Unfortunately, it is common for children to be exposed to severe, adverse life events (Cohen, Hitsman, et al., Reference Cohen, Hitsman, Paul, McCaffery, Stroud and Sweet2006; Copeland, Keeler, Angold, & Costello, Reference Copeland, Keeler, Angold and Costello2007). More than two-thirds of children under the age of 16 experience at least one trauma-level life event (Cohen, Grieve, et al., Reference Cohen, Grieve, Hoth, Paul, Sweet and Tate2006; Copeland et al., Reference Copeland, Keeler, Angold and Costello2007) and amount of exposure increases in high-risk subgroups, as seen for children growing up in unsafe neighborhoods (e.g., Boothroyd & Evans, Reference Boothroyd and Evans2001) or in countries at war (e.g., Sack, Him, & Dickason, Reference Sack, Him and Dickason1999). This is important for public health, because a broad body of research spanning the social and life sciences has established the negative impact of early stress on behavior, physiology, and long-term mental and physical health (e.g., Cohen, Janicki-Deverts, & Miller, Reference Cohen, Janicki-Deverts and Miller2007; Copeland et al., Reference Copeland, Keeler, Angold and Costello2007; Hofstra, van der Ende, & Verhulst, Reference Hofstra, van der Ende and Verhulst2002; Rosenblum, Forger, Noland, Trost, & Coplan, Reference Rosenblum, Forger, Noland, Trost and Coplan2001).
There is a dose-dependent relationship between trauma exposure and psychiatric disorder in children (Copeland et al., Reference Copeland, Keeler, Angold and Costello2007), and trauma exposure is a risk factor for future psychopathology, even in nonclinical samples (Bremner, Southwick, Johnson, Yehuda, & Charney, Reference Bremner, Southwick, Johnson, Yehuda and Charney1993).Footnote 1 The brain is the primary mediator between life stress and biobehavioral outcomes (e.g., Ganzel, Morris, & Wethington, Reference Ganzel, Morris and Wethington2010; McEwen, Reference McEwen2007), but the neurobiology underlying this mediation is not well specified. In particular, remarkably little is known about the effects of stress on brain function and structure in healthy adolescents (i.e., in samples screened to exclude clinical disorder). Nonclinical samples are of interest to this question, because the neural consequences of stressor exposure in nonpsychiatric individuals may predict risk for psychopathology, but they are less likely to be confounded with a disease state.
The neural systems underlying emotion processing are the most vulnerable to change and/or damage due to stress (Ganzel & Morris, Reference Ganzel and Morris2011; Ganzel et al., Reference Ganzel, Morris and Wethington2010). Stress-sensitive brain regions include the amygdala, the hippocampus, and the prefrontal cortex (PFC; particularly the medial and the ventromedial PFC and the anterior cingulate; Cerqueira, Mailliet, Almeida, Jay, & Sousa, Reference Cerqueira, Mailliet, Almeida, Jay and Sousa2007; McEwen, Reference McEwen2005; Vyas, Mitra, Shankaranarayana Rao, & Chattarji, Reference Vyas, Mitra, Shankaranarayana Rao and Chattarji2002). Research regarding the neural basis of fear and anxiety suggests that extreme, repeated, or chronic activation within these systems may result in their becoming sensitized (e.g., Rosen & Schulkin, Reference Rosen and Schulkin1998, Reference Rosen, Schulkin and Schulkin2004; see also McEwen, Reference McEwen2007). Acute stress-related increases in adrenal steroids may be an underlying factor in sensitization, and such increases have been associated with strong increases in resting-state activity in the hippocampus, amygdala, and PFC in rodents and healthy humans (Ferris & Stolberg, Reference Ferris and Stolberg2010; Lovallo, Robinson, Glahn, & Fox, Reference Lovallo, Robinson, Glahn and Fox2010). Currently, it is argued that the interaction between adrenal steroids (e.g., glucocorticoids) and excitatory amino acids (e.g., glutamate) drives stress-related plasticity and/or damage in these brain regions (for a review, see McEwen & Gianaros, Reference McEwen and Gianaros2010). This in turn may underlie sensitization of these brain regions, such that these areas show increased reactivity to nonspecific, low-level stimuli that is accompanied by increases in fear-related behavior (e.g., Rosen & Schulkin, Reference Rosen and Schulkin1998, Reference Rosen, Schulkin and Schulkin2004).
Stress-related neural change may take different forms in different brain regions, as observed in adult animal models. Uncontrollable stress produces extended hyperexcitability of the amygdala and related structures, which are then more readily activated by unrelated stimuli (Adamec, Blundell, & Burton, Reference Adamec, Blundell and Burton2005; Maier & Watkins, Reference Maier and Watkins1998; Rosen & Schulkin, Reference Rosen and Schulkin1998). At the neuronal level, chronic stress produces expanded dendritic arborization and increased spine density in the amygdala, along with increases in anxiety-like behavior (Vyas et al., Reference Vyas, Mitra, Shankaranarayana Rao and Chattarji2002). Similar effects have been observed even with single-event stressors (Mitra, Jadhav, McEwen, & Chattarji, Reference Mitra, Jadhav, McEwen and Chattarji2005). Conversely, stressor exposure is associated with decreased dendritic arborization and spine density of neurons in the hippocampus and medial PFC (Blanchard et al., Reference Blanchard, Spencer, Wiess, Blanchard, McEwen and Sakai1995; Blanchard, Sakai, McEwen, Weiss, & Blanchard, Reference Blanchard, Sakai, McEwen, Weiss and Blanchard1993; Radley et al., Reference Radley, Rocher, Miller, Janssen, Liston and Hof2006; Vyas et al., Reference Vyas, Mitra, Shankaranarayana Rao and Chattarji2002). The latter effects are reversible after the stressor is removed, whereas stress-related changes in the architecture and function of the amygdala (and in behavioral anxiety) appear more persistent (Adamec et al., Reference Adamec, Blundell and Burton2005; Tottenham et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011; Vyas et al., Reference Vyas, Mitra, Shankaranarayana Rao and Chattarji2002).
