Introduction
Spider crabs that belong to the superfamily Majoidea (sensu Ng et al., Reference Ng, Guinot and Davie2008) exhibit remarkable disparity in terms of body size, colouration, morphology and behaviour (Rathbun, Reference Rathbun1925; Baeza et al., Reference Baeza, Bolaños, Fuentes, Hernández, Lira and Lopez2010). Among them, the ‘Channel clinging crab’, ‘Cangrejo de la Virgen’, or ‘Caribbean king crab’ Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) is one of the largest crabs in the world and the largest native crab in the tropical and subtropical western Atlantic, reaching a carapace length up to 180 mm and weighing more than 3 kg (Tunberg & Creswell, Reference Tunberg and Creswell1988, Reference Tunberg and Creswell1991; Baeza et al., Reference Baeza, Bolaños, Fuentes, Hernández, Lira and Lopez2010, Reference Baeza, Simpson, Ambrosio, Guéron, Mora and Owen2015). Maguimithrax spinosissimus exhibits a Caribbean-wide distribution, ranging from North Carolina in North America to the north-eastern coast of Venezuela in South America (Rathbun, Reference Rathbun1925; Winfree & Weinstein, Reference Winfree and Weinstein1990; Tunberg & Creswell, Reference Tunberg and Creswell1991). The Caribbean king crab inhabits ledges and other structures in the shallow subtidal to depths approaching 200 m, remains cryptic during daylight hours, and emerges from shelter shortly after sunset to forage on macroalgae through the night (Winfree & Weinstein, Reference Winfree and Weinstein1990; Tunberg & Creswell, Reference Tunberg and Creswell1991; Wilber & Wilber, Reference Wilber and Wilber1991). Recent studies of this species have focused on adult sexual dimorphism (Baeza et al., Reference Baeza, Anderson, Spadero and Behringer2012), female reproductive performance (Baeza et al., Reference Baeza, Simpson, Ambrosio, Guéron, Mora and Owen2015) and population genetics and connectivity (Márquez et al., Reference Márquez, Hurtado-Alarcon, Isaza, Alzate and Campos2016; Hurtado-Alarcón et al., Reference Hurtado-Alarcón, Campos, Bermúdez Tobón and Márquez2017; Baeza et al., Reference Baeza, Holstein, Umaña-Castro and Mejía-Ortíz2019). The early life history of this species is less known.
Maguimithrax spinosissimus exhibits two zoea larval stages prior to megalopa stage (Brownell et al., Reference Brownell, Provenzano and Martinez1977; Tunberg & Creswell, Reference Tunberg and Creswell1988) as do all majoid crabs. However, M. spinosissimus is considered unusual among mithracids, due to a major reduction in the number of setae in the maxillae and maxillipeds, from the first to the second zoeal stage, as reported in its first larval description (Provenzano & Brownell, Reference Provenzano and Brownell1977). By contrast, other mithracids have both zoea with well-developed setae in maxillae and maxillipeds (e.g. Santana et al., Reference Santana, Pohle and Marques2003; Rhyne et al., Reference Rhyne, Fujita and Calado2006). This reduction in the number of setae could be explained by the putatively lecithotrophic strategy attributed to the larval stages of M. spinosissimus (Provenzano & Brownell, Reference Provenzano and Brownell1977; Porter et al., Reference Porter, Iglehart, Craig, Adey, Adey and Farrier1986; Creswell et al., Reference Creswell, Tunberg and Winfree1989; Tunberg & Creswell, Reference Tunberg and Creswell1991). Similar reduction in setation and spines is observed in other brachyuran crabs with abbreviated and/or direct development and lecithotrophic larvae (e.g. Taishaku & Konishi, Reference Taishaku and Konishi2001; Bolaños et al., Reference Bolaños, Cuesta, Hernández, Hernández and Felder2004, Reference Bolaños, Rivero, Hernández, Magán, Hernández, Cuesta and Felder2005; González-Gordillo et al., Reference González-Gordillo, Anger and Schubart2010). Furthermore, a brief prezoeal stage has been described in M. spinosissimus (Provenzano & Brownell, Reference Provenzano and Brownell1977; Tunberg & Creswell, Reference Tunberg and Creswell1988) as well as in the closely related Mithrax pleuracanthus (Goy et al., Reference Goy, Bookhout and Costlow1981). The occurrence of a prezoeal stage has been attributed to stress of either the gravid female or the developing embryos (Tunberg & Creswell, Reference Tunberg and Creswell1988).
The reduction in setation and spines during larval stages have led several authors to consider the need for a re-description of the larval stages of M. spinosissimus due to the uncommonly reported characteristics, the low quality of the illustrations, and the possibility of errors in the original description (Rice, Reference Rice1980; Bolaños et al., Reference Bolaños, Lares and Hernández1990; Santana et al., Reference Santana, Pohle and Marques2003, Reference Santana, Marques and Pohle2004; Rhyne et al., Reference Rhyne, Fujita and Calado2006). Detailed larval descriptions can be useful not only for taxonomic and phylogenetic studies, but are also relevant to inform fisheries management, aquaculture planning and conservation strategies (Santana et al., Reference Santana, Colavite, Bolaños, Hernández and Canepa2016; Baeza et al., Reference Baeza, Holstein, Umaña-Castro and Mejía-Ortíz2019). Indeed, due to its body size and short larval period (~6 days from zoea I to megalopa), M. spinosissimus has an enormous potential for aquaculture. This crab is also currently caught incidentally for local consumption and targeted by artisanal fisheries along the greater Caribbean (Creswell, Reference Creswell2011; Hurtado-Alarcón et al., Reference Hurtado-Alarcón, Campos, Bermúdez Tobón and Márquez2017). Various previous studies have addressed essential information needed for its cultivation (e.g. Brownell et al., Reference Brownell, Provenzano and Martinez1977; Creswell et al., Reference Creswell, Tunberg and Winfree1989; Tunberg & Creswell, Reference Tunberg and Creswell1991; Creswell, Reference Creswell2011; Baeza et al., Reference Baeza, Simpson, Ambrosio, Guéron, Mora and Owen2015).
