near-shore to the open sea stations (Figure 5). Average microphytoplankton abundances of the 0–100 layer were 3.9 ± 3.6, 3.1 ± 2.0 and 1.47 ± 0.8 × 103 cells l−1 at P-100, P-300 and P-1200, respectively. Microphytoplankton abundances in the surface layer (0–10 m) were two (at P-300 and P-1200) or three times (at P-100) greater than in the layer from 20 to 100 m. Dinoflagellates dominated (46–58%) in the microphytoplankton in April at P-100 and P-1200, while coccolithophorids prevailed (49%) at P-300. Average nanophytoplankton abundances of the 0–100 layer were 6.3, 2.3 and 1.3 × 105 cells l−1 at P-100, P-300 and P-1200, respectively. Average nanophytoplankton abundances of the 0–100 layer were 6.3 ± 2.7, 2.3 ± 0.3 and 1.3 ± 0.8 × 105 cells l−1 at P-100, P-300 and P-1200, respectively.

Fig. 5. Distribution of micro- and nanophytoplankton abundances along the investigated transect in the southern Adriatic in February, April and June 2009.
In June 2009, microphytoplankton varied from 7.5 × 102 to 1.3 × 104 cells l−1 (average of the 0–100 layer 3.8 ± 4.1 × 103 cells l−1) at P-100; from 6.2 × 102 to 9.2 × 103 cells L−1 (average of the 0–100 layer 4.6 ± 2.9 × 103 cells l−1) at P-300; and from 8.0 × 