Introduction
The genus Ficopomatus Southern, 1921 comprises six species currently considered valid, namely, F. macrodon Southern, Reference Southern1921 from Chilka Lake, India; F. enigmaticus (Fauvel, Reference Fauvel1923) from Canal de Caen, France; F. miamiensis (Treadwell, Reference Treadwell1934) from Miami River, Florida, USA; F. uschakovi (Pillai, Reference Pillai1960) from Panadura River Estuary, Sri Lanka; F. talehsapensis Pillai, Reference Pillai2008 from Taléh Sap, Thailand; and F. shenzhensis Li et al., Reference Li, Wang and Deng2012 from Guangdon, China. Little is known about three of them (F. macrodon, F. talehsapensis, F. shenzhensis) beyond the basic taxonomic description.
All Ficopomatus are tropical species, except for F. enigmaticus that is found in subtropical and warm-temperate regions. They are extremely euryhaline, and are found in freshwater, marine, and hypersaline environments such as estuaries and mangroves (ten Hove & Weerdenburg, Reference ten Hove and Weerdenburg1978; Pillai, Reference Pillai2008; Li et al., Reference Li, Wang and Deng2012), while F. enigmaticus was recently reported in living stromatolites (Miranda et al., Reference Miranda, Kupriyanova, Rishworth, Peer, Bornman, Bird and Perissinotto2016). All species of the genus may occur as solitary individuals or in dense aggregations. Ficopomatus enigmaticus is the best known ecologically and economically important reef-building species of the genus that has invaded warm-temperate estuaries worldwide (reviewed in Dittmann et al., Reference Dittmann, Rolston, Benger and Kupriyanova2009).
Morphologically, the species in the genus are characterized by an operculum with a bulbous fleshly ampulla, uncovered or covered with either a chitinous endplate or bearing numerous chitinous spines, and very distinct coarsely serrated collar chaetae (Kupriyanova et al., Reference Kupriyanova, Rzhavsky AV, ten Hove, Purschke, Böggemann and Westheide2019). Ficopomatus uschakovi has a distinct apomorphy, dorsally fused thoracic membranes. Opercular shapes and ornamentations of Ficopomatus have been traditionally used to identify to the species level (ten Hove & Weerdenburg, Reference ten Hove and Weerdenburg1978). While F. macrodon, F. miamiensis, F. shenzhensis and F. talehsapensis lack opercular spines, chitinous spines in F. enigmaticus and F. uschakovi show a remarkable variation in shape, number, size and arrangement within a species. Such a variability might be a result of intraspecific variability or attributed to ontogenetic development, but it can be an indication of cryptic species as well. Moreover, F. enigmaticus and F. uschakovi in Australia were confused by Straughan (Reference Straughan1966) who synonymized both species claiming that there is a cline between isolated populations between Sydney (temperate) and Brisbane (tropical). This was refuted by Hartmann-Schröder (Reference Hartmann-Schröder1971), Pillai (Reference Pillai1971) and ten Hove & Weerdenburg (Reference ten Hove and Weerdenburg1978).
The last decades have seen significant progress in the development of genetic tools for annelids that allow to address questions about evolution, development, gene regulation, chronobiology and behaviour on a functional level (Zantke et al., Reference Zantke, Bannister, Rajan, Raible and Tessmar-Raible2014) and documentation of cryptic species as well (Bickford et al., Reference Bickford, Lohman, Sodhi, Ng, Meier, Winker, Ingram and Das2007). In Ficopomatus only a handful of molecular studies have assessed phylogenetic relationships among species and population structure within species. The first phylogenetic analysis based on 18S rDNA and 28S rDNA sequences of F. enigmaticus from Australia, F. macrodon from Thailand and F. miamiensis from Florida, USA (Kupriyanova et al., Reference Kupriyanova, ten Hove, Sket, Zakšek, Trontelj and Rouse2009) confirmed monophyly of the brackish-water genus Ficopomatus and its sister group relationship with the freshwater species Marifugia cavatica Absolon & Hrabê, Reference Absolon and Hrabê1930. Ficopomatus shenzansis Li et al. (Reference Li, Wang and Deng2012) from China was the first species of the genus in which the morphological description was accompanied by an analysis of 18S sequence data obtained from the type material. No further molecular data on Ficopomatus species, other than F. enigmaticus, are available to date. Within F. enigmaticus, an analysis of Cytb sequences revealed three genetic lineages (tentative species) with overlapping distributions in Australia (Styan et al., Reference Styan, McCluskey, Sun and Kupriyanova2017). Two of these lineages showed opercular morphology typical for F. enigmaticus, but the third clade was morphologically distinct, having opercula similar to those in F. uschakovi, but with thoracic membrane not fused dorsally (Fig. 4 in Styan et al., Reference Styan, McCluskey, Sun and Kupriyanova2017). Recent studies revealed additional Cytb haplotype variability within the F. enigmaticus populations from New Zealand, California, Portugal and northern Spain (Yee et al., Reference Yee, Mackie and Pernet2019; Grosse et al., Reference Grosse, Pérez, Juan-Amengual, Pons and Capa2021).
In this study we examined two species of Ficopomatus: F. miamiensis and F. uschakovi. Ficopomatus miamiensis was originally described (as Sphaeropomatus) from Miami River in Florida and reported from Louisiana and Texas, as well from Caribbean localities, such as Jamaica, Barbados, Curaçao and Belize (ten Hove & Weerdenburg, Reference ten Hove and Weerdenburg1978, Bastida-Zavala et al., Reference Bastida-Zavala, McCann, Keppel and Ruiz2017), and the Colombian Caribbean (Fernández-Rodríguez et al., Reference Fernández-Rodríguez, Londoño-Mesa and Ramírez-Restrepo2016). In Mexico, F. miamiensis has been recorded (as Mercierellopsis prietoi) from Tecolutla in the southern Gulf of Mexico by Rioja (Reference Rioja1945) and recently from La Mancha, Veracruz (Ruiz Guerrero & López-Portillo, Reference Ruiz Guerrero and López-Portillo2014, Reference Ruiz Guerrero and López-Portillo2017) and Isla del Carmen, Campeche (Miranda-Salinas et al., Reference Miranda-Salinas, García-Garza and de León-González2016). Currently this species has also established on the Pacific coasts of Mexico, being associated with mangrove roots and shrimp farms in the southern Gulf of California (Salgado-Barragán et al., Reference Salgado-Barragán, Méndez and Toledano-Granados2004; Tovar-Hernández et al., Reference Tovar-Hernández, Méndez and Villalobos-Guerrero2009, Reference Tovar-Hernández, Villalobos-Guerrero, Yáñez-Rivera, Aguilar-Camacho and Ramírez-Santana2012, Reference Tovar-Hernández, Yáñez-Rivera, Villalobos-Guerrero, Aguilar-Camacho, Ramírez-Santana, Low Pfeng, Quijón and Peters2014; Tovar-Hernández & Yáñez-Rivera, Reference Tovar-Hernández, Yáñez-Rivera, Low Pfeng and Peters Recagno2012).