The purpose of this study is to re-describe and illustrate the complete larval development of M. spinosissimus and compare our results with the previous larval description by Provenzano & Brownell (Reference Provenzano and Brownell1977). The larval morphology of M. spinosissimus is also compared with allied species within the family and the possible reasons for the dissimilarities observed between this description and that of Provenzano & Brownell (Reference Provenzano and Brownell1977) are discussed.
Materials and methods
Seven ovigerous females of Maguimithrax spinosissimus were collected in January 1996, while free diving between 3–5 m depth from Laguna Central, south of Cayo Robusqui, Los Roques Archipelago, Venezuela. Females were transported to the laboratory and individually held in aquaria with average salinity of 36 psu, in a temperature-controlled room (26 ± 2 °C) until larval hatching. Larval rearing temperature was similar to that observed in the field, 23–31 °C (Brownell et al., Reference Brownell, Provenzano and Martinez1977).
Larval development and descriptions followed standard protocols (e.g. Pohle & Marques, Reference Pohle and Marques2000; Colavite et al., Reference Colavite, Santana and Pohle2014; Santana et al., Reference Santana, Colavite, Bolaños, Hernández and Canepa2016), where 10 of the most active larvae from each of a total of 7 hatches were separated in groups and placed in jars filled with 200 ml of filtered seawater. The remaining larvae were kept in mass culture in 10 jars filled with 1 litre of filtered seawater for additional specimens to be used in morphological descriptions. Larvae were fed ad libitum with diatoms and Artemia nauplii. Seawater was changed and larvae were inspected and fed daily. All glass jars were washed in fresh water and air-dried before re-using the following day. A photoperiod of 12:12 light:dark was used for larval rearing.
At least 30 specimens from each stage (plus the exuviae) were fixed in 4% buffered formalin, and at least 10 specimens of each larval stage were carefully dissected. For morphological description, whole larva and dissected appendages were stained using methylene blue, acid fuchsin and/or chlorazol black. Polyvinyl lactophenol or Canada balsam were used as mounting media for slide preparations.
The description of setae generally follows that of Pohle & Telford (Reference Pohle and Telford1981), but here we included only an analysis using light microscopy, using an Olympus BX 50 equipped with camera lucida. Appendages are described from proximal to distal end. Measurements were taken from 10 specimens of each larval stage, being: total length (TL) the distance between the tip of rostrum and posterior end of telson furca; carapace length (CL) the distance from the anterior margin between the eyes and the carapace posterior-most margin in zoeal stages, and from the tip of rostrum to the posterior margin of carapace for the megalopa; carapace width (CW) taken at the level of its widest point in megalopa.
Larval stage samples and female crabs are available upon request and are deposited in the crustacean collection of the Grupo de Investigación en Carcinología de la Universidad de Oriente, Núcleo Nueva Esparta (GICUDONE), Isla Margarita, Venezuela, accession numbers GIC-935 and GIC-936. Research ethics approval was not needed to conduct this study.
Results
The complete larval development of Maguimithrax spinosissimus consists of two zoeal stages and one megalopa. In some cases, the first zoea was preceded by a non-swimming prezoeal stage, that survived only for a few hours in the laboratory. Comparisons between specimens from different females show no major morphological differences. The duration from hatching to the first crab stage was 5–6 days at temperatures that ranged between 24–28 °C in the laboratory. Larval morphometrics are given in Table 1. Only morphological changes are described for the second zoea.
