INTRODUCTION
The swimming crabs are members of the Portunidae Rafinesque, 1815 family, which is represented in Brazil by nine genera and 22 species, including the exotic species Charybdis hellerii (Milne-Edwards, 1867). Most of these species are restricted to west Atlantic regions from eastern United States to Argentina, although they also occur in other regions (Fausto-Filho, Reference Fausto-Filho1980; Melo, Reference Melo1996; Mantelatto & Dias, Reference Mantelatto and Dias1999).
Portunid crabs of the genus Callinectes Stimpson (1860) show extensive distribution and can be found in lagoons, mangroves, estuaries and shelves at depths of up to 90 m (Melo, Reference Melo1996). Along the Brazilian coastline, this genus comprises seven species: Callinectes bocourti Milne-Edwards, 1879; C. danae Smith, 1869; C. exasperates (Gerstaecker, 1856); C. larvatus Ordway, 1863; C. ornatus Ordway, 1863; C. sapidus Rathbun, 1895; and C. affinis Fausto-Filho, Reference Fausto-Filho1980, of which only the latter cannot be found in Ilhéus (Almeida et al., Reference Almeida, Coelho, Santos and Ferraz2006). An additional species, C. maracaiboensis (Taissoun, 1972), was cited on the Brazilian coastline, although it was later considered a junior synonym of C. bocourti by Robles et al. (Reference Robles, Schubart, Conde, Carmona-Suárez, Alvarez, Villalobos and Felder2006).
On the Brazilian coastline studies conducted in the continental shelf have shown that Callinectes danae and C. ornatus are the most abundant species in these areas (Lunardon-Branco & Branco, Reference Lunardon-Branco and Branco1993; Severino-Rodrigues et al., Reference Severino-Rodrigues, Guerra and Graça-Lopes2002). In estuarine areas, however, segregation of species distribution was observed, with prevailing species changing along the estuary (Pita et al., Reference Pita, Severino-Rodrigues, Graça-Lopes and Coelho1985; Teixeira & Sá, Reference Teixeira and Sá1998; Severino-Rodrigues et al., Reference Severino-Rodrigues, Pita and Graça-Lopes2001; Carmona-Suárez, Reference Carmona-Suárez2009). Some studies have shown that a variety of factors can influence Callinectes species distribution in an estuary. Attrill et al. (Reference Atrill, Power and Thomas1999) showed the importance of temperature as a controlling variable for some species of Portunidae and other groups. Norse (Reference Norse1978) noticed that distribution of Portunidae in estuarine areas reflects low saline tolerance variations among species, as all species have shown tolerance to higher saline levels. However, Buchanan & Stoner (Reference Buchanan and Stoner1988) suggest that Callinectes spp. distribution patterns can also be the result of complex intraspecific and interspecific interactions between the congeneric species.
In addition to differences between species regarding spatial distribution, species individuals can present different distributions according to development stages. Branco & Masunari (Reference Branco and Masunari2000) and Mantelatto (Reference Mantelatto2000) showed that Callinectes females migrate to external estuarine areas during the spawning seasons. Such behaviour can cause sex proportion differences during specific times of the year. This sexual proportion disparity, expected to be a ratio of 1:1, is common in crustaceans and can be related to both migration and other aspects of species reproductive strategies, such as dispersion patterns, mortality and differential growth rates between sexes (Mantelatto & Fransozo, Reference Mantelatto and Fransozo1999). Moreover, Pita et al. (Reference Pita, Severino-Rodrigues, Graça-Lopes and Coelho1985) observed that age groups show different distributions throughout the estuarine area.
Most studies on population aspects of Callinectes occurring in Brazil were conducted on Callinectes ornatus, C. danae and C. sapidus and are mainly restricted to the subtropical zone of Brazil (Branco & Lunardon-Branco, Reference Branco and Lunardon-Branco1993; Negreiros-Fransozo & Fransozo, Reference Negreiros-Fransozo and Fransozo1995; Mantelatto & Fransozo, Reference Mantelatto and Fransozo1996, Reference Mantelatto and Fransozo1999; Branco & Masunari, Reference Branco and Masunari2000; Baptista et al., Reference Baptista, Pinheiro, Blankensteyn and Borzone2003; Branco & Fracasso, Reference Branco and Fracasso2004; Oliveira et al., Reference Oliveira, Pinto, Santos and D'Incao2006; Pereira et al., Reference Pereira, Branco, Christoffersen, Freitas-Junior, Fracasso and Pinheiro2009). The published studies on population aspects of Callinectes in the tropical zone and studies on the biology of other species found on the Brazilian coastline are still incipient.
The objective of this study is to analyse spatial and temporal distribution of the Callinectes species in the tropical Cachoeira River estuary (Ilhéus, Bahia, Brazil), observing environmental variables that influence the distribution of these organisms.
MATERIALS AND METHODS
The estuarine portion of the Cachoeira River comprises around 1.272 ha of mangroves (Projeto Mata Atlântica Nordeste, 1994). Climate is type Af (hot and humid, without defined dry season), according to the Köppen climate classification system. High rainfall periods are in May and August (namely between June and August), with mean annual rainfall of 2.179 mm; 2.628 mm maximum and 1.737 mm minimum (BAHIA, 1993).
Fluvial systems can be characterized as torrent with very abrupt outflow fluctuations and sediment input in coastal areas (Souza, Reference Souza2005).
Monthly collections were conducted between October 2007 and September 2008 in five stations distributed according to estuary salinity gradient during low tide of neap tide.
Due the characteristics of the environment, with some areas of rocky bottom, traps were used (trapdoor-type devices locally known as manzuá) built in metal and polyethylene netting. A coating net container was installed inside the device to hold the bait (Figure 1). Each trap contained approximately 100 g of bait, composed of meat and sardines (10:1). Six traps were submerged in each station for around two hours; three at the river margin and three in the channel (Figure 2).