Ficopomatus uschakovi was originally described (as Neopomatus) from Sri Lanka and subsequently reported from tropical localities in Asia, for example India (Fauvel, Reference Fauvel1931, Reference Fauvel1932, Reference Fauvel1953), Java, Indonesia (Pillai, Reference Pillai1965), Malaysia (Rosli et al., Reference Rosli, Yahya, Idris and Bachok2019), Philippines (Pillai, Reference Pillai1965), Thailand (Wangkulangkul et al., Reference Wangkulangkul, Hayeewachi and Rodcharoen2022), Oceania, for example the Solomon Islands (Gibbs, Reference Gibbs1971); tropical Australia: northern New South Wales and Queensland (Dew, Reference Dew1959 (in part); Straughan, Reference Straughan1967, Reference Straughan1968 (in part), Reference Straughan1971, Reference Straughan1972; Hartmann-Schröder, Reference Hartmann-Schröder1971); and Western Africa: Ivory coast (Rullier, Reference Rullier1955) and Nigeria (Sandison & Hill, Reference Sandison and Hill1966). In the Americas, F. uschakovi was first reported from Atlantic coast of Brazil (de Assis et al., Reference de Assis, Alonso and Christoffersen2008), Venezuela (Liñero-Arana & Díaz-Díaz, Reference Liñero-Arana and Díaz-Díaz2012), and during the last decade from Caribbean Colombia (Arteaga-Flórez et al., Reference Arteaga-Flórez, Fernández-Rodríguez and Londoño-Mesa2014; Fernández Rodríguez et al., Reference Fernández-Rodríguez, Londoño-Mesa and Ramírez-Restrepo2016), Southern Mexican Pacific (Bastida-Zavala & García-Madrigal, Reference Bastida-Zavala and García-Madrigal2012; Bastida-Zavala et al., Reference Bastida-Zavala, Rodríguez Buelna, de León-González, Camacho-Cruz and Carmona2016) and the east coast of the USA, i.e. Florida and Texas (Bastida-Zavala et al., Reference Bastida-Zavala, McCann, Keppel and Ruiz2017).
Here we provide new records of F. miamiensis and F. uschakovi from coastal localities along both Atlantic and Pacific coasts of Mexico, and a comparison of mitochondrial cytochrome b (Cytb) DNA sequences of F. miamiensis from the type locality (Florida) and Atlantic and Pacific coasts of Mexico.
Materials and methods
Sampling was carried out for Ficopomatus miamiensis and F. uschakovi on red mangrove roots (Rhizophora mangle Linnaeus), oysters (Crassostrea virginica (Gmelin)) and wood dock pilings in the Laguna de Mandinga, Laguna del Ostión and Alvarado during December 2012 (Atlantic Ocean, Gulf of Mexico, Mexico, Veracruz State) under the sampling permission granted by the Comisión Nacional de Acuacultura y Pesca (DGOPA.14011.151012.3291). Samples from red mangrove were collected from the Reserva de la Biosfera La Encrucijada (Pacific Ocean, Mexico, Chiapas state) in July 2014. Samples from a shrimp farm near Urías Estuary and del Yugo Estuary (Pacific Ocean, Mexico, Gulf of California, Sinaloa State) were collected in August 2014 and October 2014, respectively (Figure 1).

Fig. 1. Amphi-American distribution of Ficopomatus miamiensis (red circles represent records from the present study, yellow stars – records from literature) and F. uschakovi (blue triangles records from the present study, green squares – records from literature).
Mangrove roots and oysters were collected by hand and transported to the laboratory in seawater-filled containers. Tubeworms from shrimp farm pilings were scraped from substrate. Worms were removed from tubes, some were fixed in 95% ethanol and others in formaldehyde as indicated in the material examined sections. A Leica MZ75 stereomicroscope and Olympus CH30 compound microscope were used for identification and digital photographs were taken with an attached Canon S5 digital camera. Several specimens were processed and examined after final dehydration in two changes of 100% ethanol at the Laboratorio de Microscopía Electrónica de Barrido (Facultad de Ciencias, Universidad Autónoma de México). Specimens were critical point dried using CO2, mounted on stubs with platinum tape and coated with gold (200 Å thickness). They were then viewed using a Cambridge 250 scanning electron microscope (SEM).
Specimens were measured to record total body length, peduncle plus operculum length, operculum diameter, thoracic length and thoracic width. The numbers of left and right radioles and thoracic chaetigers were counted, and the position of peduncle insertion (left or right) was recorded. In Tables 1 and 2, measurements are expressed as mean values + SD, number of specimens analysed (N) and the range of such values. For example, total body length 7.35 mm (6.17 + 2.92, N = 8, r: 3.1–11.4 mm).
Table 1. Measurements of Ficopomatus miamiensis from Western Atlantic and Eastern Pacific populations

Table 2. Measurements of Ficopomatus uschakovi from Western Atlantic and Eastern Pacific populations

The species distribution map (Figure 1) was produced using SimpleMappr (Shorthouse, Reference Shorthouse2010). It includes new reports of F. miamiensis and F. uschakovi from this study, as well as previous literature records of F. miamiensis and F. uschakovi in Tropical America (Rioja, Reference Rioja1945; ten Hove & Weerdenburg, Reference ten Hove and Weerdenburg1978; Bastida-Zavala, Reference Bastida-Zavala2008; Bastida-Zavala & García-Madrigal, Reference Bastida-Zavala and García-Madrigal2012; Liñero-Arana & Díaz-Díaz, Reference Liñero-Arana and Díaz-Díaz2012; Arteaga-Flórez et al., Reference Arteaga-Flórez, Fernández-Rodríguez and Londoño-Mesa2014; Ruiz Guerrero & López-Portillo, Reference Ruiz Guerrero and López-Portillo2014, Reference Ruiz Guerrero and López-Portillo2017; Tovar-Hernández et al., Reference Tovar-Hernández, Yáñez-Rivera, Villalobos-Guerrero, Aguilar-Camacho, Ramírez-Santana, Low Pfeng, Quijón and Peters2014; Miranda-Salinas et al., Reference Miranda-Salinas, García-Garza and de León-González2016; Bastida-Zavala et al., Reference Bastida-Zavala, McCann, Keppel and Ruiz2017).