Table 1. Dimensions (mm) of larval structures of Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818)

Note: Values are given as the mean ± standard deviation, with range in parentheses.
Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818)
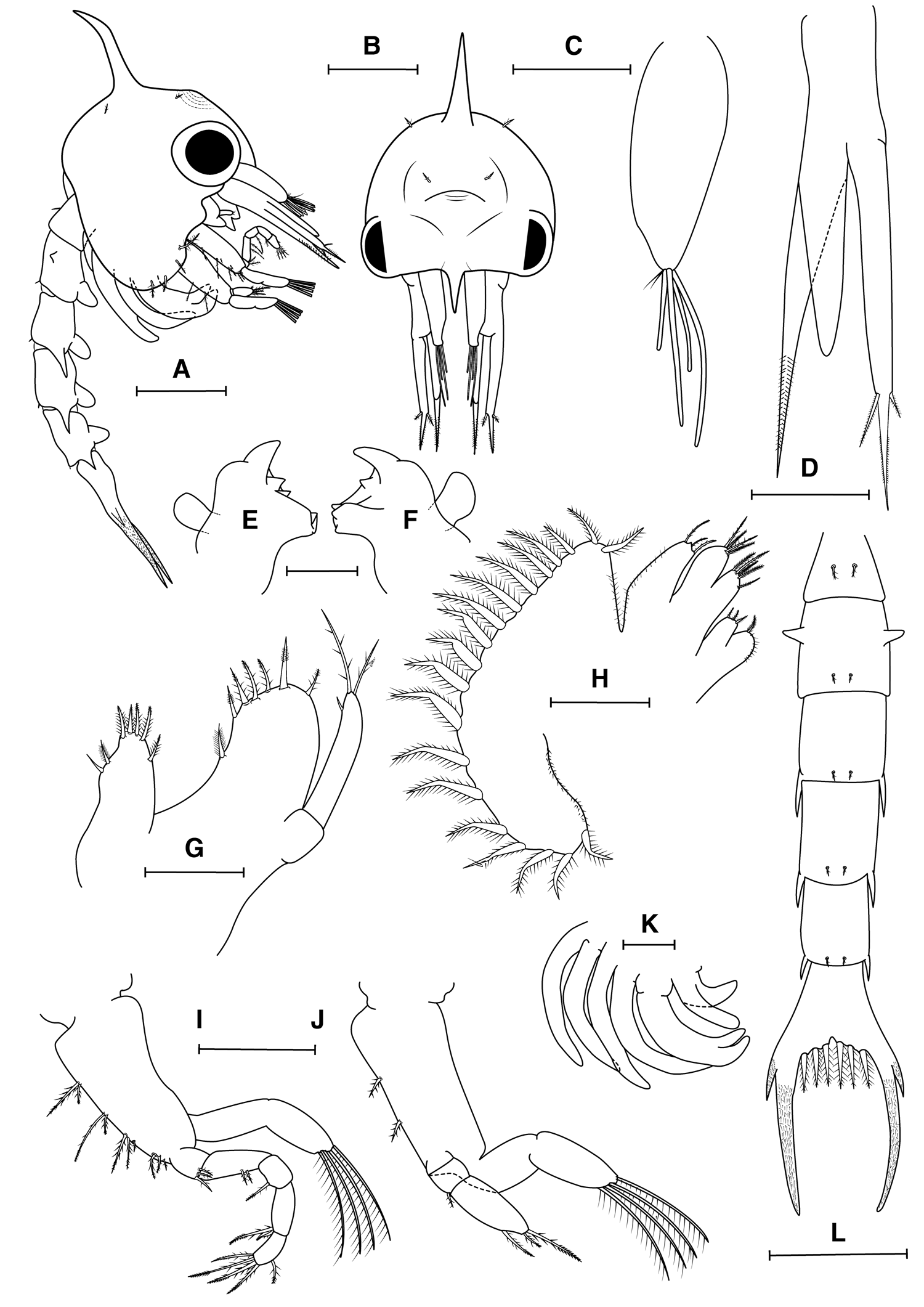
Fig. 1. Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) first zoeal stage. (A) Lateral view; (B) frontal view; (C) antennule; (D) antenna; (E) right mandible; (F) left mandibles; (G) maxillule (H) maxilla; (I) maxilliped I; (J) maxilliped II; (K) maxilliped III and pereiopods; (L) dorsal view of pleon and telson. Scale bars: A, B, H, K: 0.5 mm; C, D, I, J: 0.2 mm; E, F, G: 0.1 mm.
First zoea
Carapace (Figure 1A, B). Dorsal spine slightly curved posteriorly and distally. Rostral spine short, not reaching exopod of antenna, lateral spines absent. A pair of simple setae near dorsal organ and a pair of simple setae postero-lateral to dorsal spine. Ventral margin with long, densely plumose seta posterior to scaphognathite notch (anterior seta), with three smaller plumose setae. Eyes sessile. Small indistinct prominence frontally between dorsal spine and rostrum, bearing cuticular dorsal organ (sensu Martin & Laverack, Reference Martin and Laverack1992; Lerosey-Aubrill & Meyer, Reference Lerosey-Aubrill and Meyer2013). No yolk granules observed in the cephalothorax.
Antennule (Figure 1C). Uniramous, endopod (accessory flagellum) absent; exopod (primary flagellum) unsegmented, with four aesthetascs (two long, two short), and two very short simple setae distally.
Antenna (Figure 1D). Biramous. Protopod long, pointed, bearing two rows of spinules in distal third. Endopod bud reaching more than half of protopod. Exopod slightly longer than protopod, with a spinulated distal process bearing two serrulate setae (one long, one short) approximately one third of tip.
Mandible (Figure 1E, F). Asymmetrical, with medial toothed molar process and enlarged lateral incisor processes. Internal margin of incisor process of right mandible with one acute tooth, left mandible with two acute teeth. Palp bud prominent.
Maxillule (Figure 1G). Coxal endite with four terminal setae (three graded plumodenticulate, one plumodenticulate), three subterminal setae (two graded plumodenticulate, one simple). Basial endite with three terminal plumodenticulate cuspidate setae, three subterminal plumodenticulate setae and one plumose seta proximally. Endopod with two articles, proximal without setae, distal with one subapical short, simple seta, two apical plumodenticulate setae. Exopod absent.
Maxilla (Figure 1H). Coxal endite bilobed, proximal lobe with one plumodenticulate; distal lobe with three short setae (two plumose, one plumodenticulate). Basial endite bilobed, proximal and distal lobes with five and 3–4 plumodenticulate setae, respectively. Endopod unsegmented, unilobed, with three plumodenticulate setae, a short apical protuberance present. Proximal lobe of coxal endite and endopod with microtrichia on lateral margin. Scaphognathite with 16–20 densely plumose setae on lateral margin, microtrichia on both margins.
First Maxilliped (Figure 1I). Coxa without seta; basis with 10 plumodenticulate setae arranged 2 + 2 + 3 + 3. Endopod with five articles, with 3,2,1,2,5 (distal segment with one subapical, four apical) plumodenticulate setae. Exopod incompletely 2-segmented, with four terminal plumose natatory setae.
Second Maxilliped (Figure 1J). Coxa without seta; basis with two plumodenticulate setae; endopod with three articles, with 0,1,3 (distal segment with one subapical; two apical, one long, one short) plumodenticulate setae; exopod incompletely 2-segmented with four terminal plumose natatory setae.
Third Maxilliped (Figure 1K). Present as a small bud, endopod and exopod distinguishable.
Pereiopods (Figure 1A, K). Present as elongated buds, chela distinct, segmentation apparent in some pereiopods.