102 to 6.1 × 103 cells l−1 (average of the 0–100 layer 2.9 ± 1.9 × 103 cells l−1) at P-1200 (Figure 5). The bulk of microphytoplankton were made up of dinoflagellates (52–70%) at all stations. Nanophytoplankton abundances decreased in the offshore direction, and their average values in the 0–100 layer were 2.1 ± 1.1, 1.9 ± 1.0 and 1.8 ± 1.1 × 105 cells l−1 at stations P-100, P-300 and P-1200, respectively.
Abundance of three bloom-forming species (Noctiluca scintillans, Sa lpa fusiformis, S. maxima), calanoid copepods and appendicularians
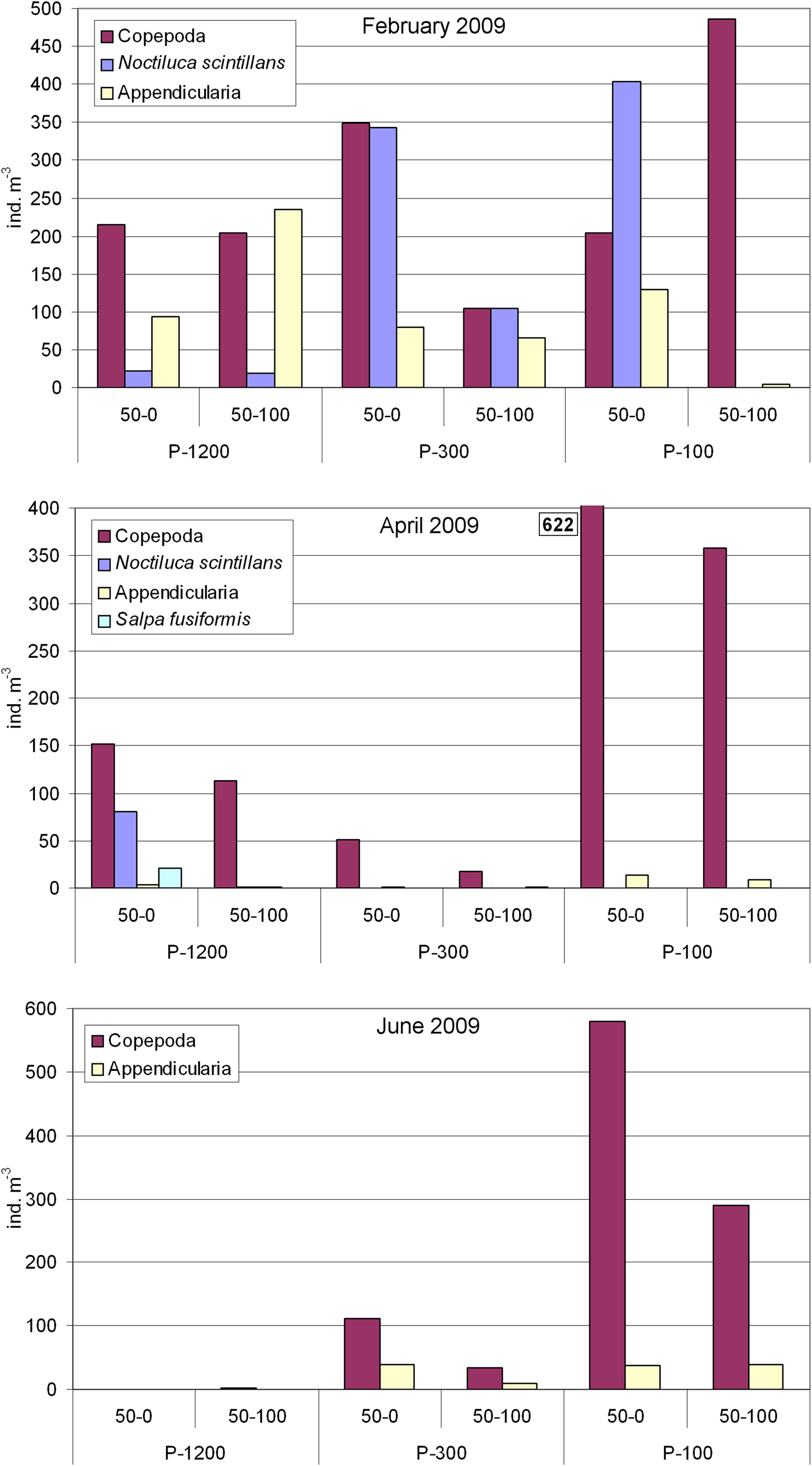
In February 2009, Noctiluca scintillans occurred in high abundance of 21.4 cells m−3 and 343 cells m−3 in the 0–50 m layer of open sea stations P-1200 and P-300, respectively. Maximal abundance of 403 cells m−3 in the 0–50 m layer was registered at shallower station P-100. Abundance of calanoid copepods increased from the open sea towards the coast (max. 486 ind. m−3 at station P-100, 50–100 m), while appendicularian abundance increased in the offshore direction (maximum abundance of 236 ind. m−3 at station P-1200, 50–100 m layer) (Figure 6).
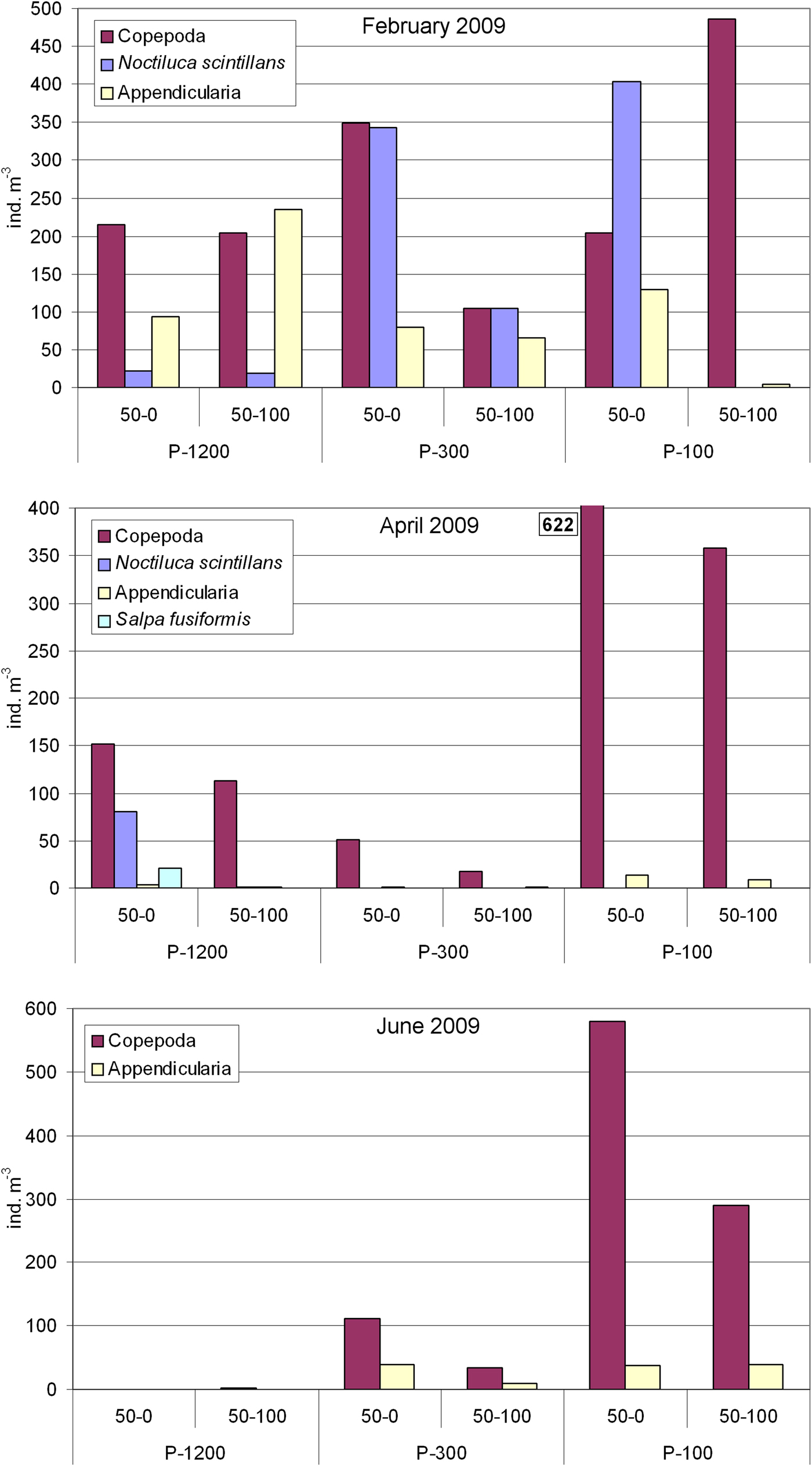