Specimens were deposited in the Collection of Reference of El Colegio de la Frontera Sur, Chetumal (ECOSUR-OH-P), the Colección Poliquetológica de la Universidad Autónoma de Nuevo León (UANL), the Colección Regional de Invertebrados Marinos, Instituto de Ciencias del Mar y Limnología, Universidad Nacional Autónoma de México (ICML–EMU) and the Australian Museum (AM).
DNA extraction, amplification and sequencing
Genomic DNA was extracted from posterior parts of abdomens using the Bioline Isolate II genomic DNA kit according to the manufacturer's protocol. Stock DNA was diluted 1:10 with deionized water to produce template strength DNA for Polymerase Chain Reactions (PCR). The Cytb gene fragments (~350 bp) were amplified with the primer pair Cytb424F (GGWTAYGTWYTWCCWTGRGGWCARAT) and cobr825 (AARTAYCAYTCYGGYTTRATRTG) (Halt et al., Reference Halt, Kupriyanova, Cooper and Rouse2009). PCR conditions were as follows: an initial denaturation step at 94°C for 3 min, 45 cycles at 94°C for 1 min, 50°C for 30 s, 72°C for 1 min, with a final extension at 72°C for 8 min. PCR success was detected using gel electrophoresis (1% agarose gel stained with gel red (Biotium TM, San Francisco)) and visualized using a Bio-Rad XR + Gel Documentation System. Successful PCR products were sent to Macrogen TM, South Korea for purification and standard Sanger sequencing. Sequences were edited and aligned using Geneious. A BLAST search confirmed the correct gene regions had been amplified (Altschul et al., Reference Altschul, Gish, Miller, Myers and Lipman1990) and the new sequences were submitted to GenBank (Table 3).
Table 3. Terminals used in phylogenetic analysis with registration numbers and collection localities

The analysed dataset included 13 Cytb published sequences of Ficopomatus enigmaticus, F. macrodon, F. cf. uschakovi and F. miamiensis available to date in GenBank as well as three new sequences from Tropical America (Table 3). Galeolaria caespitosa was used as an outgroup.
The phylogenetic relationships were inferred using maximum likelihood analysis in IQ-TREE (Minh et al., Reference Minh, Schmidt, Chernomor, Schrempf, Woodhams, von Haeseler and Lanfear2020). Nucleotide substitution model selected using the Bayesian information criterion in ModelFinder (Kalyaanamoorthy et al., Reference Kalyaanamoorthy, Minh, Wong, von Haeseler and Jermiin2017) was HKY + F + I + G4. Branch support was estimated using 1000 ultrafast bootstraps (Hoang et al., Reference Hoang, Chernomor, von Haeseler, Minh and Vinh2018).
Results
Taxonomy
Serpulidae Rafinesque, Reference Rafinesque1815
Genus Ficopomatus Southern, 1921
Ficopomatus miamiensis (Treadwell, Reference Treadwell1934)
Figures 1–4, Table 1
Sphaeropomatus miamiensis Treadwell, Reference Treadwell1934: 339–341, figs 1–5, 9 (Miami River, FL, USA).
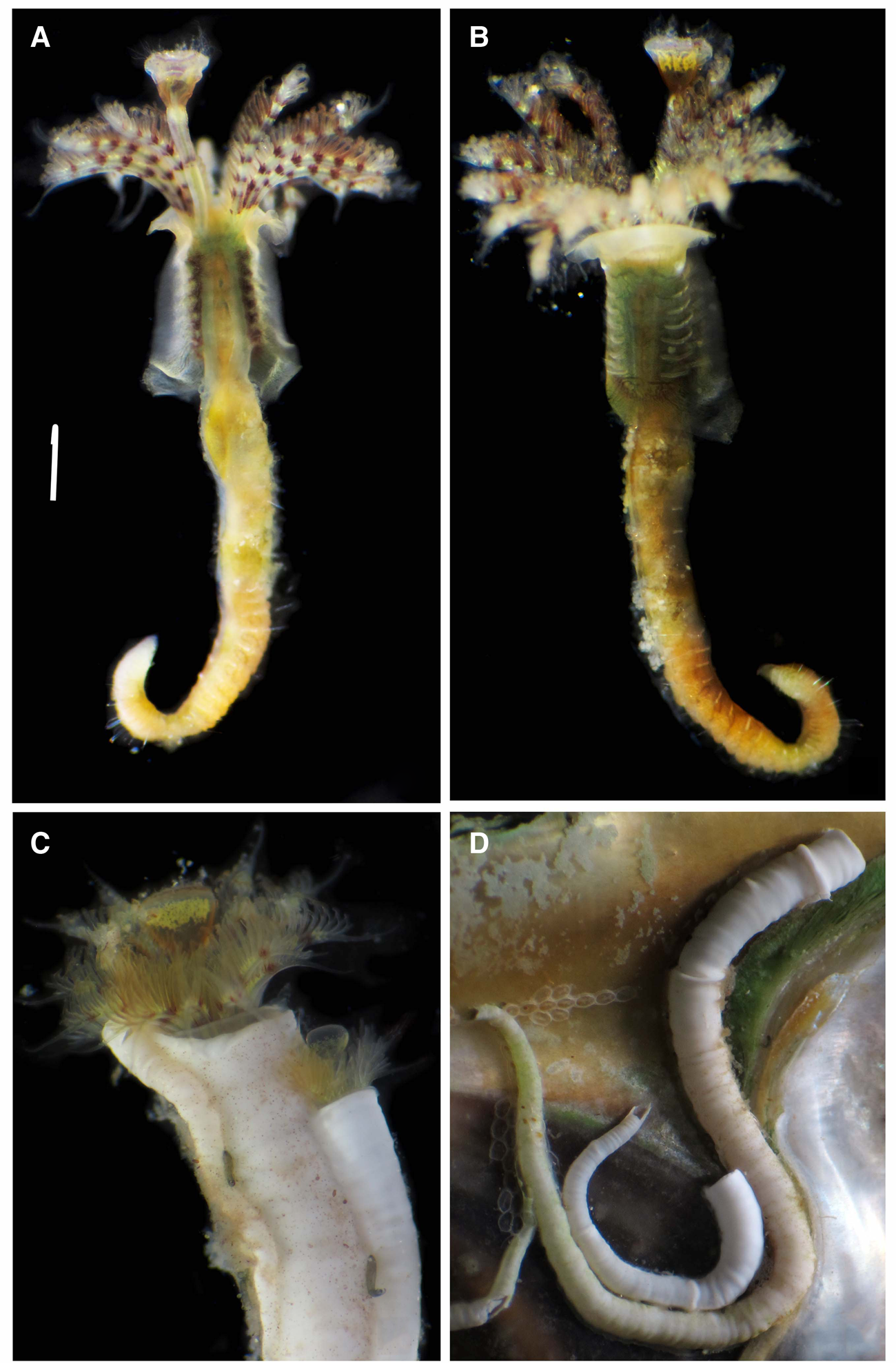
Fig. 2. Ficopomatus miamiensis from the southern Gulf of Mexico, live worms: (A) body, dorsal view; (B) same, ventral view; (C) radiolar crowns and opercula; (D) tubes.