There is accumulating evidence for long-term effects of stress on the nonclinical human brain. In adults, exposure to severe stressors is associated with later amygdala hyperexcitability, accompanied by increased anxiety (Ganzel, Kim, Glover, & Temple, Reference Ganzel, Kim, Glover and Temple2008) and enhanced recollection of the originating stressor (Sharot, Martorella, Delgado, & Phelps, Reference Sharot, Martorella, Delgado and Phelps2007). Stress-related atrophy and disrupted neural connectivity have been observed in the adult human amygdala, hippocampus, and PFC (Ganzel, Casey, Glover, Voss, & Temple, Reference Ganzel, Casey, Glover, Voss and Temple2007; Ganzel et al., Reference Ganzel, Kim, Glover and Temple2008; Gianaros et al., Reference Gianaros, Jennings, Sheu, Greer, Kuller and Matthews2007; Liston, McEwen, & Casey, Reference Liston, McEwen and Casey2009). When these effects occur in response to relatively moderate stressors (e.g., examination stress), they were reversible (e.g., in the PFC; Liston et al., Reference Liston, McEwen and Casey2009). However, reversibility is understudied in humans and may depend on the repetition, chronicity, and/or intensity of the stressor. There is currently only limited evidence for the reversibility of stress-related increases in amygdala reactivity or behavioral indicators of anxiety in animals or humans (e.g., Adamec et al., Reference Adamec, Blundell and Burton2005; Ganzel et al., Reference Ganzel, Kim, Glover and Temple2008; Vyas et al., Reference Vyas, Mitra, Shankaranarayana Rao and Chattarji2002; although see Ganzel et al., Reference Ganzel, Casey, Glover, Voss and Temple2007).
Research on the effects of stress on the developing brain has focused on disruptions in caregiving during infancy, when the amygdala, the hippocampus, and the hypothalamic–pituitary–adrenal axis are likely to be undergoing rapid development (e.g., Moriceau, Roth, Okotoghaide, & Sullivan, Reference Moriceau, Roth, Okotoghaide and Sullivan2004; Plotsky & Meaney, Reference Plotsky and Meaney1993). In rodents, maternal separation during this postnatal period is reported to have long-term neural effects, including acceleration of amygdala development (Ono et al., Reference Ono, Kikusui, Sasaki, Ichikawa, Mori and Murakami-Murofushi2008), increases in amygdala corticotropin-releasing hormone messenger RNA expression (e.g., Hatalski, Guirguis, & Baram, Reference Hatalski, Guirguis and Baram1998), greater hypothalamic–pituitary–adrenal axis reactivity to stress, and increased anxiety, aggression, and social instability in adulthood (Kikusui & Mori, Reference Kikusui and Mori2009). For human infants, disruptions in caregiving are also associated with increases in anxiety and long-term impairments in social behavior (e.g., Tottenham et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011; Zeanah et al., Reference Zeanah, Egger, Smyke, Nelson, Fox and Marshall2009), accompanied by amygdala hypertrophy (Tottenham, Hare, et al., Reference Tottenham, Hare, Quinn, McCarry, Nurse and Gilhooly2009) and heightened amygdala response to emotional faces; Tottenham et al., Reference Tottenham, Hare, Millner, Gilhooly, Zevin and Casey2011).Footnote 2
There is emerging evidence that other types of stressor exposure in childhood and adolescence may also have long-term effects on the brain, as well as on behavior. Much of this evidence is indirect. Healthy adults who report growing up with low perceived socioeconomic status (Gianaros et al., Reference Gianaros, Horenstein, Hariri, Sheu, Manuck and Matthews2008) or risky family environments (Taylor, Eisenberger, Saxbe, Lehman, & Lieberman, Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006) show abnormal amygdala reactivity and connectivity relative to adults reporting fewer comparable childhood stressors. These results are consistent with a pattern of avoidant coping in response to stressors that can be ignored and behavioral hyppereactivity to stressors that cannot be ignored (Taylor, Reference Taylor2010). In addition, nonclinical adults who report a greater number of negative early life events are more likely to have increased problems with anxiety and addiction, which in turn have been associated with atypical brain electrophysiology (McFarlane et al., Reference McFarlane, Clark, Bryant, Williams, Niaura and Paul2005) and smaller medial PFC (Cohen, Grieve, et al., Reference Cohen, Grieve, Hoth, Paul, Sweet and Tate2006). Altered white matter integrity has also been observed in nonclinical adults and youth with a history of multiple negative early life events (Paul et al., Reference Paul, Henry, Grieve, Guilmette, Niaura and Bryant2008). In one of the very few neuroimaging studies of nonclinical youth in the extant literature, reductions in white matter integrity in the corpus callosum were observed in children (ages 8 to 18 years) who had three or more negative life events, relative to age-matched controls exposed to fewer life events (Seckfort et al., Reference Seckfort, Paul, Grieve, Vandenberg, Bryant and Williams2008). In this study, differences in white matter integrity were not related to processing speed, verbal memory, or cognitive flexibility, so that the behavioral consequences of early life stress remain unclear.
Neuroimaging studies of pediatric posttraumatic stress disorder (PTSD) may provide some insight into the ways in which development modulates the neural embedding of stress and trauma in nonclinical youth. In particular, the neural sequelae of trauma exposure in PTSD appears to differ between children and adults (e.g., De Bellis et al., Reference De Bellis, Keshavan, Shifflett, Iyengar, Beers and Hall2002; Rauch, Shin, & Wright, Reference Rauch, Shin and Wright2003). In adults, PTSD is associated with amygdala hyperexcitability and decreases in PFC function, accompanied by decreased regional gray matter volume in the rostral and dorsal anterior cingulate cortex, and possibly also in the amygdala (Kasai et al., Reference Kasai, Yamasue, Gilbertson, Shenton, Rauch and Pitman2008; Rauch et al., Reference Rauch, Shin and Wright2003; Rogers et al., Reference Rogers, Yamasue, Abe, Yamada, Ohtani and Iwanami2009). In children, however, PTSD is primarily characterized by a more generalized impact on the brain, as evidenced by smaller total brain volumes, including regional volumetric decreases observed in the ventral PFC, the right temporal lobe, the corpus callosum and the cerebellum, but not in the amygdala (De Bellis et al., Reference De Bellis, Keshavan, Shifflett, Iyengar, Beers and Hall2002; De Bellis, Keshavan, et al., Reference De Bellis, Baum, Birmaher, Keshavan and Ryan1999; De Bellis & Kuchibhatla, Reference De Bellis and Kuchibhatla2006; see also Carrion, Weems, Richert, Hoffman, & Reiss, Reference Carrion, Weems, Richert, Hoffman and Reiss2010). The persistence of these effects across time and development is unclear.