Fig. 6. Distribution of abundances of Noctiluca scintillans, Salpa fusiformis, calanoid copepods and appendicularians at the three stations in two layers (0–50 m and 50–100 m) in February, April and June 2009.
In April 2009, the abundance of N. scintillans markedly increased in the offshore direction. A maximum of 80 cells m−3 was registered in the upper 50 m at the deep open sea station, P-1200, while very low values were registered at stations P-300 and P-100 in the upper 50 m (0.04–0.06 cells m−3). Noctiluca scintillans was absent from the 50–100 m layer of P-300 and P-100 (Figure 6), while at P-1200 0.7 cells m−3 were recorded. Similarly, Salpa fusiformis reached a high abundance of 21 ind. m−3 at the open sea station P-1200 in the 0–50 m layer. Very low abundances of Salpa fusiformis were registered on stations P-300 and P-100 (0.08–1.8 ind. m−3 and it was absent at P-100 in the 50–100 m layer) (Figure 6). Calanoid copepods were the most abundant mesozooplankton group with maximum of 622 ind. m−3 at P-100 in the 0–50 m layer. In general, the lowest abundance of calanoid copepods was at P-300. Abundance of appendicularians was low, less than 14 ind. m−3 at the coastal station and 6 ind. m−3 at the open sea stations (Figure 6).