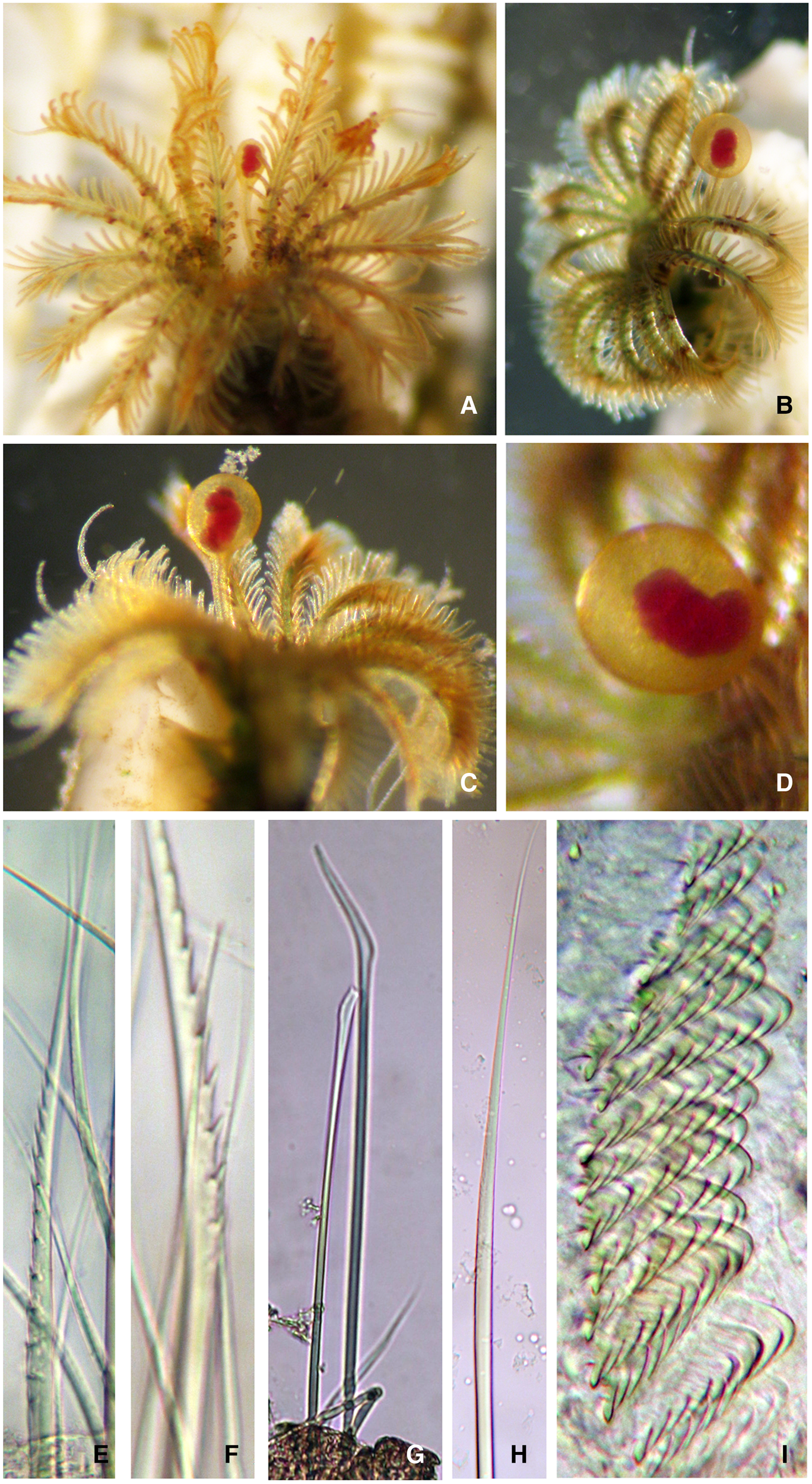
Fig. 3. Ficopomatus miamiensis from the southern Gulf of California: (A–C) radiolar crowns of live worms, (D) detail of operculum showing the internal blood vessel, (E–F) coarsely serrated collar chaetae, (G) abdominal chaeta, (H) thoracic chaeta, (I) saw-shaped anterior abdominal uncini.

Fig. 4. Ficopomatus miamiensis reefs in a shrimp farm from the southern Gulf of California: (A, D) empty pond, soil covered with reefs of tubeworms, (B) reefs at border of a pond, (C) reef fragment removed from a wood piling, (E, G) detail of a reefs, (F) aquaculture infrastructure covered by tubeworms, (H) detail of a colony, (I) tubeworms on mangrove roots.
Mercierellopsis prietoi Rioja, Reference Rioja1945: 411–417, plates 1, 2 (Mexico, Tecolutla (Gulf of Mexico)).