In sum, the literature suggests that stress may alter limbic structure and function, especially at the level of the amygdala. Stress may impact children and adolescents differently, in that ongoing dynamic development of the brain is likely to interact with stress processes at the neural level. Thus, it would not be surprising if stress-related neural change appeared systemically and coincided with global alterations in brain structure, as evidenced by prior research on PTSD.
In the present study, we used functional and structural magnetic resonance imaging (MRI) to examine the neural correlates of prior life stress and current anxiety in a sample of healthy adolescents. Building on previous assays of amygdala responsiveness (Guyer et al., Reference Guyer, Monk, McClure-Tone, Nelson, Roberson-Nay and Adler2008; Thomas et al., Reference Thomas, Drevets, Dahl, Ryan, Birmaher and Eccard2001), participants passively viewed blocks of fearful and calm faces while undergoing functional neuroimaging. We assessed amygdala reactivity (fearful vs. calm contrast) and brain volume in order to distinguish the relative contributions of these factors to the association between prior life stress and current anxiety.
Methods
Participants
Seventeen adolescents were recruited, screened, and imaged for this study. We obtained informed parental consent and child assent according to Cornell University institutional guidelines for the protection of human subjects. Exclusion criteria included contraindications for neuroimaging; present or past psychiatric, endocrine, neurological, and other major medical illness; current use of prescription medication; and left-handedness. Imaging data from 2 adolescents were excluded from the data set owing to technical difficulties with the scanner, and one family did not complete behavioral data collection, leaving a final sample of 14 adolescents (10 male, 4 female; mean age = 13.1 ± 2.2 years, range = 10–15 years of age). Pubertal development was assessed using the Peterson Developmental Scale (Peterson, Crockett, Richards, & Boxer, Reference Peterson, Crockett, Richards and Boxer1987). Handedness was assessed using the 12 handedness items from the Revised Physical and Neurological Examination for Subtle Signs (Denkla, Reference Denkla1985).
Behavioral assessments
Current PTSD and major depression were assessed as exclusion criteria using the relevant modules of the University of Michigan National Comorbidity Survey Composite International Diagnostic Interview (CIDI; Kessler et al., Reference Kessler, McGonagle, Zhao, Nelson, Hughes and Eshelman1994). The CIDI is a comprehensive, fully structured diagnostic interview for assessment of current and lifetime mental disorders following criteria from the revised third edition of the Diagnostic and Statistical Manual for Mental Disorders (DSM; American Psychiatric Association [APA], 1987). Standardized CIDI interview protocols were followed. There is good concordance between diagnoses obtained with the CIDI and from clinician interview, with κs ranging from 0.75 to 0.96 for major depression and the anxiety disorders, including PTSD (Kessler, Sonnega, Bromet, Hughes, & Nelson, Reference Kessler, Sonnega, Bromet, Hughes and Nelson1995; Reed et al., Reference Reed, Gander, Pfister, Steiger, Sonntag and Trenkwalder1998). The CIDI PTSD interview also assesses lifetime exposure to life events, as defined by the DSM criterion for PTSD (using the “A1” stressor criterion; e.g., APA, 1987). It has been used in major epidemiological studies to quantify prior trauma exposure across the lifetime (e.g., Breslau et al., Reference Breslau, Kessler, Chilcoat, Schultz, Davis and Andreski1998; Kessler et al., Reference Kessler, McGonagle, Zhao, Nelson, Hughes and Eshelman1994; Young & Breslau, Reference Young and Breslau2004). Categories of life events include interpersonal or sexual violence, life-threatening accidents or illness to self or a loved ones, sudden death of a loved one, human-made and natural disaster, and witnessing or perpetrating violence, as well as open-ended questions regarding other types of adverse life events (e.g., “Did you ever experience any other extremely traumatic or life-threatening event that I haven't asked about yet?”). In this structured interview, each type of event is assessed separately. For example, if the child was involved in a life-threatening auto accident in which a close family member was killed, then both types of event are endorsed (see Stein et al., Reference Stein, Chiu, Hwang, Kessler, Sampson and Alonso2010). The number of each type of life event and the child age at each event were recorded. Responses to trauma history probes from the CIDI PTSD interview were used to derived values for number of trauma-level life events (which we term life events) from birth to the time of imaging, years since the worst event, years since the most recent event, age at the first event, and age at the worst event. We adhered to standardized CIDI interview protocols, paying significant attention to questionnaire methodology in order to maximize respondents’ recall and to minimize reporting differences. The event history questions in the CIDI PTSD modular interview are derived from the widely used Trauma History Questionnaire, which shows relatively good stability over several months in clinical and nonclinical samples (e.g., Green, Reference Green, Stamm and Varra1996). To minimize bias owing to mood-congruent recall, parents provided information about adverse life events on behalf of their children (e.g., see protocols from the Yale Child Study Center Child & Family Traumatic Stress Intervention; Berkowitz & Stover, Reference Berkowitz and Stover2005; Berkowitz, Stover, & Marans, Reference Berkowitz, Stover and Marans2011).
The Screen for Child Anxiety Related Emotional Disorders (SCARED; Birmaher et al., Reference Birmaher, Brent, Chiappetta, Bridge, Monga and Baugher1999) was used to assess current anxiety in this sample of adolescents. The SCARED is a 41-item child self-report measure of daily anxiety with five factors assessing DSM-IV (APA, 1994) classifications for generalized anxiety disorder, somatic/panic disorder, separation anxiety, social phobia, and school phobia. The SCARED has good internal consistency (α = 0.74–0.93) and good discriminative and test–retest reliabilities (intraclass correlation coefficients = 0.70–0.90; Birmaher et al., Reference Birmaher, Brent, Chiappetta, Bridge, Monga and Baugher1999). Scores on the SCARED can range from 0 to 82. Scores above 25 may indicate an anxiety disorder, whereas scores higher than 30 are a more specific indicator. This instrument has been used for research purposes with clinical and nonclinical samples (Birmaher et al., Reference Birmaher, Brent, Chiappetta, Bridge, Monga and Baugher1999; Thomas et al., Reference Thomas, Drevets, Dahl, Ryan, Birmaher and Eccard2001).