Pleon (Figure 1A, L). Five pleonites. Pleonites I–V with a pair of plumodenticulate setae (pleonite I with longer middorsal pair, pleonite II–V with shorter posteromedial pair). Pleonite II with a pair of distinct dorsolateral processes. Posterolateral margin of pleonite II with blunt process, pleonites III–V with long acute spines. Pleopod buds uniramous on pleonites II–V, endopods absent.
Telson (Figure 1L). Bifurcated, small median notch. Three pairs of plumodenticulate setae on inner margin; each furcal shaft proximally bearing one distinct lateral spine, furcal shafts covered with rows of spinules to just below tips.
Second zoea
Carapace (Figure 2A). Eyes stalked. Five pairs of small plumodenticulate setae dorsally. Ventral margin with five setae (two densely anterior plumose, three shorter plumose). Protuberance bearing cuticular dorsal organ enlarged. No yolk granules observed in the cephalothorax.

Fig. 2. Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) second zoeal stage. (A) Lateral view; (B) antennule; (C) antenna; (D) right mandible; (E) left mandible; (F) maxillule; (G) maxilla; (H) maxilliped III and pereiopods; (I) dorsal and ventral view of pleon. Scale bars: A, H, I: 0.5 m; B, C: 0.2 mm; D, E, F, G: 0.1 mm.
Antennule (Figure 2B). Peduncle uniramous; endopod bud present, without setae; exopod with two annuli, proximal without setae, distal with eight long aesthetascs and two short simple setae.
Antenna (Figure 2C). Endopod with five articles, reaching more than two-thirds of endopod.
Mandible (Figure 2D, E). Palp bud enlarged.
Maxillule (Figure 2F). Exopodal seta pappose.
Maxilla (Figure 2G). Proximal and distal lobes of coxal endite with one short simple seta each. Basial endite with shorter setae. Endopod without short apical protuberance. Scaphognathite with 30–36 marginal plumose setae.
First Maxilliped (Figure 2A). Exopod with 6 plumose natatory setae.
Second Maxilliped (Figure 2A). Exopod with 6 plumose natatory setae.
Third Maxilliped (Figure 2H). Endopod with five articles. Exopod long, unsegmented.
Pereiopods (Figure 2A, H). Longer, first with distinct chela. Segmentation apparent.
Pleon (Figure 2A, I). Six pleonites. Pleonite II with an extra pair of middorsal plumodenticulate setae. Pair of unsegmented, biramous pleopods on pleonites II–V (exopods long, endopods small). Sixth pleonite with uniramous uropod buds present.
Megalopa
Carapace (3A, B). Longer than wide, subrectangular. Rostral spine short, acute, slightly deflected ventrally. Hepatic region projected, forming two knob-like lateral expansions, gastric region swollen with distinct protogastric and metagastric regions. Protogastric region bearing the dorsal organ medially. Branchial, cardiac and intestinal regions inconspicuously defined. Carapace surface covered mostly with simple setae as illustrated. No yolk granules observed in the cephalothorax.
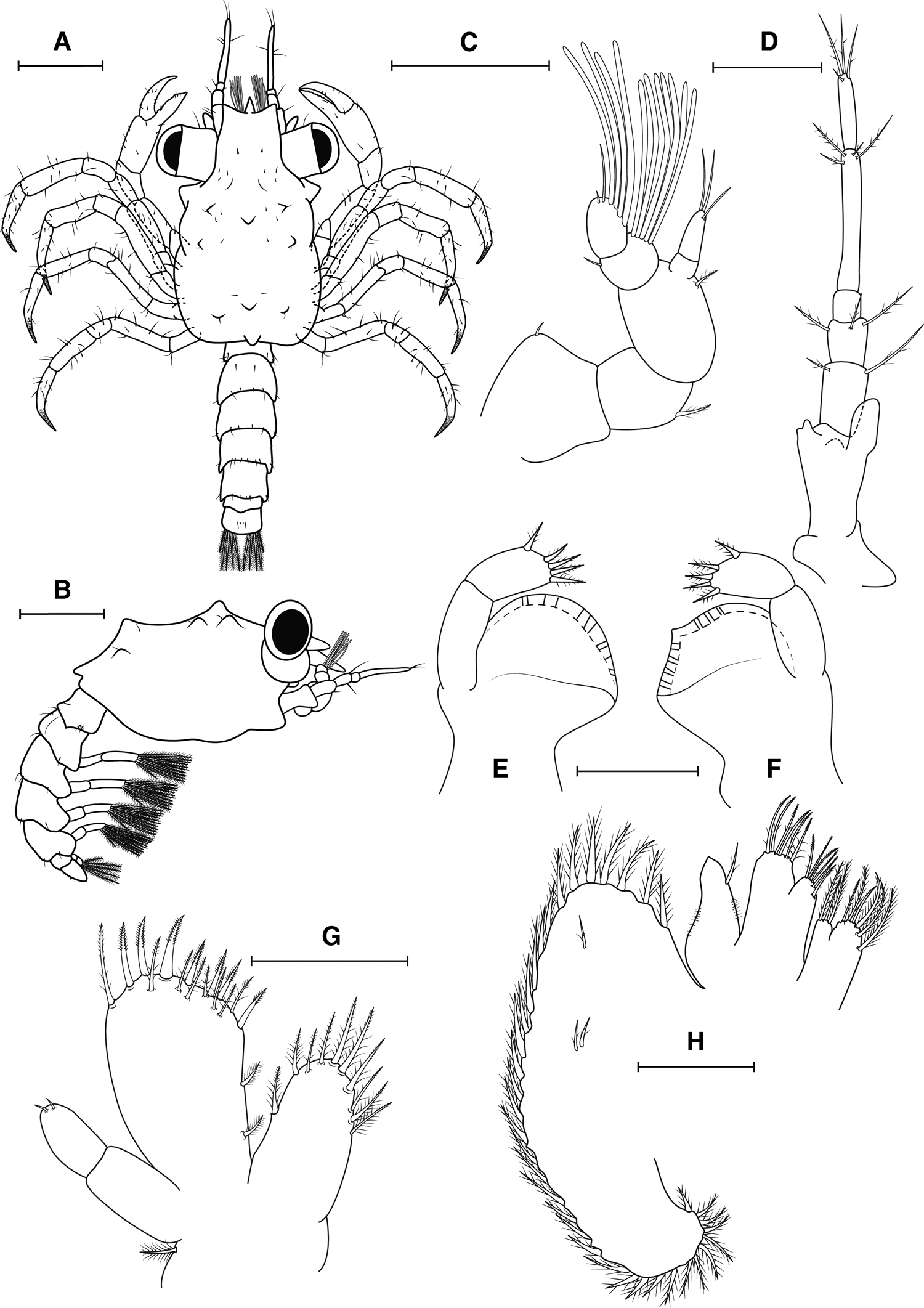
Fig. 3. Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) megalopa stage. (A) Dorsal view; (B) lateral view; (C) antennule; (D) antenna; (E) left mandible; (F) right mandible; (G) maxillule; (H) maxilla. Scale bars: A, B: 0.5 mm; C, D: 0.2 mm; E–H: 0.1 mm.