Ficopomatus miamiensis. – Hartman, Reference Hartman1956: 300 (Indian and Miami Rivers, Florida). – ten Hove & Weerdenburg, Reference ten Hove and Weerdenburg1978: 106–109, figs 1f–i, 3c, 4 h–i, q, v–w, ee–ii, xx, 5a–b (Florida, Louisiana, Jamaica, Barbados, Curaçao, Belize, Panama (canal zone)). – Lacalli, Reference Lacalli1976: 301–303 (larval development). – Bastida-Zavala, Reference Bastida-Zavala2008: 19, 21, figs 5B–D (Estero de Urías, Sinaloa, Mexico and Miraflores, Panama). – Tovar-Hernández et al., Reference Tovar-Hernández, Méndez and Villalobos-Guerrero2009, 327–328, Figs 3g–i, 6a, 7a–c (El Confite Estuary, Sinaloa, Mexico). – Ruiz Guerrero & López-Portillo, Reference Ruiz Guerrero and López-Portillo2014: 1316, Reference Ruiz Guerrero and López-Portillo2017: 752 (La Mancha, Veracruz, Mexico, ecology). – Tovar-Hernández et al., Reference Tovar-Hernández, Yáñez-Rivera, Villalobos-Guerrero, Aguilar-Camacho, Ramírez-Santana, Low Pfeng, Quijón and Peters2014: 386, fig 2i (La Paz, Baja California Sur, Mexico). – Bastida-Zavala et al., Reference Bastida-Zavala, McCann, Keppel and Ruiz2017: 19–21, figs 2I–K, 3 (Jacksonville, Indian River, Biscayne Bay, Tampa Bay, Pensacola, FL; Galveston and Corpus Christi TX, USA; Chetumal, Mexico). – Miranda-Salinas et al., Reference Miranda-Salinas, García-Garza and de León-González2016: 10 (Isla del Carmen, Campeche, Mexico).
Examined material
Atlantic Ocean, Gulf of Mexico, Mexico, Veracruz
ECOSUR-OH-P-1116 (1 spec), UANL 8118 (28 spec), AM (W.43523, W.43664, W.43665, W.43666, W.43667, W.43668, W.43669, W.43670, W.43671, W.43672, W.43673) (11 spec): Mandinga, Laguna Grande, Station 26, 19°2′20.64″N 96°4′24.24″W, on oyster C. virginica, 0.5 m deep, 10.74 ppt, 26.63°C, 4.31 mg O2/l, 10 December 2012, legit JM. Aguilar & TF. Villalobos. Fixed in 96% ethanol.
ECOSUR-OH-P1114 (1 spec), ECOSUR-OH-P1115 (1 spec), ECOSUR-OH-P1117 (1 spec), ECOSUR-P3240 (28 spec), UANL 8119 (12 spec): Mandinga, Laguna Grande, Station 27, 19°01.847′N 96°04.845′W, on mangrove roots, 0.5 m deep, 10.87 ppt, 27.23°C, 5.78 mg O2/l, 10 December 2012, legit JM. Aguilar & TF. Villalobos. Fixed in 96% ethanol.
UANL 8120 (30 spec): Mandinga, Laguna Grande, Station 28, 19°01.916′N 96°04.135′W, on oyster C. virginica, 0.5 m deep, 11.41 ppt, 27.24°C, 6.32 mg O2/l, 10 December 2012, legit JM. Aguilar, TF. Villalobos & MA. Tovar. Fixed in 96% ethanol.
ICML–EMU 13,276 (3 spec): Alvarado, 18°44′1.26″N 95°47′1.2″W, on wooden dock, 0.5 m deep, salinity, temperature and dissolved oxygen not determined, 6 December 2012, legit AD. Vera & JM. Aguilar. Fixed in formalin.
ICML–EMU 13277 (8 spec): Alvarado, 18°45′20.34″N 95°46′29.04″W, on car battery, 1 m deep, salinity, temperature and dissolved oxygen not determined, 6 December 2012, legit JM. Aguilar. Fixed in formalin.
ICML–EMU 13278 (23 spec): Alvarado, 18°46′32.76″N 95°46′54.60″W, on a crown-shaped plastic wire, 6 December 2012, 1 m deep, salinity, temperature and dissolved oxygen not determined, legit AD. Vera & JM. Aguilar. Fixed in formalin.
Pacific Ocean, Mexico, Gulf of California, Sinaloa
ECOSUR-OH-P1104 (1 spec), ECOSUR-OH-P1105 (1 spec), ECOSUR-OH-P1106 (1 spec), ECOSUR-OH-P1107 (1 spec), ECOSUR-OH-P1108 (1 spec), ECOSUR-P3241 (3 spec), UANL 8121 (17 spec), ICML–EMU 13279 (38 spec). Shrimp Farm, Urías Estuary, 23°09′30.29″N 106°17′58.35″W, on bivalves covering dock pilings, 5 August 2014, 0.5 m deep, 42 ppt, 29°C, dissolved oxygen not determined. Legit MA. Tovar. Fixed in 96% ethanol.
AM (W.43522, W.43654, W.43655, W.43656, W.43657, W.43658, W.43659, W.43660, W.43661, W.43662, W.43663) (11 spec). El Confite Estuary, 23°0.9′32.1″N 106°18′14.2″W, on mangrove roots, 30 April 2008, 0.3 m deep, salinity, temperature and dissolved oxygen not determined, legit MA. Tovar & J. Salgado. Fixed in 96% ethanol.
UANL 8124 (38 spec): Del Yugo Estuary, 23°18′9.40″N 106°28′59.59″W, on wooden dock pilings and rocks, 9 October 2014, 0.5 m deep, salinity, temperature and dissolved oxygen not determined. Legit MA. Tovar. Fixed in 96% ethanol.
Diagnosis
Tubes gregarious or solitary, white (Figures 2C, 4E, G, H), with 3–5 peristomes, lacking keels or alveoli (Figure 2D). Operculum spherical, smooth, without spines (Figure 3A–D). Collar entire (Figure 2B), collar chaetae coarsely serrated (Figure 3E–F). Thoracic membrane not fused dorsally (Figure 2A), ventrally united to form a short apron (Figure 2B). Thorax with limbate chaetae (Figure 3H) and saw-shaped uncini with 6–8 teeth. Abdomen with true trumpet-shaped chaetae. Anterior abdominal uncini saw to rasp-shaped (Figure 3I), with 8–9 teeth visible in profile. Posterior abdominal uncini rasp-shaped and smaller than anterior ones. Measurements of body and radiolar structures from Atlantic and Pacific populations are shown in Table 1.