An assessment of current PTSD symptoms was obtained using an eight-item subset of the Impact of Events Scale (IES-8; Horowitz, Wilner, & Alvarez, Reference Horowitz, Wilner and Alvarez1979) that has been validated for use with children (Dyregrov & Yule, Reference Dyregrov and Yule1995; Smith, Perrin, Dyregrov, & Yule, Reference Smith, Perrin, Dyregrov and Yule2003). The IES-8 is an eight-item measure of pediatric PTSD symptoms that occur in the week prior to assessment (in this case, in the week prior to imaging). In a study of 2,976 war-exposed Bosnian children (ages 9–14 years), the IES-8 showed good internal consistency: total symptom scale, α = 0.75; intrusion subscale, α = 0.75; and avoidance subscale, α = 0.73 (Smith et al., Reference Smith, Perrin, Dyregrov and Yule2003).
Stimuli and imaging methods
Stimuli
Stimuli presentation and procedures followed Ganzel et al. (Reference Ganzel, Casey, Glover, Voss and Temple2007). During imaging, adolescents passively viewed images of fearful and calm faces from a standardized picture set (the MacBrain Face Stimulus Set; Tottenham, Tanaka, et al., Reference Tottenham, Tanaka, Leon, McCarry, Nurse and Hare2009) presented on an overhead liquid crystal panel. Images consisting of a fixation cross (+), fearful faces (F), or calm faces (C) were presented in a pseudorandom sequence of nine blocks (see Figure 1). In each block of faces, 10 images were presented for 4 s each. Fixation blocks were 30 s long. The order of block presentation was counterbalanced across subjects in the following sequences: +CF+CF+CF or +FC +FC+FC. The total stimulus presentation was 330 s.

Figure 1. (Color online) Stimuli consisted of blocks of fearful (F) and calm (C) faces separated by a fixation (+) cross, counterbalanced +FC + FC + FC or +CF + CF + CF.
Image acquisition
Imaging was performed using a General Electric Signa 3-tesla MRI scanner (General Electric Medical Systems, Milwaukee, WI). After an initial three-plane localizer, anatomical images were acquired to correct for head position in three dimensions using a fast spin-echo sequence (repetition time [TR] = 4000 ms, echo time [TE] = 68 ms, flip = 90°, field of view [FOV] = 20 cm, 29 slices, 5-mm slice thickness, no gap, axial–oblique orientation). During the same session, a three-dimensional spoiled gradient recalled T1-weighted anatomical scan was acquired for normalization (124 axial slices, TR = 25 ms, TE = 5 ms, flip = 20°, FOV = 24 cm, 1.5 mm thickness, 0 mm gap). Functional imaging data was acquired while each subject viewed the stimuli described above. Twenty-nine slices per volume (5 mm thick, no gap) were collected in the same spatial prescription as the fast spin-echo using a spiral in–out sequence (Preston, Thomason, Ochsner, Cooper, & Glover, Reference Preston, Thomason, Ochsner, Cooper and Glover2004) with a 2000 ms TR and a 30 ms TE. All MRI images were inspected by a board-certified neuroradiologist to rule out gross brain abnormalities.
Functional MRI data analysis
We analyzed the functional imaging data using SPM8 (Wellcome Trust Centre for Neuroimaging, University College, London) implemented on MatLab 7.12.0 R2011a (MathWorks, Natick, MA). During preprocessing, the first four volumes were deleted and images were realigned using a six-parameter rigid body transformation. Images were then smoothed with a 4-mm full width at half-maximum (FWHM) Gaussian kernel to facilitate application of a motion adjustment algorithm to correct interpolation errors from excessive head movement (Mazaika, Hoeft, Glover, & Reiss, Reference Mazaika, Hoeft, Glover and Reiss2009). The realigned images were normalized within standard Montreal Neurological Institute (MNI) space via 12-parameter affine transformation and smoothed again using a 7-mm FWHM kernel to minimize noise (the two smoothing operations are approximately equal to one smoothing with an 8-mm FWHM kernel; Mazaika et al., Reference Mazaika, Hoeft, Glover and Reiss2009; Mazaika, Whitfield-Gabrieli, & Reiss, Reference Mazaika, Whitfield-Gabrieli and Reiss2007). Standard adult templates have been shown to be appropriate for normalization of MRI data from older children and adolescents (Burgund et al., Reference Burgund, Kang, Kelly, Buckner, Snyder and Petersen2002).
Statistical analyses were based on fixed-effects models at the level of the individual participant and on random effects models at the level of the group (Friston, Holmes, Price, Buchel, & Worsley, Reference Friston, Holmes, Price, Buchel and Worsley1999). At the individual level, statistical models were constructed using a general linear model with a delayed box-car reference function. Comparison contrasts (t tests) were performed for each stimulus class versus the others (e.g., fearful vs. calm faces, fearful faces vs. fixation, and calm faces vs. fixation). This study focuses on the comparison contrast of fearful versus calm faces.
At the group level, models were constructed to examine the associations among life events, brain function, and current anxiety in this sample. Whole brain voxelwise covariate analyses (fearful vs. calm contrast) were conducted to examine the role of emotion processing in the association between lifetime accumulation of life events and current anxiety.
Whole brain Monte Carlo simulations (10,000 iterations) were conducted in the AlphaSim program within Analysis of Functional NeuroImages software package (Cox, Reference Cox1996). Clusterwise false positive rates of p < .05 (corrected for multiple comparisons) were determined for whole-brain analyses and for regions of interest in the amygdala and the hippocampus. Throughout the text, p values with correction for multiple comparisons are identified. Coordinates presented in the text represent the peak voxel for the cluster or region of interest (ROI). Volumetric analyses for the amygdala and the hippocampus were conducted using the Wake Forest University PickAtlas (Maldjian, Laurienti, Burdette, & Kraft, Reference Maldjian, Laurienti, Burdette and Kraft2003).