In June 2009, large zooids of S. maxima (avg. 10 cm in length) accumulated in high numbers at the surface (Figure 7) of the deep open sea station P-1200. According to the visual census, we estimated their abundance to be 1.5 ind. m−2. Presumably due to the high feeding presure of S. maxima and competition for the same food, only 1 ind. m−3 of calanoid copepods was found and appendicularians were almost absent (max. 0.1 ind. m−3) (Figure 6). Salpa maxima was not recorded at the station P-300 and P-100 where calanoid copepods dominated with 111 and 579 ind. m−3 in the 0–50 m layer, respectively. Appendicularian abundance was moderate at both stations, with maximum of 40 ind. m−3 at station P-300 in the 0–50 m layer.
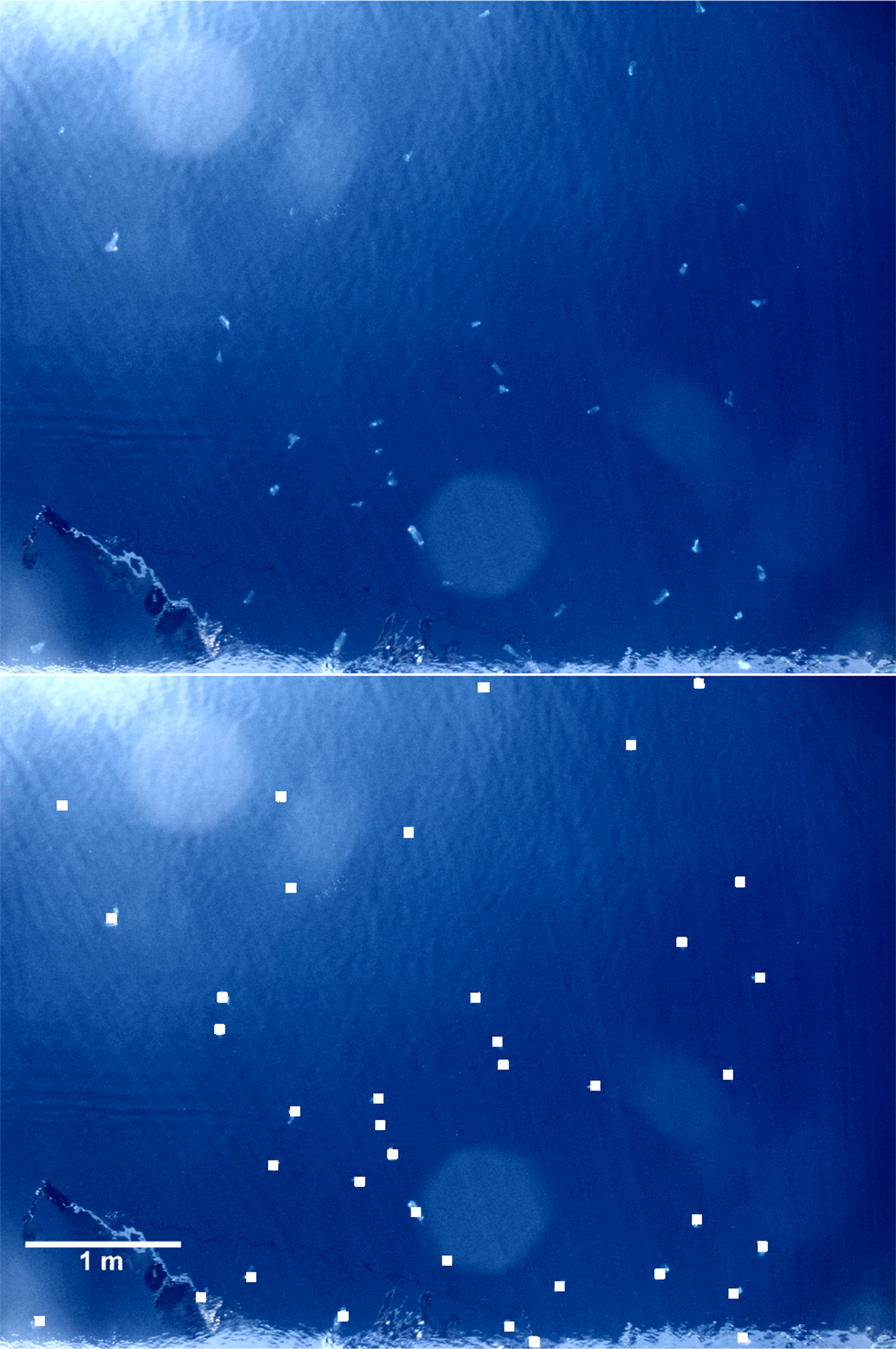
Fig. 7. Salpa maxima accumulation near the surface. White squares indicate individual zooids.