Fig. 1. Manzuá-type trap used in collections.

Fig. 2. Study area and layout of traps in each collection station (1 to 5). ▴, channel; •, margin.
Collected crabs were stored in containers with ice and later frozen. In the laboratory, individuals were identified according to species and sex. Morphological maturity was determined through form and adherence of abdominal somites to thoracic sternites. Adults were considered as being those without abdominal somites adhered to sternites (Taissoun, Reference Taissoun1969; Williams, Reference Williams1974).
Sediment samples were collected with Van Veen-type grabs in each collection station for granulometric characterization and organic matter content levels according to Dean (Reference Dean1974). In addition, temperature, salinity and pH of bottom water at margins and channels were also recorded with a portable meter, and transparency with a Secchi disc at the start of each collection. Depth of each collection point was measured with a portable probe (HawkEye® with 0.03 m precision) during low tide of neap tide (height of 0.7 m).
The Student's t-test for dependent samples was used to verify possible differences in species capture between channel and margin of each station.
Canonical correlation analysis (ACC) was conducted on abundance data of each intraspecific group, divided according to species, sex and morphological maturity stage (juvenile, adult and ovigerous) for ordering in accordance with variables of salinity, temperature, transparency, pH, depth, margin distance, organic matter content and granulometric composition. Intraspecific classes with very low abundance (less than eight individuals) were discarded. ACC was conducted on software R (version 2.7) using the vegan package (Oksanen et al., Reference Oksanen, Kindt, Legendre, O'Hara, Simpson, Solymos, Stevens and Wagner2009). The Monte Carlo test with 1000 permutations was used to verify significant variables in species ordering. A significance level of 0.05 was assumed in all analyses.
RESULTS
Salinity presented an upstream reduction pattern due to mixture of seawater with continental drainage. There were no expressive variations throughout the study period. However, collections from October to December 2007 and from July to September 2008 showed slightly higher values in most stations, with the exception of Station 5. All stations except Station 3 showed a clear difference between channel and margin caused by a salt wedge (Figure 3). This could have been caused by bathymetric characteristics and circulation of this station, which allow salt wedge passage near the margin.

Fig. 3. Salinity values (median and quartile) for channels and margin of each collection station in the estuary of Cachoeira River between October 2007 and September 2008.
The pH values did not present a gradient along the estuary, without expressive differences between channel and margin of stations. Highest pH values were recorded in Station 5, which constantly receives nutrients from the nearby sewage treatment station (ETE), possibly increasing primary production with a corresponding dissolved CO2 consumption and consequent elevation of pH values in the area (Table 1).
Table 1. Mean and standard deviation of temperature, pH, transparency and depth values recorded for channel and margin of each station.