ROI analyses were conducted to test a priori hypotheses regarding the association among life events, anxiety, and amygdala activation in response to emotional faces. Post hoc ROI analyses were limited to clusters that included the amygdala. Associations between the behavioral measures and blood oxygen level dependent (BOLD) signal change in the amygdala for the fearful–calm contrast in each ROI were examined via linear regression using Statistical Package for the Social Sciences software (Armonk, New York). If an outlier of 2 SD or more was observed in a given analysis, the outlier was removed and the analysis was rerun. In this sample of adolescents, age and pubertal status were collinear (r = .86, p < .001), so that age and sex were used as the primary control variables in these analyses.
MRI data analysis
Total brain volume (gray matter, white matter, and cerebral spinal fluid), total white matter volume, and total gray matter volume were calculated for each participant from her or his T1-weighted anatomical scan using voxel-based morphometry (C. Gaser, Department of Psychiatry, University of Jena, Germany; http://dbm.neuro.uni-jena.de/vbm8) executed in Matlab 7.12.0 R2011a (e.g., Bergouignan et al., Reference Bergouignan, Chupin, Czechowska, Kinkingnéhun and Le Bastard2009; Yassa & Stark, Reference Yassa and Stark2009). Structural images were segmented into gray matter, white matter, and cerebrospinal fluid using the hidden Markov random field option. Rigid-body aligned segmented images were normalized to the MNI template using the high-dimensional diffeomorphic anatomical registration exponentiated lie algebra registration method (Ashburner, Reference Ashburner2007). Individual-level values for total brain volume, total gray matter volume, and total white matter volume are produced during the normalization process.
Results
Behavioral data
Life events
Mean number of life events was reported to be 3.1 (±1.9 SD), with a range between zero and six events for the group as a whole. This is consistent with the mean accumulation of trauma-level life events for youth from the New York metropolitan area (G. A. Bonanno, personal communication, March 3, 2010; see also Cohen, Hitsman, et al., Reference Cohen, Hitsman, Paul, McCaffery, Stroud and Sweet2006; Gupta & Bonnano, Reference Gupta and Bonaanno2010). The mean time since the most recent life event was 2.9 ± 3.8 years, and the mean time since the worst life event was 4.3 ± 2.8 years. The mean age at the first life event was 7.0 ± 3.2 years, and the mean age at the worst life event was 8.8 ± 3.4 years. In this sample of adolescents, no life event was reported before 4 years of age. Participant age and sex were not associated with number of life events.
Current anxiety
The mean score on the anxiety measure for this sample of adolescents was 15.9 ± 9.4. Anxiety was not statistically associated with years since most recent life event, years since worst event, age at first event, age at worst event, or IES-8 score.
Prior life events predict current anxiety
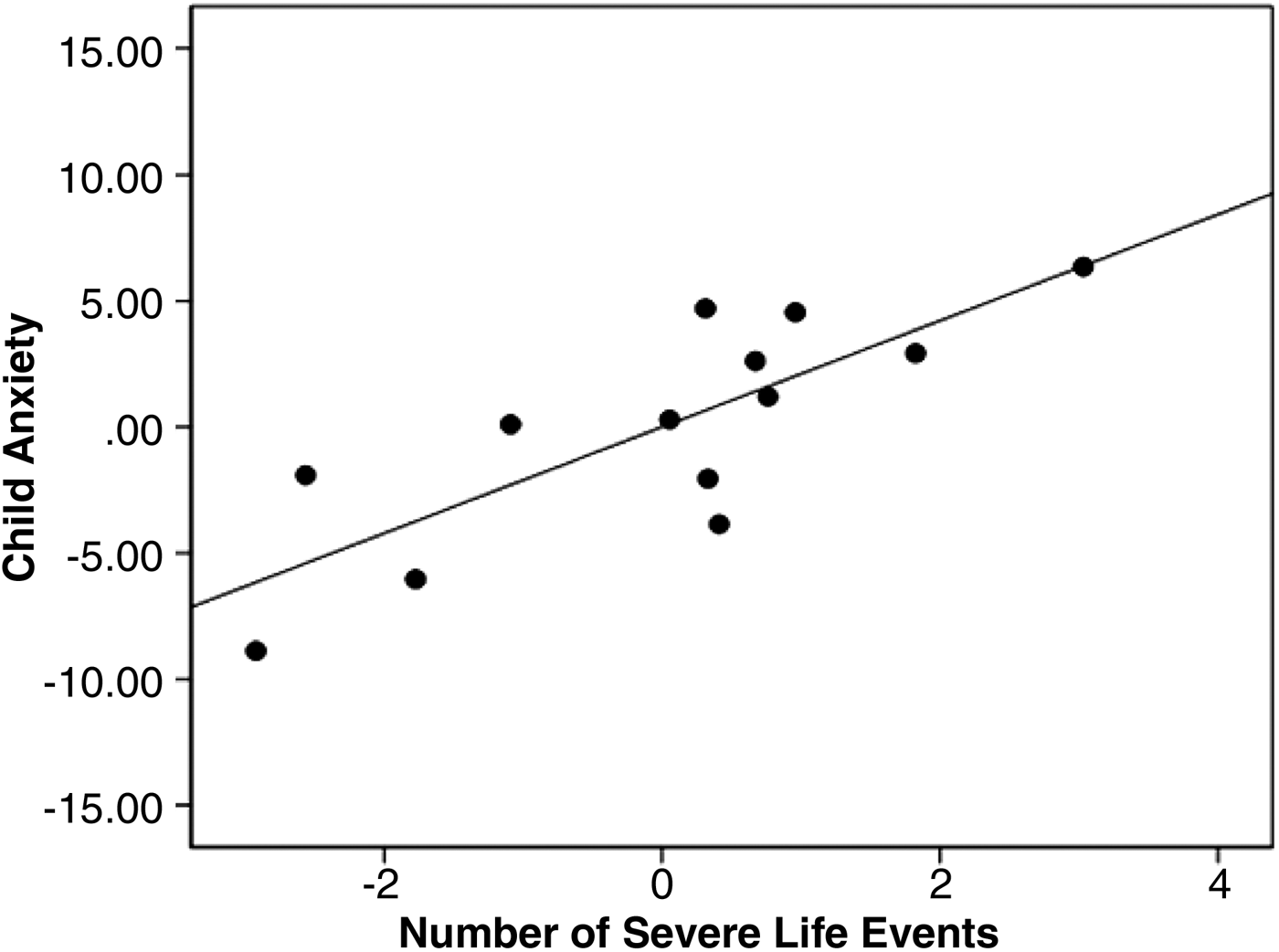
From linear regression, more prior life events predicted higher current levels of anxiety: β = 0.70, p = .004, adjusted R 2 = .48 (β = 0.43, p = .005, with control for total gray matter volume, age, and sex; see Figure 2).