Antennule (Figure 3C). Peduncle with three articles, proximal with very short simple seta; medial and distal segments with one plumodenticulate seta each. Accessory flagellum with 2 annuli, with three long simple setae (one subterminal and two terminal). Primary flagellum with 2 annuli, with 7 aesthetascs ventrally + one simple seta dorsally; 4–5 aesthetascs + two simple setae (one long subapical and one very short apical), respectively.
Antenna (Figure 3D). Articles proximally to distally with 0, 2, 3, 0, 4, 4 simple setae, respectively. Basal article with distinct exopod bud. Articles V and VI fused.
Mandibles (Figure 3E, F). Asymmetrical, scoop-shaped process with cutting edge and small acute tooth in left mandible, right mandible without acute tooth. Palp with two articles, with five plumodenticulate setae on the distal segment.
Maxillule (Figure 3G). Coxal endite with 10 setae (six graded plumodenticulate, four plumodenticulate). Basial endite with 17 setae (seven terminal plumodenticulate cuspidate, eight subterminal plumodenticulate, two plumose setae on proximal margin). Endopod reduced, segmented, with two very short simple setae on distal segment. Exopod with a plumodenticulate seta.
Maxilla (Figure 3H). Coxal endite bilobed, proximal lobe with six setae (four plumose and two plumodenticulate), distal lobe with three setae (two plumose and one plumodenticulate). Basial endite bilobed with six plumodenticulate setae on each lobe. Endopod reduced, with microtrichia in both margins and one terminal plumodenticulate setae. Scaphognathite with 35–38 marginal plumose setae and three small plumodenticulate setae on blade.
First Maxilliped (Figure 4A). Coxa bearing seven setae (five plumodenticulate; two plumose, one long and one short). Basis endite with 11 plumodenticulate setae (eight long and three short) arranged as illustrated. Endopod unsegmented without setae. Exopod with two articles, proximal smooth, distal with six setae (four plumose and two short plumodenticulate). Epipod elongated with five long plumodenticulate setae (one proximal, two medial, two distal).

Fig. 4. Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) megalopa stage. (A) maxilliped I; (B) maxilliped II; (C) maxilliped III; (D) pleopods; (E) telson; (F) pereiopods; Scale bars: A–C: 0.5 mm; D, E: 0.3 mm, F: 0.5 mm.
Second Maxilliped (Figure 4B). Coxa and basis not clearly differentiated. Endopod with five articles, proximally to distally with 0, 0, 1, 3, 6 plumodenticulate setae, respectively. Exopod with two articles, proximal without setae, distal with six setae (four plumose, two short simple setae). Epipod not present on examined specimens.
Third Maxilliped (Figure 4C). Coxa with 11 plumodenticulate setae. Basis fused to ischium with protuberances on mesial margin, indicative of crista dentata. Endopod with five articles, proximally to distally with 12, 5 + 3, 4 + 2, 4 + 2, 4 plumodenticulate setae. Exopod with two articles, proximal smooth, distal with 2 short subapical, 4 long plumose apical setae. Epipod with five long plumodenticulate setae (one proximal, 1 subterminal, 3 distal).
Pereiopods (Figure 4F). Cheliped and pereiopods with mostly simple setae as figured. Coxa of P2–5 with an acute spine on ventral margin. Dactyls of P2–5 with rows of spinules distally. Coxa of cheliped bearing 10 possible plumose setae in a semicircle shape.
Pleon (Figure 3A, B). Pleonites I–V with 6, 8, 6, 8, 6 simple setae arranged as illustrated. Pleonite VI without seta.
Pleopods (Figure 4D, E). Pleonites II–V with a pair of biramous pleopods. Exopod of pleopods I–IV with 11, 11, 11, 9 plumose natatory setae, respectively. Endopod with two cincinnuli each. Pleonites VI with a pair of uropods, uniramous, 2-segmented, with five natatory setae on distal segment.
Telson (Figure 4E). Rounded posteriorly, bearing a pair of simple setae on middorsal margin and a pair of simple setae on midventral margin.
Discussion
We have described and illustrated in detail the complete larval development of M. spinosissimus using specimens collected from Los Roques Archipelago, Venezuela, the same locality from which Provenzano & Brownell (Reference Provenzano and Brownell1977) collected material for their study. In the following, we compare our results with (i) a previous larval description by Provenzano & Brownell (Reference Provenzano and Brownell1977) and (ii) allied species within the family (see Windsor & Felder, Reference Windsor and Felder2014, Reference Windsor and Felder2017).