Depth and transparency showed an upstream tendency to reduction, with higher values in channels. Temperature presented an inverse tendency, with higher values recorded in margins.
Organic matter content clearly increased upstream. This gradient was most expressive when recording margin values, which were higher than those observed in their corresponding channels (Figure 4).

Fig. 4. Organic matter content in channel and margin of collection stations in the Cachoeira River estuary. S, standard deviation.
A total of 1051 individuals were collected pertaining to five species of the genus Callinectes (793 Callinectes danae, 133 C. ornatus, 66 C. exasperatus, 58 C. larvatus and 1 C. bocourti). Species C. danae, C. exasperatus and C. larvatus presented individuals of both sexes and all stages of maturity in the study area. Callinectes ornatus did not present juvenile females and C. bocourti was represented by a single adult female in the study area (Table 2).
Table 2. Abundance of intraspecific classes of Callinectes in the Cachoeira River estuary.

The dominant species during most of the study period was Callinectes danae, showing lesser abundance from October to November 2007 and September 2008. Highest abundance was recorded in December 2007 (Figure 5).

Fig. 5. Number of Callinectes individuals captured in each collection. Callinectes bocourti was not included as only one individual was collected.
Callinectes ornatus reached highest abundance levels in November 2007, March, July and September 2008, a period in which higher salinity values were observed. This species did not occur or recordings of frequency were very low in other months.
Frequency of other species was low during the entire study period. Callinectes larvatus was not captured from October 2007 to January 2008, and C. exasperatus was not recorded in October 2007 and April 2008, being that highest abundance levels were recorded between July and August 2008.
Only one Callinectes bocourti individual was captured during the entire study period and recorded in the month of August 2008.
Callinectes danae and C. exasperatus were the only species captured in all stations (Figure 6). There was a restricted occurrence of Callinectes ornatus in outer estuarine areas, with collection in Stations 1 and 2. Callinectes larvatus was predominant in the outer and intermediary zones (Stations 1, 2 and 3) with collection of only one individual in Station 5, whereas C. bocourti was only recorded in Station 1.

Fig. 6. Abundance of Callinectes species in channel and margin of each collection station of Cachoeira River estuary. *, significant difference in monthly specimens captured between channel and margin of each station (P < 0.01).
In addition to species distribution differences along the estuary, species also presented different distributions in relation to the transversal estuarine profile. This behaviour was observed through differences in species capture between channel and margin of each station (P < 0.01), with the exception of Callinectes ornatus (P = 0.13). Callinectes danae was the only species found both in channel and margin of all stations (Figure 6). Despite the occurrence of C. exasperatus in all stations, this species was restricted to margins. Similar behaviour was observed for species C. larvatus, which occurred mainly in the margins of Stations 1, 2 and 3, being that only one individual was collected in the channel of Stations 1 and 3 and the margin of Station 5. Callinectes ornatus occurred both in the channel and margin of Station 1, and was recorded in the channel of Station 2.
Callinectes danae was the dominant species in most salinity levels, with the exception of zones with salinity levels between 33 and 37, in which C. ornatus was predominant.
Callinectes larvatus was not recorded to salinity levels 13 to 17, while C. exasperatus showed lesser participation as salinity increased (Figure 7).
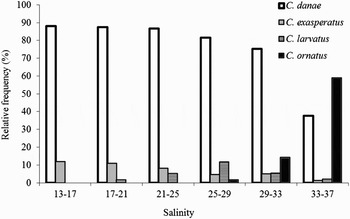
Fig. 7. Relative frequency of Callinectes species in each salinity level. Calinectes bocourti was not included as only one individual was captured.
The Monte Carlo test with ACC data showed the importance of salinity variables, transparency, depth, margin distance, organic components and granulometric fractions (grains, very course sand, course sand, medium sand, fine sand, very fine sand and silt + clay) in intraspecific and species ordering. Grain fractions presented a value of P = 0.02, whereas all variables presented P < 0.01. The pH (P = 0.45) and temperature (P = 0.15) did not show significant correlations in species ordering (Figure 8).