Discussion
The open southern Adriatic (OSA) is oligotrophic and phytoplankton abundance and biomass are typically low (Viličić et al., Reference Viličić, Vučak, Škrivanić and Gržetić1989, Reference Viličić, Kršinić and Bićanić1994; Viličić, Reference Viličić1991, Reference Viličić1998; Turchetto et al., Reference Turchetto, Bianchi, Boldrin, Malagutti, Rabitti, Socal and Strada2000; Cerino et al., Reference Cerino, Bernardi A, Coppola, La Ferla, Maimone, Socal and Totti2012). High abundance of Noctiluca scintillans, Salpa fusiformis and S. maxima in February, April and June 2009 in the two open sea stations (P-300 and P-1200) indirectly indicates the possibility that the OSA is not exclusively oligotrophic, at least at certain times of the year, from winter to early summer. In February and April 2009, a N. scintillans bloom (with maximal abundance up to 343 cells m−3) was registered for the first time in the OSA. In contrast, based on long-term studies, the shallow highly eutrophic northern Adriatic is the site of a prominent spring-summer bloom (order of magnitude 103–106) of this species, with some blooms (order of magnitude 103) also in winter (Fonda-Umani et al., Reference Fonda-Umani, Beran, Parlato, Virgilio, Zollet, De Olazabal, Lazzarini and Cabrini2004; Mikaelyan et al., Reference Mikaelyan, Malej, Shiganova, Turk, Sivkovitch, Musaeva, Kogovšek, Taisia and Lukasheva2014). Unusual high abundance for the open sea of N. scintillans in February 2009 in the OSA indicates some changes of the trophic status of the area may have occurred. According to Harrison et al. (Reference Harrison, Furuya, Glibert, Xu, Liu, Yin, Lee, Anderson, Gowen, Al-Azri and Ho2011) Noctiluca blooms, in coastal or offshore areas, may be a manifestation of eutrophication, since increase in nutrients leads to increase in phytoplankton abundance, which is the preferred food of N. scintillans. This presumption is supported by satellite chl-a which showed medium-high chl-a at the open sea till mid-February. Moreover, recent investigation in the OSA indicates increase of phytoplankton abundance and biomass during winter (Ljubimir et al., Reference Ljubimir, Jasprica, Čalić, Hrustić, Dupčić Radić and Car2017). However, very low abundance of microphytoplankton (102 cells l−1 in the first 10 m) registered at the time of sampling in the OSA, from 23–24 February, is likely due to a combination of grazing pressure by Noctiluca and other herbivorous zooplankton (calanoid copepods and appendicularians), and vertical mixing of moderate intensity which did not exceed 400 m (Ljubimir et al., Reference Ljubimir, Jasprica, Čalić, Hrustić, Dupčić Radić and Car2017). According to a study in Sagami Bay, reduction in phytoplankton abundance was mainly controlled by Noctiluca grazing (Baek et al., Reference Baek, Shimode, Kim, Han and Kikuchi2009). Diatoms, which are the preferred food source for Noctiluca scintillans (Harrison et al., Reference Harrison, Furuya, Glibert, Xu, Liu, Yin, Lee, Anderson, Gowen, Al-Azri and Ho2011), dominated in the microphytoplankton population at the open sea station P-1200 in February. In April 2009, N. scintillans was still present at the OSA deepest station (P-1200) in relatively high abundance in the 0–50 m depth layer alongside with S. fusiformis. Accordingly, low abundance of microphytoplankton, particularly diatoms, was registered at station P-1200 likely due to the grazing pressure of N. scintillans and S. fusiformis. It is interesting that, unlike February 2009, in April 2009 these two species were not registered at the open sea station P-300 and nearshore station P-100. This indicates that they had enough food at the surface layer of the deepest open sea station (P-1200), which is confirmed by satellite chl-a prior to the sampling date (Figure 4). A bloom of another salp species, S. maxima, occurred in June 2009 at the same station. Records of salp blooms in the OSA are rather scant in the literature. The possible reason is that gelatinous zooplankton blooms are usually of short duration and might be overlooked by standard net monitoring and therefore have not been reported in the past. The only reports of S. maxima blooms in the OSA are from July 1940 (Babnik, Reference Babnik1948), June 1998 and March–May 2013 (Boero et al., Reference Boero, Belmonte, Bracale, Fraschetti, Piraino and Zampardi2013). For S. fusiformis the only report is from