Molecular results
The results of maximum likelihood analysis (Figure 5) placed sequences of F. miamiensis from Gulf of Mexico and Gulf of California into a perfectly supported monophyletic clade (100%) with F. miamiensis previously collected in Florida. The F. miamiensis clade formed the sister group to the clade (91.8%) including F. macrodon from Thailand and the F. cf. uschakovi from Southern NSW, Australia (100%). The Ficopomatus enigmaticus clade was well supported (98.7%) and included three well-supported clades including specimens from Australia, California and Spain (Balearic Islands).

Fig. 5. Maximum likelihood phylogram resulting from the analysis of 16 Cytb sequences of Ficopomatus rooted with Galeolaria caespitosa. Numbers above branches are bootstrap values from ML analysis. Only bootstrap values over 70 are shown.
Remarks
We found no phenotypic differences between specimens of F. miamiensis from the Atlantic and the Pacific coasts of Mexico (also see Tovar-Hernández et al., Reference Tovar-Hernández, Méndez and Villalobos-Guerrero2009 (Figs 3g–i, 6a, 7a–c), Tovar-Hernández et al., Reference Tovar-Hernández, Villalobos-Guerrero, Yáñez-Rivera, Aguilar-Camacho and Ramírez-Santana2012 (text figures on pages 12–13), Tovar-Hernández & Yáñez-Rivera, Reference Tovar-Hernández, Yáñez-Rivera, Low Pfeng and Peters Recagno2012 (Figures 1, 5–6)). This species is easy to identify by its smooth spherical operculum lacking spines (Tovar-Hernández et al., Reference Tovar-Hernández, Méndez and Villalobos-Guerrero2009). The examined population of the Atlantic side of Mexico has a larger average body size than that from the Pacific population (7.35 vs 13.61 mm, Table 1). It might be because the Pacific population was collected in culture ponds of a shrimp farm where the food availability is higher than in natural habitats (oyster banks) of the Atlantic population.
Like all species of the genus, F. miamiensis worms are either dioecious (or likely protandric hermaphrodites) lacking sexual dimorphism, their oocytes have asynchronous development and vitellogenesis is extra-ovarian (Lacalli, Reference Lacalli1976; reviewed in Dittmann et al., Reference Dittmann, Rolston, Benger and Kupriyanova2009). External fertilization takes place in the water column and typical planktotrophic larval development from early trochophore to settlement takes place in 13 days at 27°C (Tovar-Hernández et al., Reference Tovar-Hernández, Méndez and Villalobos-Guerrero2009; Tovar-Hernández & Yáñez-Rivera, Reference Tovar-Hernández, Yáñez-Rivera, Low Pfeng and Peters Recagno2012).
Ficopomatus uschakovi (Pillai, Reference Pillai1960)
Figures 1, 6–7, Table 2
Neopomatus uschakovi Pillai, Reference Pillai1960: 28–32, fig. 10H, 11A–H, 12A–H, plate I, figs 1–2 (Panadura River estuary, Madu Gamga estuary at Balapitiya and Ratgama Lake, Donanduwa, Sri Lanka). – Hartman, Reference Hartman1965: 80 (catalogue). – Pillai, Reference Pillai1965: 172 (Surabaja and Madura Island, East Java); Reference Pillai1971: 118–123, 127, fig. 9G, 10 (Hikkaduwa and Panadura, Sri Lanka). – Hartmann-Schröder, Reference Hartmann-Schröder1971: 7–27, fig. 2, 3, 5, 7b–d, 11–14 (Nambucca River, New South Wales; Brisbane, Queensland, Australia). – Zibrowius, Reference Zibrowius1973: 64 (West coast of Africa).
Mercierella enigmatica (non Fauvel, Reference Fauvel1923). – Fauvel, Reference Fauvel1931: 1067, Reference Fauvel1932: 249, Reference Fauvel1933: 185. – Dew, Reference Dew1959: 31, fig. 22. – Straughan, Reference Straughan1966: 143–144, fig. 2–3, Reference Straughan1967: 204, Reference Straughan1968: 59–64, plates 1–5 (in part, plates 1, 2, 3A are F. uschakovi fide ten Hove & Weerdenburg, Reference ten Hove and Weerdenburg1978), 1971: 171–173, 1972 (along entire text, ecological studies).
Neopomatus uschakovi var. lingayanensis Pillai, Reference Pillai1965: 170–172, fig. 23A–I (Lingayan Gulf, Luzon Island, Philippines).
Neopomatus similis Pillai, Reference Pillai1960: 32–33, fig. 12 I–M, plate II, fig. 1 (Negombo Lagoon, Maha Alamba, Pitipana, Sri Lanka). – Hartman, Reference Hartman1965: 80 (catalogue).
Neopomatus similis var. rugosus Pillai, Reference Pillai1960: 33–35, plate II, fig. 2 (Negombo Lagoon, Sri Lanka). – Hartman, Reference Hartman1965: 80 (catalogue).
Ficopomatus uschakovi. – ten Hove & Weerdenburg, Reference ten Hove and Weerdenburg1978: 109–113, fig. 2a–d, 3a, f–k, 4j–n, r, x–z, 5d (Panadura River Estuary, Maha Almba, Ratgama Lake (Sri Lanka), Madras (India), Java (Indonesia), Luzon (Philippines), Guadalcanal (Solomon Islands), Northern New South Wales and Queensland (Australia), Lagos (Nigeria), Abidjan (Ivory Coast), Noordwijk (the Netherlands). – Bastida-Zavala & García-Madrigal, Reference Bastida-Zavala and García-Madrigal2012: 48–52, fig. 1A–E, 2A–I (La Encrucijada, Chiapas, Mexico). – Liñero-Arana & Díaz-Díaz, Reference Liñero-Arana and Díaz-Díaz2012: 235–237, figs 1a–j (Caño Morocoto, Venezuela). – Arteaga-Flórez et al., Reference Arteaga-Flórez, Fernández-Rodríguez and Londoño-Mesa2014: 5–9, fig. 2A–J (Golfo de Urabá, Caribbean Colombia). – Bastida-Zavala et al., Reference Bastida-Zavala, McCann, Keppel and Ruiz2017: 21–22, figs 2L–O, 3 (Florida: Jacksonville, Biscayne Bay; Texas: Galveston, Corpus Christi, USA).