Fig. 8. Canonical ordering diagram based on abundance data by sex and morphological development stage of each species and environmental variable of the Rio Cachoeira estuary. Cd, Callinectes danae; Ce, Callinectes exasperatus; Cl, Callinectes larvatus; Co, Callinectes ornatus; JF, juvenile female; AF, adult female; OF, ovigerous female; JM, juvenile male; AM, adult male; S, salinity; D, depth; T, transparency; DM, distance of margin; O, organic matter content; SC, silt + clay; VFS, very fine sand; FS, fine sand; MS, medium sand; CS, course sand; VCS, very course sand; G, grains.
Analysis showed that Callinectes ornatus occurred mainly in areas of higher salinity, depth, transparency and proportion of medium to fine sand, corresponding to areas of higher marine influence (Stations 1 and 2). Callinectes danae, with occurrence in all stations, was grouped close to the central vector variable regions. Callinectes larvatus was most frequent in areas with intermediary values of salinity, depth and transparency and higher fine fraction sediment and organic matter values. This species also presented a clear negative relation with margin distance. Callinectes exasperatus showed a positive relation with fine fractions of sediment and organic matter content, occurring in areas with lesser salinity, transparency and depth. As in the case of C. larvatus, C. exasperatus was recorded in regions closer to the margin.
Canonical correlation analysis also showed that sex and morphological maturity stage (intraspecific groups) of each species can present a different distribution pattern. Adult females of C. danae, C. exasperatus and C. larvatus tended to occur in outer estuarine areas, with higher values of salinity, transparency and depth. Ovigerous and non-ovigerous females of C. ornatus also present slightly different distribution with ovigerous females occurring in outer estuarine areas.
Juvenile individuals of Callinectes danae and C. larvatus showed different ordering of adult species, which were mainly recorded in inner estuarine areas and near the margin.
DISCUSSION
Considering the environmental variables measured in the estuary of Cachoeira River in conditions of neap tide, species responded to two main gradients. In the first, variables reflect strongly areas of lesser and greater marine influence, while in the second, variables represent variations of water and sediment characteristics found between channel and margin of each estuarine region.
Callinectes danae is shown as the dominant species in most station collections during the study period, supporting significant salinity variation. Due to the ammonia site responsible for osmotic equilibrium, this species can tolerate high salinity variations (Masui et al., Reference Masui, Furriel, McNamara, Mantelatto and Leone2002, Reference Masui, Furriel, Mantelatto, McNamara and Leone2003), which was not observed with C. ornatus (Garçon et al., Reference Garçon, Masui, Mantelatto, McNamara, Furriel and Leone2007). However, despite the physiological capability of C. danae to occur in high saline areas, C. ornatus was more abundant in areas of higher marine influence, possibly indicating higher efficiency of C. ornatus in these conditions.
Among other factors, expressive differences in the capture of individuals between margin and channel of stations may be caused by the difference in salinity observed in this study caused by the saline wedge and characteristic sediment differences, considering that margins tend to present higher participation of fine fractions and higher organic matter content than corresponding channels. In addition to salinity influence, Mansur (Reference Mansur1997) observed a negative relation of Callinectes ornatus with very course, course and medium sand fractions and a positive relation between the species and very fine sand and silt + clay fractions. This behaviour differed from findings in this study in that the species also presented a negative relation with very course and course sand but responded negatively to silt + clay and medium and fine sand areas.
Percentage of organic matter in sediment is considered an important distribution factor of benthic organisms. In this study, Callinectes exasperaus and C. larvatus showed a positive relation with this variable, while C. ornatus occurred in areas of lesser organic matter content. This relation, however, was not observed for species of Callinectes by Mansur (Reference Mansur1997), who only found a significant relation between organic matter content present in the water and abundance of C. sapidus.
The tendency of females, mainly ovigerous females, to occur in outer estuarine areas may be related to migration of these females in the search for more saline waters with better conditions for spawning and subsequent development of larvae stages (Branco & Masunari, Reference Branco and Masunari2000; Mantelatto, Reference Mantelatto2000). This can explain the occurrence of a Callinectes bocourti female in Station 1, as this species predominantly occurs in low salinity (Norse, Reference Norse1978).