March 1990 (Kršinić, Reference Kršinić1998). During the S. maxima bloom in June 2009, calanoid copepods and appendicularians, which are strong competitors for the same food resources, were almost completely absent from the surface up to 100 m depth (<1 ind. m−3). Abundance of microphytoplankton was low with very low diatom contribution (Figure 5). Satellite chl-a was also low (Figure 4). Salps have extremely high clearing rates and can feed on all sizes of phytoplankton, from viruses to protists, competing with crustacean filter feeders (Bone, Reference Bone1998). Because of the swarming potential of these large microphages they are able to control phytoplankton blooms (Fortier et al., Reference Fortier, Le Fèvre and Legendre1994) and to increase the transfer of biogenic carbon to the bottom through their fast-sinking, resistant, faecal pellets.
Blooms of Noctiluca and salps at the OSA might be indirect indicators of eutrophication of the area, since an increase in nutrients leads to an increase in phytoplankton, their main food source as a grazer. Recently, in situ and satellite chl-a data have indicated that phytoplankton blooms in the OSA are more frequent than we previously assumed and with some interannual variability (Santoleri et al., Reference Santoleri, Banzon, Marullo, Napolitano, D'Ortenzio and Evans2003; D'Ortenzio & d'Alcala, Reference D'Ortenzio and d'Alcala2009). This variability is dependent on the meteorological conditions that affect the open-ocean convection which drives the mixing and uplifting of nutrients from the rich intermediate layers into the euphotic zone. It is further modulated by incursions of different water masses into the OSA, especially during winter, that modify not only thermohaline properties, but also chemical properties (different nutrient load) of the area (Gačić et al., Reference Gačić, Civitarese, Cardin, Crise, Miserocchi and Mauri2002; Civitarese et al., Reference Civitarese, Gačić, Lipizer and Eusebi Borzelli2010). Water exchange between the Southern Adriatic and Ionian Sea is intimately linked by means of the Bimodal Oscillating System (BiOS) mechanism that changes the circulation of the North Ionian Gyre (NIG) from cyclonic to anticyclonic and vice versa, on a decadal time scale (Gačić et al., Reference Gačić, Eusebi Borzelli, Civitarese, Cardin and Yari2010). When the NIG is anticyclonic, Atlantic Water (AW) enters the SA, decreasing salinity and increasing nutrients from upwelling at the periphery of the NIG. Conversely, a cyclonic phase in the NIG depresses the nitricline along the borders of the Northern Ionian Gyre, favouring inflow of nutrient-poorer but saltier Levantine Intermediate Water (LIW) (Civitarese et al., Reference Civitarese, Gačić, Lipizer and Eusebi Borzelli2010). In 2009, the OSA was enriched by nutrients in two ways: due to inflow of AW (salinity did not exceed 38.68) and by winter vertical mixing which injects nutrients into the euphotic zone from deeper layers, thereby stimulating phytoplankton increase in the otherwise oligotrophic OSA. This is in accordance with uniform salinity and temperature (Figure 3), and high nutrient values, particularly nitrate, evident at the deepest station P-1200 in February (max. 6 µM) and April 2009 (max. 4.5 µM) (Ljubimir et al., Reference Ljubimir, Jasprica, Čalić, Hrustić, Dupčić Radić and Car2017). During February 2009, prior to our sampling date, several episodes of strong wind (the region's cold, dry northwind bura) were responsible for substantial heat loss at the air–sea interface. This produced dense surface water and consequent convective mixing (Gačić et al., Reference Gačić, Civitarese, Cardin, Crise, Miserocchi and Mauri2002) that, at offshore station P-1200, extended to 400 m (Ljubimir et al., Reference Ljubimir, Jasprica, Čalić, Hrustić, Dupčić Radić and Car2017). The evolution of this convective event was also captured by a series of surface chlorophyll images taken by satellites. The images show moderately high chl-a concentration at the beginning of February and subsequent chl-a minimum (due to convection-driven sinking of phytoplankton to the deep) extending over a wide area of the Southern Adriatic at the end of February. The chl-a minimum corresponds with the cooling event a few days before (16–20 February), after which a significant increase of chl-a was observed over the following 2–3 weeks (beginning of March).