Examined material
Atlantic Ocean, Gulf of Mexico, Mexico, Veracruz State
AM W.43524, W.43674, W.43675, W.43676 (4 spec), ECOSUR-OH-P1109 (1 spec), ECOSUR-OH-P1110 (1 spec), ECOSUR-OH-P1111 (1 spec), ECOSUR-OH-P1112 (1 spec), ECOSUR-OH-P1113 (1 spec), ECOSUR-P3242 (4 spec), UANL 8122 (41 spec): Barrillas, Laguna del Ostión, Station 5, 18°11′38.8″N 94°35′51.27″W, mangrove roots, 0.5 m deep, salinity, temperature and dissolved oxygen not determined, 5 December 2012, legit J. Cruz. Fixed in 96% ethanol.
UANL 8123 (15 spec): Granja Sociedad Cooperativa, Station 4, 18°11′20.15″N 94°35′56.97″W, on oyster C. virginica, 5 December 2012, 0.5 m deep, salinity, temperature and dissolved oxygen not determined, legit J. Cruz. Fixed in formalin.
Pacific Ocean, Mexico, Chiapas State
ECOSUR-OH-P1099 (1 spec), ECOSUR-OH-P1100 (1 spec), ECOSUR-OH-P1101 (1 spec), ECOSUR-OH-P1102 (1 spec), ECOSUR-OH-P1103 (1 spec), ECOSUR-P3243 (1 jar with many tubes attached to mangrove roots), UANL 8125 (1 jar): Reserva de la Biosfera La Encrucijada, Barra San Juan, 14°55′16.78″N 92°37′18.40″W, on mangrove roots, 0.5 m deep, salinity, temperature and dissolved oxygen not determined, 5 July 2014, legit G. Mejía & Y. Siu. Fixed in 96% ethanol.
Diagnosis
Tubes gregarious or solitary, red in live material (Figure 6A–C), changing to brown or orange in preserved material. Tube with prominent to shallow peristomes or only low growth rings. Alveoli absent, a keel sometimes present in specimens from southern Gulf of Mexico (Figure 6C). Operculum spherical to oval in shape, with flat, slightly convex or slightly concave horny endplate (Figure 7B–E). Opercula with 1–5 concentric rows of spines (Figure 7B–E); the rows sometimes incomplete or converging with other rows. Collar entire (Figure 7A). Collar chaetae include coarsely serrated chaetae and narrowly limbate chaetae (Figure 7H). Thoracic membranes fused dorsally (Figure 6D–E), ventrally forming a small apron (Figure 7A). Thorax with limbate chaetae (Figure 7G) and saw-shaped uncini with 6–7 teeth (Figure 7I, K). Abdomen with true trumpet-shaped chaetae (Figure 7F). Anterior abdominal uncini rasp-shaped (Figure 7J) and partly saw-to-rasp-shaped with 9 teeth. Measurements of body and radiolar structures from Atlantic and Pacific populations are given in Table 2.

Fig. 6. Ficopomatus uschakovi from Mexico: (A) tubes on mangrove roots; (B) tubes on oyster C. virginica shells; (C) detail of tubes; (D–E) thoracic membranes, dorsal views, in (E) arrow points to fused membrane. (A–D) Specimens from the southern Gulf of Mexico, (E) specimen from the southern Gulf of California.

Fig. 7. Ficopomatus uschakovi from southern Gulf of Mexico. SEM images: (A) body, ventral view; (B) radiolar crown and operculum; (C–E) opercula, different views; (F) trumpet chaetae from abdomen; (G) chaetae from last thoracic segment; (H) collar chaetae; (I) pegs of uncini from mid-thorax; (J) uncini from anterior abdomen; (K) uncini from mid-thorax. Scale bars: A, 250 μm; B–E, 100 μm; F, 30 μm; G, 20 μm; H, 15 μm; I, 2 μm; J, 10 μm; K, 3 μm.
Remarks
Specimens identified here as F. uschakovi show a distinctive opercular variation, from an operculum being spherical to oval in shape, with a slightly convex, flat or slightly concave horny plate and 1–5 concentric rows of spines, these rows are sometimes incomplete or converge with other rows. This remarkable variation was also noted by Bastida-Zavala & García Madrigal (Reference Bastida-Zavala and García-Madrigal2012) to all descriptions from all American F. uschakovi records.
Unfortunately, attempts to amplify either cytochrome oxidase I (COI) (Beatriz Yáñez Rivera, Luis Fernando Carrera, pers. comm.) or Cytb fragments of F. uschakovi from Mexican Pacific (La Encrucijada) were not successful, perhaps due to the damage of DNA as worms were fixed within their tubes and stored in a hot place for a month after sampling.
Discussion
This study demonstrated that non-native F. miamiensis and F. uschakovi are established on both coasts of Mexico. However, our understanding of taxonomic status, invasive pathways, and ecological impact differs for these two species.
Ficopomatus miamiensis is an apparently single species (as evidenced by morphological and preliminary molecular data here) with the Caribbean native range introduced to and established in the Pacific localities within the last 40 years. According to ten Hove & Weerdenburg (Reference ten Hove and Weerdenburg1978), Ficopomatus miamiensis was originally restricted to tropical and sub-tropical localities along the Atlantic coast of North and Central America and the Caribbean. The authors reported an isolated locality at the Pacific end of the Panama Canal, which could have been the initial point of introduction to the Pacific Ocean. Alternatively, the successful invasion of F. miamiensis to the Gulf of California was attributed to shrimp aquaculture practices four decades ago, when white shrimp (Litopenaeus vannamei) larvae were imported from laboratories in Florida to the farms in the Gulf of California in a time when aquaculture practices were not as controlled as they are now (Tovar-Hernández & Yáñez-Rivera, Reference Tovar-Hernández, Yáñez-Rivera, Low Pfeng and Peters Recagno2012).
In the southern Gulf of Mexico, which apparently constitutes a part of its native range, F. miamiensis inhabits coastal lagoons, often being attached to mangrove roots and oyster (Crassostrea virginica) shells but does not form large aggregations. In the southern Gulf of California, where it is considered an introduced species, F. miamiensis specimens build thousands of small reefs (20–80 cm diameter) per hectare in shrimp farm ponds during every culture cycle (Figure 4A–B, D–E, G–H). The tubes cover any submerged object such as ropes and wood pilings (Figure 4C), but they also grow on mangrove roots in aquaculture ponds (Figure 4I: dry tubes once ponds are drained) (Tovar-Hernández & Yáñez-Rivera, Reference Tovar-Hernández, Yáñez-Rivera, Low Pfeng and Peters Recagno2012).