Figure 2. A partial regression plot showing the association between the number of prior life events and current anxiety in this sample of healthy adolescents (β = 0.43, p = .005), controlling for age and sex. This association remains significant with or without control for total gray matter volume (see text).
Functional brain imaging
Life events predict amygdala reactivity
We conducted a whole-brain voxelwise covariate analysis (fear vs. calm contrast) with number of life events as the covariate of interest. This analysis identified a region in the dorsal amygdala and the anterior hippocampus in which BOLD signal (fearful vs. calm faces) covaried with number of prior life events: MNI (−16, −10, −14), T = 3.04, p cluster < .05 (correction for multiple comparisons), 86 contiguous voxels, with control for total brain volume. In this association, a greater number of prior life events was associated with increased amygdala reactivity to fearful versus calm faces. Of the voxels in this cluster, 22 fell within the anatomical amygdala and 53 fell within the anatomical hippocampus (see Figure 3). The results were comparable with or without control for total brain volume.

Figure 3. (Color online) Life events as a covariate of interest in a voxelwise whole-brain analysis within the entire group. This analysis identified a region in the amygdala and the anterior hippocampus, cluster peak at MNI (−16, −10, −14), T = 3.04, p cluster-corrected < .05, k = 86, in which the blood oxygen level dependent signal in response to fearful versus calm faces covaried with the number of prior life events. This association remained significant with or without control for total brain volume (see text). The results are displayed on a canonical individual T1 image.
Amygdala reactivity predicts anxiety
A whole-brain voxelwise covariate analysis (fearful vs. calm contrast) with current anxiety as the covariate of interest identified a significant association between adolescent anxiety and signal in dorsal amygdala: MNI (−18, −12, −16), T = 2.90, p cluster < .05 (correction for multiple comparisons), k = 21. This association between anxiety and amygdala reactivity was rendered nonsignficant by control of total brain volume (i.e., there was no significant activation at p cluster < .5, correction for multiple comparisons). The association between anxiety and global measures of brain volume are discussed below; its implications are reviewed in the Discussion Section.
Conjunction analysis
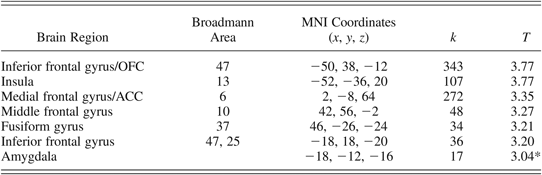
Having demonstrated that signal in the dorsal amygdala (fear vs. calm) covaried with prior life events and current anxiety (above), we wished to determine whether there were any other brain regions in which BOLD signal (fear vs. calm contrast) covaried both with prior life events and with current anxiety. Along with the amygdala, such regions would be candidate neural mediators of the association between life events and anxiety (for a discussion of this point, see Ganzel et al., Reference Ganzel, Casey, Glover, Voss and Temple2007). To do this, we performed a whole-brain voxelwise conjunction analysis (e.g., Friston et al., Reference Friston, Holmes, Price, Buchel and Worsley1999; Friston, Penny, & Glaser, Reference Friston, Penny and Glaser2005; Nichols, Brett, Andersson, Wager, & Poline, Reference Nichols, Brett, Andersson, Wager and Poline2005). We first created a mask of the significant regions of activation in the covariate analysis with anxiety (fear vs. calm contrast) using the XJView toolbox in SPM8 (http://www.alivelearn.net/xjview8/). This was used to inclusively mask the covariate analysis with number of life events (fear vs. calm contrast). At our threshold of significance, there were several regions in which signal remained significant within the area encompassed by the inclusive mask (i.e., activation was common to both covariate analyses). These included regions in the orbitofrontal cortex, the insula, the anterior cingulate, as well as the amygdala (via ROI analysis; see Table 1).
Table 1. Results of voxelwise conjunction analysis

Note: The results are for activation foci of significant clusters remaining in the covariate analysis with life events in the fearful versus calm contrast, after inclusive masking with significant regions of activation from the covariate analysis with anxiety. Only regions that were previously determined to be significant in separate group-level covariate analyses were included in the conjunction analysis (Nichols et al., Reference Nichols, Brett, Andersson, Wager and Poline2005). OFC, orbitofrontal cortex; ACC, anterior cingulate cortex.
For each cluster peak, we provide associated Broadmann areas, coordinates defined in Montreal Neurological Institute (MNI) stereotactic space (mm), cluster size in contiguous voxels (k), and Z statistic scores. Peak activity is reported; p < .05 (corrected for total brain); voxel size 3 × 3 × 3.
*Clusterwise false positive rate of p < .05, corrected for multiple comparisons within the region of interest.
Structural brain imaging
From linear regression, individual differences in total brain volume were associated with participant age at the level of a trend (β = 0.52, p = .06, adjusted R 2 = .23, with control for sex), such that older adolescents were more likely to have larger total brain volumes. Total white matter volume was associated more strongly with age (β = 0.65, p = .009, adjusted R 2 = .47, controlling for sex), and gray matter volume was not associated with age, suggesting that increases in white matter were the primary contributor to age-related increases in brain volume (note that this is consistent with prior research; e.g., Bartzokis et al., Reference Bartzokis, Mace Beckson, Lu, Nuechterlein, Edwards and Mintz2001; Giedd et al., Reference Giedd, LaLonde, Celano, Wallace, Lee and Lenroot2009).