Dissimilarities between larval descriptions in M. spinosissimus
A comparison between our re-description and that of Provenzano & Brownell (Reference Provenzano and Brownell1977) reveals major differences in larval morphology of M. spinosissimus, especially in zoeal stages, although the material was collected from the same region, but with a difference of nearly 20 years. The setal meristics strongly differ in almost all appendages, carapace and pleon in the zoeal stages (see Tables 2 and 3). Also, the segmentation of the endopod of the antenna is 5-segmented and in Provenzano & Brownell (Reference Provenzano and Brownell1977: Figure 3) we can observe a 4-segmented endopod. The few similarities are in the basial endites of the maxillule and the coxal endites of the maxilla of the second zoea (Table 3). In the megalopa, similarities are only found in the basial endite, endopod and scaphognathite of the maxilla, the maxilliped II, the pleopods and the uropod (Table 4). Importantly, the setal types were poorly described and not illustrated for most appendages in Provenzano & Brownell (Reference Provenzano and Brownell1977). Missing information on the carapace setation of the zoeal stages and the pleon setation for the zoeal stages and megalopa are here described for the first time.
Table 2. Comparison of larval characters of the first zoea of Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) and Mithrax Latreille, Reference Latreille1816

cox: coxal endite; bas: basis or basial endite; end: endopod; sca: scaphognathite; seg: segments; s: simple setae; ss: sparsely setose setae; pld: plumodenticulate setae; pl: plumose setae; pap: papose setae; ae: aesthetascs; n/d: not described; ?: number or setal type not specified.
a Observation from figure. Data from: 1 Santana et al. (Reference Santana, Pohle and Marques2003); 2 Goy et al. (Reference Goy, Bookhout and Costlow1981); 3 Magalhães et al. (Reference Magalhães, Souza-Carvalho, Biagi, Cuesta and Mantelatto2017); 4 Provenzano & Brownell (Reference Provenzano and Brownell1977); 5 Present study.
Table 3. Comparison of larval characters of the second zoea of Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) and Mithrax Latreille, Reference Latreille1816

cox: coxal endite; bas: basis or basial endite; end: endopod; exo: exopodite; sca: scaphognathite; seg: segments; s: simple setae; ss: sparsely setose setae; pld: plumodenticulate setae; pl: plumose setae; pap: papose setae; ae: aesthetascs; n/d: not described; ?: number or setal type not specified.
a Observation from figure. Data from: 1 Santana et al. (Reference Santana, Pohle and Marques2003); 2 Goy et al. (Reference Goy, Bookhout and Costlow1981); 3 Magalhães et al. (Reference Magalhães, Souza-Carvalho, Biagi, Cuesta and Mantelatto2017); 4 Provenzano & Brownell (Reference Provenzano and Brownell1977); 5 Present study.
Table 4. Comparison of larval characters of the megalopa of Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) and Mithrax Latreille, Reference Latreille1816

cox: coxa; bas: basis; end: endopod; exo: exopod; epi: epipod; sca: scaphognathite; ped: peduncle; seg: segments; Pl: pleonites; P: pleopods; s: simple setae; pld: plumodenticulate setae; pl: plumose setae; pap: papose setae; ae: aesthetascs; n/d: not described; ?: number or setal type not specified.
a Observation from figure. Data from: 1 Santana et al. (Reference Santana, Pohle and Marques2003); 2 Goy et al. (Reference Goy, Bookhout and Costlow1981); 3 Magalhães et al. (Reference Magalhães, Souza-Carvalho, Biagi, Cuesta and Mantelatto2017); 4 Provenzano & Brownell (Reference Provenzano and Brownell1977); 5 Present study.
Previous re-descriptions of larval development in other species of decapod crustaceans, including crabs, have revealed, in most cases, only minor differences between the first description and the latter (Gore, Reference Gore1970; Santos & Paula, Reference Santos and Paula2003; Calado et al., Reference Calado, Bartilotti, Narciso and dos Santos2004; Santana et al., Reference Santana, Marques and Pohle2004). Major differences, as those observed here, may point to errors during dissection (e.g. broken setae), slide preparation (e.g. overlapping setae), or problems in the observation of larval traits due to the use of inadequate microscopes (e.g. Santana et al., Reference Santana, Pohle and Marques2003), which we argue here explain the differences between our re-description and that of Provenzano & Brownell (Reference Provenzano and Brownell1977). Furthermore, some of the detected differences suggest a problem in the identification of the second zoeal stages during larval rearing by Provenzano & Brownell (Reference Provenzano and Brownell1977). For instance, the setation of the antennule, with no increment of setae from the first to the second zoea, and the similar number of setae on the scaphognathite of the maxilla of the first zoea described by Provenzano & Brownell (Reference Provenzano and Brownell1977) might be explained if these authors mistakenly classified zoea I as zoea II larvae (and vice versa).
One of the main concerns raised by some authorities (Rice, Reference Rice1980; Bolaños et al., Reference Bolaños, Lares and Hernández1990; Santana et al., Reference Santana, Pohle and Marques2003, Reference Santana, Marques and Pohle2004; Rhyne et al., Reference Rhyne, Fujita and Calado2006) regarding the description provided by Provenzano & Brownell (Reference Provenzano and Brownell1977) is the reduction in the number of setae in several appendages from the first to the second zoeal stage, a feature uncommon in the Brachyura, in general, and the Majoidea and Mithracidae, in particular (Clark, Reference Clark2000). In Provenzano & Brownell (Reference Provenzano and Brownell1977), the reduction in the number of setae occurred in the basis of the maxilla and in the endopod of the maxilliped I and II. In the present study, this reduction is not so pronounced, and we observed a reduction in the number of setae only in the coxa of the maxilla and the basis of maxilliped I from zoea I to II (Tables 2 and 3). Nevertheless, the notion that setae represent a well conserved trait in the zoeal stages of crabs (Clark, Reference Clark2000) is narrow and restraining. The stasis or reduction in the number of setae could be explained by facultative lecithotrophy attributed to the larvae of Maguimithrax spinosissimus, as observed in other brachyurans (e.g. Bolaños et al., Reference Bolaños, Rivero, Hernández, Magán, Hernández, Cuesta and Felder2005; González-Gordillo et al., Reference González-Gordillo, Anger and Schubart2010).