Juveniles of C. danae and C. larvatus present different distribution patterns to that of the adults of these species. Similar results were observed by Mansur (Reference Mansur1997), who recorded a majority of juvenile individuals in areas of lower salinity with mean size of individuals increasing towards the sea. In Baia de Ubatuba, Chacur & Negreiros-Fransozo (Reference Chacur and Negreiros-Fransozo2001) also observed that most juveniles of C. danae were found near the mouth of Rio Grande.
Although the study by Attrill et al. (Reference Atrill, Power and Thomas1999) in the Thames estuary, UK, has demonstrated the importance of temperature on the abundance of some species of Portunidae, this relationship was not found in our study. This difference may be due to the regions where the studies were conducted. The Thames estuary is located in a temperate region, with high variation in temperature throughout the year, while the Cachoeira River estuary is located in a tropical region, without major changes (Table 1). On the other hand, internal areas, which tend to present higher temperatures, may contribute to higher growth rates of young individuals, albeit the absence of a significant relation between temperature and abundance of intraspecific groups. Moreover, internal estuarine zones may provide shelter against predators to which adult individuals are more vulnerable.
Adult and juvenile distribution difference may also be caused by different foraging strategies that possibly diminish intraspecific competition, as intraspecific and interspecific interference competition is easily observed in the field. In addition to the scope of this study, aggressive interaction between the Callinectes species and individuals of the same species in the presence of food was observed.
In general terms, the observed distribution pattern agrees with previous group study results (Mansur, Reference Mansur1997; Pita et al., Reference Pita, Severino-Rodrigues, Graça-Lopes and Coelho1985; Buchaman & Stoner, 1988; Branco & Lunardon-Branco, Reference Branco and Lunardon-Branco1993; Negreiros-Fransozo & Fransozo, Reference Negreiros-Fransozo and Fransozo1995; Pereira et al., Reference Pereira, Branco, Christoffersen, Freitas-Junior, Fracasso and Pinheiro2009). However, Teixeira & Sá (Reference Teixeira and Sá1998) recorded in Lagoa de Mundaú (AL) that despite the predominance of Callinectes ornatus in high salinity areas, peak abundance occurred between 20 and 25. The result was significantly different from that found in this study, where the predominant species was found in salinity above 33 and was not recorded in salinity levels below 25. Such stenohaline behaviour corroborates the previous assumption of Mantelatto & Fransozo (Reference Mantelatto and Fransozo1999) that this species' entire life cycle is lived out in marine areas and adult individuals may eventually enter the estuary in search of food resources.
Callinectes bocourti and C. sapidus are the most tolerant to low salinity (Norse, Reference Norse1978) and are generally found in inner estuarine area, which was not sampled in the present study. Callinectes sapidus, recorded in a previous study in the Cachoeira River estuary (Almeida et al., Reference Almeida, Coelho, Santos and Ferraz2006), was not captured in this work. Occurrence of C. bocourti—a female captured in August in Station 1 and two individuals in an additional collection in December 2007 after abundant rainfall—suggest that, with the reduction of salinity in greater rainfall periods, or during the stage in which females search for more saline waters for spawning, these species may occur in outer estuarine areas.
In addition to the strong influence of environmental variables, the distribution pattern presented by species in this study apparently supports the proposal of Buchanan & Stoner (Reference Buchanan and Stoner1988), who observed the complex intraspecific and interspecific interactions between congeneric species. However, studies on physiology, distribution of prey and predators, behavioural interaction between species of Callinectes and their corresponding age groups are required for a better understanding of factors that favour the observed habitat sharing of this group.
ACKNOWLEDGEMENTS
We thank Dr M.F.L. Souza and Dr F.L.M. Mantelatto for advice that greatly improved this study. We also thank E.A. Souza-Carvalho, R. Cavalcante, colleagues of Laboratório de Oceanografia Biológica of UESC and Instituto Bios—INIBIO members for help with field and laboratory work. Financial support was provided by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq CT-Hidro 14/2005 grant number 133342/2006-9) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (F.L.C. Master's scholarship).