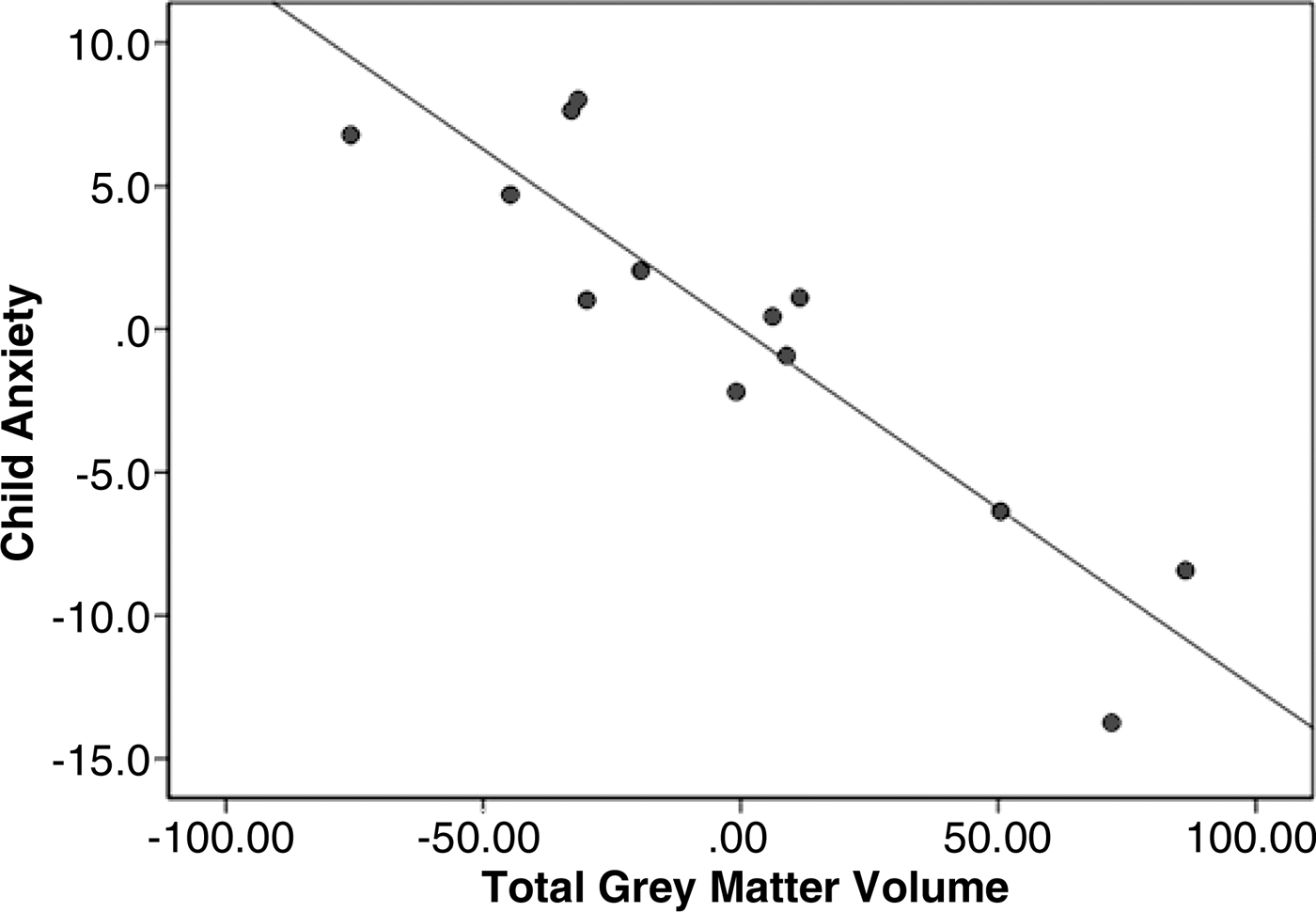
In addition, total brain volume was inversely associated with current anxiety at the level of a trend (β = −0.47, p = .07 adjusted R 2 = .40, controlling for age and sex). Adolescents with higher scores on the anxiety measure were more likely to have lower total brain volumes. Total gray matter volume showed a robust inverse association with current anxiety (β = −0.89, p = .001, adjusted R 2 = .62, controlling for age and sex; see Figure 4). Total white matter volume showed no association with anxiety, suggesting that differences in gray matter were the primary contributor to anxiety-related differences in total brain volume. The association between total gray matter volume and number of prior life events was in the same direction, although not significant (β = −0.16, p = .15, adjusted R 2 = −.02, controlling for age and sex). There were no other significant associations between whole-brain volumetric measures (total brain volume, total white matter volume, or total gray matter volume) and behavioral/life experience measures (age at first life event, age at worst life event, time since last event, time since worst event, or symptoms of current PTSD).

Figure 4. The total gray matter volume was inversely associated with the participant score on the measure of current anxiety (β = −0.89, p = .001), controlling for age and sex.
Discussion
This study expands current understanding of the neural links between life stress and biobehavioral outcomes during development. No life events were reported before 4 years of age in this sample, rendering it less likely that these results are driven by stressor exposure during a perinatal sensitive period. Thus, while the focus of research on the developmental psychobiology of stress has been on perinatal stressors (for a review, see Ganzel & Morris, Reference Ganzel and Morris2011), data from the present study provide evidence that stressors that occur in middle childhood and adolescence have long-term neural and behavioral sequelae.