Maguimithrax spinosissimus is widely distributed throughout the tropical Atlantic (Rathbun, Reference Rathbun1925; Williams, Reference Williams1984; Wagner, Reference Wagner1990; Felder et al., Reference Felder, Álvarez, Goy, Lemaitre, Felder and Camp2009; Creswell, Reference Creswell2011). Despite that, the connectivity among populations of M. spinosissimus seems to be very low, indicating the existence of an isolated or semi-isolated subpopulation in the south of the Caribbean (Baeza et al., Reference Baeza, Holstein, Umaña-Castro and Mejía-Ortíz2019). This could explain possible differences in larval morphology throughout its distribution but not within the same locality, as shown here. As for now, we suggest that the differences between the two descriptions are due to methodological problems (see above) in Provenzano & Brownell (Reference Provenzano and Brownell1977). In this sense, to solve geographic morphological variation problems in the future, one possible approach is to sequence the DNA of the spent female crab (see Li et al., Reference Li, Shih, Ho and Jiang2019), which could also provide useful data for geographic comparisons. An alternative is to sequence the DNA barcode region of larvae collected at different geographic locations and examine their morphology, a methodology successfully applied to identify other marine organisms, such as fish and stomatopod larvae (Chu et al., Reference Chu, Loh, Ng, Ooi, Konishi, Huang and Chong2019; Wong et al., Reference Wong, Tsao, Tsai, Hsieh, Li, Machida and Chan2021).
Affinities of M. spinosissimus with other Mithracidae
A recent phylogenetic hypothesis, based on molecular data, proposed for the family Mithracidae positioned Maguimithrax spinosissimus as sister to a clade comprising Mithrax hispidus (Herbst, Reference Herbst1790), Mithrax pleuracanthus (Stimpson, Reference Stimpson1871) and Mithrax tortugae (Rathbun, Reference Rathbun1920) (Windsor & Felder, Reference Windsor and Felder2014). Thus, considering the phylogenetic position of Maguimithrax, herein we compare its larval development mostly with those available for species of Mithrax (Table 5).
Table 5. Species of Mithrax Latreille, Reference Latreille1816 and Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) with larval development described indicating source and stages described

PZ: prezoea; ZI: zoea I; ZII: zoea II; M: megalopa; CI: first crab.
Although Mithracidae comprise a well-supported monophyletic family, with most of the species in the clade having a remarkably uniform larval morphology (Yang, Reference Yang1967; Wilson et al., Reference Wilson, Scotto and Gore1979; Santana et al., Reference Santana, Pohle and Marques2003; Rhyne et al., Reference Rhyne, Fujita and Calado2006), it is a consensus that larvae of Maguimithrax spinosissimus are unique when compared with other known species of the family. For example, as with the adults, the zoeal stages are distinctly larger than the other species, and the megalopa is bigger than most species, with the exception of Mithrax pleuracanthus which features a megalopa with approximately the same size as Maguimithrax spinosissimus (Table 6). However, the present re-description shows a larval morphology much more similar to Mithrax than previously thought due to the description of Provenzano & Brownell (Reference Provenzano and Brownell1977). It is worthwhile to mention that Mithrax pleuracanthus presents considerable morphological differences to the other species of the genus. For instance, the setation of the antennule in the first zoea, maxillule in the second zoea and scaphognathite of the maxilla of the megalopa sets this species apart from the other Mithrax species. These characters need verification and perhaps re-description of the larvae of Mithrax pleuracanthus could clarify these discrepancies.
Table 6. Comparison of carapace length of larval stages of Maguimithrax spinosissimus (Lamarck, Reference Lamarck1818) and Mithrax Latreille, Reference Latreille1816 species

Note: Values are given as the mean ± standard deviation, with range in parentheses.
a Standard deviation and range not described. Data from: 1 Santana et al. (Reference Santana, Pohle and Marques2003); 2 Goy et al. (Reference Goy, Bookhout and Costlow1981); 3 Magalhães et al. (Reference Magalhães, Souza-Carvalho, Biagi, Cuesta and Mantelatto2017); 4 Provenzano & Brownell (Reference Provenzano and Brownell1977); 5 Present study.
Maguimithrax and Mithrax share the following characters in the first zoeal stage: the setation of the carapace dorsally, aesthetascs of antennule (except Mithrax pleuracanthus), coxal and basial endites of maxillule, and basial endites of maxilla and first maxilliped (Tables 2–4). In the second zoea Maguimithrax and Mithrax share the following characters: the exopod of maxillule (except in Mithrax tortugae), and endopod of first maxilliped, and exopod of second maxilliped. The megalopa have a more heterogeneous morphology, with Maguimithrax and Mithrax having in common the setation of the coxal and basial endites of maxilla, pleopods and uropod. On the other hand, Maguimithrax spinosissimus can be easily distinguished from other Mithracidae by: (i) the setation of the endopod of maxillule, coxal endite and endopod of maxilla, and endopod of the maxilliped II in both zoeal stages; (ii) the scaphognathite of maxilla in the first zoeal stage; (iii) the basis of maxilliped I in the second zoeal stage and megalopa; (iv) the morphology of the antennule and antenna in the second zoeal stage presenting a segmentation similar to that observed in the megalopa, which can be a consequence of rapid facultative lecithotrophic larval development; (v) and the setation of the antennule, coxal endite of maxilla and exopod of maxilliped II in the megalopa (Tables 2–4). All these dissimilarities support the erection of the genus Maguimithrax sensu Klompmaker et al. (Reference Klompmaker, Portell, Klier, Prueter and Tucker2015) to accommodate Maguimithrax spinosissimus.