During the period from 2009 to 2013, only in 2011 did winter vertical mixing not occur in the OSA (Ljubimir et al., Reference Ljubimir, Jasprica, Čalić, Hrustić, Dupčić Radić and Car2017). It is likely that blooms of salps, and possible Noctiluca (only registered in this investigation), occurred also in other years following open sea vertical mixing but were not observed because of the dynamics and methodologies of open sea sampling. The salp blooms are indicated as a potential culprit for high Sv values from acoustic current meters in the OSA during the summer period when generally net plankton is low (Ursella et al., Reference Ursella, Cardin, Batistić, Garić and Gačić2018). The rapid population growth of Noctiluca is in the order of a few days (do Rosário Gomes et al., Reference do Rosário Gomes, Goes, Matondkar, Buskey, Subhajit, Parab and Thoppil2014), after enrichment of the surface layers with nutrients by winter vertical mixing and development of phytoplankton. Since winter vertical mixing is not a single event, but is the sum of many sinking episodes caused by cold bura wind outbreaks, the phytoplankton community is constantly depleted from the surface layer by being transported to the aphotic zone (Batistić et al., Reference Batistić, Jasprica, Carić, Čalić, Kovačević, Garić, Njire, Mikuš and Bobanović-Ćolić2012). In this way, the full bloom of phytoplankton is prevented from forming until wind-driven vertical convective events cease and insolation increases in March or April (Ursella et al., Reference Ursella, Cardin, Batistić, Garić and Gačić2018). This is confirmed by satellite surface chlorophyll data with highest chl-a concentration in April (Figure 4). The spring phytoplankton bloom could trigger salp blooms as we recorded in this study. It is possible that spring salp blooms at the open sea deplete the surface layer of nutrients and transport organic matter to the deep via their faecal pellets. By this process they could be the controlling factor that limits primary production at the open sea in summer and autumn by depleting the surface layers of nutrients in spring.
This investigation sheds new light on the trophic status of the OSA in the context of its biological characteristics through blooms of Noctiluca and salps. Future investigations should focus on continuous monitoring of open SA waters to determine frequency and duration of such blooms and possible changes of open SA trophic status due to climate and circulation changes. Also, here we highlight the potentially important role of physical processes such as vertical mixing and inflow of water masses of different properties, driven by the BiOS mechanism, on the biology of the OSA in winter and spring. Such exceptional bloom events as we registered in February (N. scintillans), April (N. scintillans and S. fusiformis) and June (S. maxima) 2009 probably had a significant impact on the functioning of the marine ecosystem of the southern Adriatic, in particular on the carbon transfer across the pelagic food web and the proportion of sinking carbon. These hypotheses need to be confirmed in future investigations.
Acknowledgements
We would like to thank Jakica Njire for her help with illustrations and the Captains and crews of research vessels ‘Naše more’ and ‘Baldo Kosić’ for their assistance during sampling cruises. This paper was presented at the 52nd European Marine Biology Symposium held in Piran, Slovenia.
Funding
This research was funded by Croatian Ministry of Science and Education (grant number 275-0000000-3186) and Croatian Science Foundation (IP-2014-09-2945, project AdMedPlan).