The presence of the introduced F. miamiensis does not seem to bother aquaculture owners as the worms do not negatively affect shrimp production. Moreover, it is considered a beneficial filter feeder that cleans the water column of shrimp faeces and wasted shrimp food (purina pellets). Once the culture cycle is over and ponds are drained, Ficopomatus tube aggregations are removed, but when the ponds are re-filled to start a new culture cycle, larvae of F. miamiensis re-colonize the farm ponds (Tovar-Hernández & Yáñez-Rivera, Reference Tovar-Hernández, Yáñez-Rivera, Low Pfeng and Peters Recagno2012).
Ecological impacts of F. miamiensis in the estuary El Confite in the southern Gulf of California are unknown. This tubeworm is among the six dominant species (along with mussels, barnacles, oysters and a crab) present throughout the year on mangrove roots. The populations of this species show a remarkable 70% increase in abundance in November/December (Salgado-Barragán, Reference Salgado-Barragán2002), which coincides with the end of culture cycles in October when nutrient-rich water of ponds, containing larvae of F. miamiensis, is directly discharged to the nearest estuary. Larvae of F. miamiensis subsequently settle in large numbers on mangrove roots (Tovar-Hernández & Yáñez-Rivera, Reference Tovar-Hernández, Yáñez-Rivera, Low Pfeng and Peters Recagno2012). Even though economic and ecological impacts of F. miamiensis are unknown, it is included in the list of invasive for Mexico species (Diario Oficial de la Federación, 2016), whose introduction is forbidden in natural protected areas and critical habitats for wildlife conservation as well as in refuge areas to protect native aquatic species.
Unlike F. miamiensis, specimens of F. uschakovi show significant morphological variability suggesting that the nominal species might be an unresolved complex of multiple species, some of which are highly invasive. Thus, as in F. enigmaticus where the existence of cryptic species has been demonstrated with genetic data (Styan et al., Reference Styan, McCluskey, Sun and Kupriyanova2017; Yee et al., Reference Yee, Mackie and Pernet2019; Grosse et al., Reference Grosse, Pérez, Juan-Amengual, Pons and Capa2021), multiple species within F. uschakovi are also likely to be revealed with DNA data. Unfortunately, no published sequences of nominal F. uschakovi are currently available from any locality, except for F. cf. uschakovi from temperate Southern NSW, Australia in Styan et al. (Reference Styan, McCluskey, Sun and Kupriyanova2017). These animals are morphologically distinct from F. uschakovi sensu stricto in having thoracic membranes free, not fused dorsally, suggesting the existence of an undescribed species with F. uschakovi opercular morphology in temperate Australia.
Ficopomatus uschakovi was originally described from Sri Lanka, but its native range in Southeast Asia is unknown. It is clearly a highly invasive taxon that was first introduced to America about a decade ago, but introductory pathways are unknown. In the Biosphere Reserve ‘La Encrucijada’ (Southern Mexican Pacific) the nearest port is 70 km away and there are no shrimp farms that support the hypothesis of ships/marine traffic or aquaculture activities as pathways of introduction (Bastida-Zavala & García-Madrigal, Reference Bastida-Zavala and García-Madrigal2012). The nominal species has been reported in Tropical America forming small patches attached to mangrove roots (de Assis et al., Reference de Assis, Alonso and Christoffersen2008; Bastida-Zavala & García-Madrigal, Reference Bastida-Zavala and García-Madrigal2012; Arteaga-Flórez et al., Reference Arteaga-Flórez, Fernández-Rodríguez and Londoño-Mesa2014) and found in discrete groups under rocks (Liñero-Arana & Díaz-Díaz, Reference Liñero-Arana and Díaz-Díaz2012). Either ecological or economic impacts of this invader are unknown, except for the fact that fishing boats in the southern Mexican Pacific require frequent cleaning of F. uschakovi tube encrustations (Bastida-Zavala & García-Madrigal, Reference Bastida-Zavala and García-Madrigal2012).
Molecular genetics studies are needed to clarify the F. uschakovi taxonomic status and invasive pathways. In particular, specimens from the type locality (Sri Lanka) and other reportedly native localities in Southeast Asia need to be sequenced and compared with the sequences obtained from specimens collected along American coasts. Further ecological studies are needed to evaluate potential impacts of these invasive species on native biota and economic activities.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Acknowledgements
We thank Gamaliel Mejía and Yadira Siu Rodas (ECOSUR-Tapachula) who sent us samples from Chiapas and their sampling efforts are much appreciated. Facilities in La Laguna del Ostión and Granja Sociedad Cooperativa Purificadora de Ostión were kindly provided by Sr. José Cruz Terrón. Silvia Espinosa Matías (Facultad de Ciencias, UNAM) processed SEM photographs and images included in Figure 5. Photographs in Figures 2 and 5 were taken by Humberto Bahena-Basave (ECOSUR-Chetumal), and those from Figure 4 by Sergio Rendón Rodríguez (ICML, UNAM). Jose María Aguilar-Camacho (Hebrew University of Jerusalem), Tulio F. Villalobos-Guerrero and Dalia Vera-Hidalgo (Universidad Autónoma de Chiapas) were all very helpful during sampling in Veracruz and sorting of materials. Special thanks to Guillemine Daffe (Université de Bordeaux, France) for sequencing Cytb of F. miamiensis at the Australian Museum and to Beatriz Yáñez Rivera (ICML, UNAM) and Luis F. Carrera Parra (ECOSUR Chetumal) for their efforts to obtain COI sequences of the species reported here. We would like to thank Rolando Bastida-Zavala (Universidad del Mar, México) for sending us literature.
Author contributions
MATH, conceptualization; funding acquisition; sampling; writing; original draft preparation, review and editing. JAdLG: analysis; writing; drafting the work; editing and final approval of version to be published. EK: analysis; funding acquisition; writing, review and editing.
Financial support
This work was supported by Instituto Nacional de Ecología (grants INE/ADE-013/2011 and INE/PC-020/2012), Fondo Sectorial de Investigación Ambiental (grant SEMARNAT-CONACYT A3-S-73811) and Australian Biological Resources Study (grant RG18–21).
Conflict of interest
The authors declare none.