In summary, we have re-described in detail the larval development of Maguimithrax spinosissimus. The short larval period here observed is in line with that reported by Provenzano & Brownell (Reference Provenzano and Brownell1977) and supports the notion that this species has considerable potential for aquaculture in the region. Furthermore, due to its short larval development, various previous studies have suggested that Maguimithrax spinosissimus is either a lecithotrophic (e.g. Creswell et al., Reference Creswell, Tunberg and Winfree1989; Baeza et al., Reference Baeza, Holstein, Umaña-Castro and Mejía-Ortíz2019) or a facultative lecithotrophic species (e.g. Provenzano & Brownell, Reference Provenzano and Brownell1977; Porter et al., Reference Porter, Iglehart, Craig, Adey, Adey and Farrier1986; Tunberg & Creswell, Reference Tunberg and Creswell1991). Facultative lecithotrophy is not common but known to occur in some brachyuran crabs (Anger, Reference Anger1995) and other decapod groups, and may be a physiological response to different temperature/food density combinations in natural habitats (Thessalou-Legaki et al., Reference Thessalou-Legaki, Peppa and Zacharaki1999). Here we fed all larval stages ad libitum (see Materials and methods section), but no assessment of food ingestion was made. However, strict lecithotrophic majoid species usually have a mass of yolk granules in the larval stages (e.g. Taishaku & Konishi, Reference Taishaku and Konishi2001), which is not the case for M. spinosissimus. Indeed, Tunberg & Creswell (Reference Tunberg and Creswell1988) indicated that survival and size of the larvae of M. spinosissimus were greater when specimens were fed daily. Altogether, the information above suggests that rearing efforts need to provide nutrition to larvae so as to speed up development during the larval phase of M. spinosissimus. Partial lecithotrophy needs to be further explored in M. spinosissimus and, if confirmed, this trait will aid in the development of efficient culturing techniques in the species.
The complete larval development of M. spinosissimus provides support for conservation strategies of this species as well as coral reefs. This crab achieves moderate to high abundance in the Florida Keys, USA and a wide distribution in the greater Caribbean Sea. Importantly, the structure of coral reef benthic communities, in particular those located in the Florida Keys, is changing quickly, turning from stony-coral-dominated communities into others in which soft corals, sponges and algae are among the main constituents (Norström et al., Reference Norström, Nyström, Lokrantz and Folke2009; McMurray et al., Reference McMurray, Finelli and Pawlik2015). As a herbivore, Maguimithrax spinosissimus has the potential to control algal growth in the field. Its larval re-description can help optimizing protocols for laboratory rearing, and the production of large quantities of juveniles could subsequently be used for ‘seeding’ this species to control the algal overgrowth of coral reefs that are already subject to major local disturbances and global climate change. Lastly, we have improved the battery of resources available for a reef-dwelling invertebrate inhabiting an already morphed seascape (coral reefs) that will likely continue to change given the increasing frequency and scale of contemporary disturbances (McMurray et al., Reference McMurray, Finelli and Pawlik2015 and references therein). This new reef seascape dominated by a species-rich sponge and algal assemblage might become the dominant community state in the greater Caribbean (Norström et al., Reference Norström, Nyström, Lokrantz and Folke2009; Bell et al., Reference Bell, Davy, Jones, Taylor and Webster2013; McMurray et al., Reference McMurray, Finelli and Pawlik2015 and references therein). Together with major changes in coral reefs, for example, continuous decrease in coral cover in several localities across the Caribbean as has been well documented in recent years (McMurray et al., Reference McMurray, Finelli and Pawlik2015 and references therein), we expect the behaviour of these coral-dwelling species to change concomitantly while they acclimatize (in the short term) and adapt to these morphing seascapes. Ultimately, the development of the present and other resources are also of utmost importance as they will improve the understanding of the biology of M. spinosissimus and its responses to local, regional and global anthropogenic disturbance for a species inhabiting an environment already facing considerable environmental threats.
Acknowledgements
All authors dedicate this work to the memory of our great friend Juan A. Bolaños, who passed away during the preparation of this manuscript. His work and efforts to develop carcinology in Latin America were invaluable. We thank Dr Antônio L. Castilho, Dr Maria Lúcia Negreiros-Fransozo and Dr Adilson Fransozo from the Núcleo de Estudos em Biologia, Ecologia e Cultivo de Crustáceos (NEBECC), UNESP, Botucatu – SP, Brazil, for providing optical resources and working space. We thank Carlos Lira, Gonzalo Hernández, (Universidad de Oriente) and Juan ‘Diablo’ for their help during the field and lab work. We thank the editor, Dr Benny Chan (Biodiversity Research Centre, Academia Sinica, Taiwan), and three anonymous reviewers for the valuable comments that improved our manuscript.
Financial support
This work was supported by the Fundação de Amparo à Pesquisa do Estado de São Paulo – FAPESP [grants 2012/20564-3, 2013/01201-0 and 2014/15549-0] and the National Council for Scientific and Technological Development (CNPq) to WS [Research Scholarship PQ #315185/2020-1]. Finally, we thank the Universidad de Oriente – UDO, Venezuela for supporting studies on the systematics of decapod crustaceans.