Exposure to negative life events during childhood has been associated with long-term increases in anxiety (e.g., Copeland et al., Reference Copeland, Keeler, Angold and Costello2007; Rosenblum et al., Reference Rosenblum, Forger, Noland, Trost and Coplan2001). Based on prior research, we hypothesized that the neural link between prior life stress and current anxiety would include either stress-related amygdala hyperreactivity to standardized emotional stimuli (fearful vs. calm faces) or potentially stress-related systemic differences in global brain volume. In this sample of nonclinical adolescents, we did identify associations (a) between number of prior trauma-level life events (parent report) and current anxiety (child report), (b) between number of life events and amygdala reactivity to fearful versus calm faces, and (c) between amygdala reactivity (fear vs. calm) and current anxiety. Furthermore, we found heightened reactivity (fear vs. calm) in the dorsal amygdala that was associated with increases in prior life events and with increases in current anxiety. While limited sample size prevents formal tests of mediation (Baron & Kenny, Reference Baron and Kenny1986), these results are consistent with the hypothesis that the dorsal amygdala plays a role in neural mechanisms linking prior life events and current anxiety in this sample of healthy adolescents. Regions within the orbitofrontal cortex, the anterior insula, and the supplemental motor area showed similar covariation between regional reactivity (fear vs. calm) and both life events and anxiety. Thus, these regions are also candidate members of the system(s) responsible for the neural mediation between prior stress and current anxiety.
We did not find an association between number or timing of prior life events and current total brain volume. However, we did identify a modest inverse association between total brain volume and current anxiety in this sample. This effect was, in turn, driven by a robust inverse association between total gray matter volume and current anxiety. Given the ongoing dynamic development of the brain during childhood and adolescence, it would not be surprising if change may appear systemically and may coincide with global alterations in structure as evidenced by prior research on PTSD.
It is important to note that previous research (e.g., De Bellis, Keshavan, et al., Reference De Bellis, Keshavan, Clark and Casey1999; De Bellis & Kuchibhatla, Reference De Bellis and Kuchibhatla2006) shows systemic effects of traumatic events on the dynamically developing brain. Our morphometric data suggests global influences of anxiety on brain development in childhood and adolescent years may mask real functional effects; in these data, control of total brain volume hid significant amygdala-specific functional differences. In particular, total gray matter volume may explain much the same variance as amygdala reactivity in predicting current anxiety.Footnote 3 Such findings emphasize the importance of circuit-based neural changes and the complexity of how experiences may dynamically alter interacting circuits that are involved in processing and responding to emotional cues and contexts. In the more stable system of the adult brain, such global alterations have not been reported with chronic or acute anxiety (e.g., Schienle, Ebner, & Schafer, Reference Schienle, Ebner and Schafer2011; Spampinato, Wood, De Simone, & Grafman, Reference Spampinato, Wood, De Simone and Grafman2009), so that these findings may reflect a uniquely developmental phenomenon.
We note that these data cannot speak to the adaptive nature of stress-related increases in amygdala activity and anxiety. It has been argued that such changes are likely to be useful in the short term but may become maladaptive in the long run (Sterling & Eyer, Reference Sterling, Eyer, Fisher and Reason1988). The question of reversibility is key here. This is particularly true if stress drives changes in physiology and behavior that become “fixed automatisms” (Sterling & Eyer, Reference Sterling, Eyer, Fisher and Reason1988), which increase the burden of wear and tear on the system over time (McEwen & Stellar, Reference McEwen and Stellar1993). In contrast to the clear evidence for reversibility of stress-related change in the PFC and the hippocampus, there is minimal evidence for reversibility in the amygdala (for a discussion, see Ganzel et al., Reference Ganzel, Morris and Wethington2010).
Study limitations
Study limitations include small sample size, which limits statistical power and increases risk of error during statistical hypothesis testing. Thus, these results should be considered preliminary. In addition, these findings cannot demonstrate causal relationships. While we found significant relationships among adolescents’ current level of anxiety, amygdala reactivity, and prior exposure to trauma-level life events, the present data cannot establish causation among these factors. In addition, the use of parent report for child life events is a limiting factor, as well as being a strength of this study. Retrospective report of life events can be substantially biased by mood state, including increased state anxiety (Hardt & Rutter, Reference Hardt and Rutter2004; Moffit, Caspi, & Rutter, Reference Moffitt, Caspi and Rutter2006). This study used parent's report of their child's life stress history, which limited the possibility that child state anxiety drove individual differences in reported life events. However, the possibility remains that the child had experienced life events of which the parent had no knowledge, so that these results may be conservative (Berkowitz & Stover, Reference Berkowitz and Stover2005; Berkowitz et al., Reference Berkowitz, Stover and Marans2011). In addition, exposure to trauma-level events is only one measure of an adolescent's accumulation of life stress. It remains for future research to examine the impact of brain development on the neural consequences of stressors of different types, intensities, and chronicities (Alleva & Santucci, Reference Alleva and Santucci2001; Pruessner et al., Reference Pruessner, Dedovic, Khalili-Mahani, Engert, Pruessner and Buss2008; Wheaton, Reference Wheaton, Horwitz and Scheid1999), and to distinguish the biologically critical components within complex stressors such as growing up in poverty (Gianaros et al., Reference Gianaros, Horenstein, Hariri, Sheu, Manuck and Matthews2008) or in risky family environments (Repetti, Taylor, & Seeman, Reference Repetti, Taylor and Seeman2002; Taylor et al., Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006). Finally, this study employed a task that was specifically designed to elicit activation in the amygdala (passive viewing of fearful vs. calm faces). As described in the Introduction, there are other stress-sensitive brain regions (e.g., the hippocampus and the PFC). The contributions of these other brain regions to the neural mechanisms linking prior stress and current anxiety are less likely to be revealed using this task.
Conclusion
Overall, these data suggest a central role for amygdala in the association between prior life stress and current anxiety in healthy adolescents. These effects may be modulated or overlaid by broad anxiety-related differences in gray matter volume that may be developmental in origin. Consistent with current theory, these effects are not dependent on psychopathology but may constitute a vulnerable preclinical state (e.g., Ganzel et al., Reference Ganzel, Morris and Wethington2010; McEwen, Reference McEwen2007).
Thus, the present study provides evidence for the neural embedding of life events in healthy (nonclinical) adolescents. Amygdala reactivity to emotional stimuli was identified as a possible neural link in the association between accumulated prior life events and current anxiety. Study of the effects of life stress within the healthy developing brain will help to clarify the basic neural mechanisms underlying the stress process, which, in turn, will provide a basis for understanding the role of development in stress-related psychopathology.