Introduction
The phenology of adult insect development can be an important determinant of individual and population reproductive success (Singer, Reference Singer1972; Taylor, Reference Taylor1981; Cushman et al., Reference Cushman, Boggs, Weiss, Harvey, Murphy and Ehrlich1994; Weiss & Weiss, Reference Weiss and Weiss1998; Weiss et al., Reference Weiss, Murphy, Ehrlich and Metzler1993). Significant asynchrony in the emergence of males and females (Weiss et al., Reference Weiss, Murphy, Ehrlich and Metzler1993) or emergence when food resources are unavailable can lead to reproductive failure. For example, the oviposition of the phytophagous winter moth (Operophtera brumata) coincides with host plant availability (Visser & Holleman, Reference Visser and Holleman2001). Developmental synchrony of males and females may be more critical in short-lived insects or in environments where mate location is difficult (Calabrese & Fagan, Reference Calabrese and Fagan2004). In such contexts, males often emerge shortly before females. This ‘protandric emergence’, which is common in the Lepidoptera (Thomas, Reference Thomas1989; Wiklund et al., Reference Wiklund, Kaitala, Lindfors and Abenius1993; Savopoulou-Soultani et al., Reference Savopoulou-Soultani, Stavridis, Vassiliou, Stafilidis and Iraklidis1994), shortens the pre-reproductive period of proovogenic females (females emerging with a fixed egg load), because most males are available for mating when they emerge (Fagerström & Wiklund, Reference Fagerström and Wiklund1982), increasing the probability of mating (Carvalho et al., Reference Carvalho, Queiroz and Ruszczyk1998). The dynamics of the emergence of the two sexes within each generation may be a key factor in the reproductive success of the population.
The duration of larval development is also an important component of fitness, especially in the presence of competition and/or parasitism. A shorter developmental time is often favored because it reduces exposure to parasitoids and reduces the risk of death due to stochastic events. These parameters are critical for the survival of larvae in the wild (Haggstrom & Larsson, Reference Haggstrom and Larsson1995; Benrey & Denno, Reference Benrey and Denno1997; Thiéry & Moreau, Reference Thiéry and Moreau2005). In addition, multivoltine organisms typically have shorter development times. The consequential reduction of adult size can have consequences on fitness, because insect body size is often associated with fecundity (Honek, Reference Honek1993).
Numerous studies have shown that the duration of larval development and the timing of adult emergence vary significantly among populations and between years within individual populations (see Weiss et al., Reference Weiss, Murphy, Ehrlich and Metzler1993 for an example). This may be due to numerous interacting factors, such as genetic differences among individuals and populations, environmental factors (weather conditions, photoperiod, and temperature), and local habitat characteristics (Taylor, Reference Taylor1981; Curry & Feldman, Reference Curry and Feldman1987; Danks, Reference Danks1994, Reference Danks2002; Weiss & Weiss, Reference Weiss and Weiss1998; Chuche & Thiéry, Reference Chuche and Thiéry2012; but see Forrest & Miller-Rushing, Reference Forrest and Miller-Rushing2010 for a review). The effect of varying thermal regimes on larval development has been investigated (Weiss et al., Reference Weiss, Murphy, Ehrlich and Metzler1993; Ellwood et al., Reference Ellwood, Diez, Ibanez, Primack, Kobori, Higushi and Silnader2012), but the effect of juvenile resource quality on larval development is often neglected. In phytophagous insects, the larval host plant may have profound effects on the growth rate of juveniles and consequently on the dynamics of emergence (Scriber, Reference Scriber1981; Nylin & Janz, Reference Nylin and Janz1993; Thiéry & Moreau, Reference Thiéry and Moreau2005). Indeed, there can be significant variation in host plant quality and in host choice by ovipositing females, and these can have important effects on the offspring. Nevertheless, the effect of the host plant on the pattern of offspring emergence has often been neglected. Tikkanen et al. (Reference Tikkanen, Niemela and Keranen2000) showed that changes in the host plant can significantly disrupt the timing of male and female emergence and lead to a reduction of population density. In general, lack of information regarding the effect of host plants on insect emergence limits our understanding of the natural variation in the reproductive success of phytophagous insects.
The purpose of the present study was to determine the effect of larval host plants on the emergence phenology of a wild population of Lobesia botrana (Lepidoptera: Tortricidae, Denis & Schiffermueller), a significant insect pest in vineyards of Europe, North Africa, and West Asia (Roehrich & Boller, Reference Roehrich, Boller, Van der Gesst and Evenhuis1991; Thiéry, Reference Thiéry2005). In particular, we measured the emergence phenology of males and females that developed on six different grape varieties during a single year in an experimental vineyard in southwestern France. Lobesia botrana is an ideal model organisms for such studies because larval fitness and adult reproductive success of this species are strongly related to the nature of the host plants on which the larvae have fed (Savapoulou-Soultani et al., Reference Savopoulou-Soultani, Stavridis and Tzanakakis1990; Torres-Vila et al., Reference Torres-Vila, Rodriguez-Molina, Roehrich and Stockel1999; Thiéry & Moreau, Reference Thiéry and Moreau2005; Moreau et al., Reference Moreau, Arruego, Benrey and Thiéry2006a,Reference Moreau, Benrey and Thiéryb,Reference Moreau, Benrey and Thiéryc, Reference Moreau, Thiéry, Troussard and Benrey2007). In addition, adult emergence is an important determinant of reproductive success; so it is very important to understand the effect of host characteristics on the pattern of adult insect emergence in order to implement effective pest management practices.
Materials and methods
Study vineyard and grape varieties
All experiments were performed on a wild population of the European grapevine moth (L. botrana) in a 30-year-old experimental vineyard of about 1 ha that was planted with different grape varieties and located in the INRA Bordeaux-Aquitaine of south-western France (GPS: N44°47′29.58″W0°34′46.50″). This study was performed from August to November. The six studied varieties were Chardonnay, Chasselas, Merlot, Pinot, Riesling, and Noah, the first five of which were used in a previous study of variation in reproductive life history traits in laboratory experiments (Moreau et al., Reference Moreau, Benrey and Thiéry2006b,Reference Moreau, Benrey and Thiéryc). The experimental plot was surrounded by vineyards of classical Bordeaux varieties (Merlot, Cabernet Sauvignon, and Sauvignon). The moth population was monitored by pheromone sticky delta traps (Thiéry, Reference Thiéry2008).
Model insect and experiments
Lobesia botrana, whose original food source is believed to be the Thymeleacea Daphne gnidium, has become a significant pest in European vineyards (Maher & Thiéry, Reference Maher and Thiéry2006). Its larvae are polyphagous and can develop on almost all grape varieties and more than 25 other hosts (Thiéry & Moreau, Reference Thiéry and Moreau2005). In our latitude, L. botrana has three or four generations. First-generation larvae appear in June and damage the inflorescences, second-generation larvae appear in July, and third-generation larvae appear in August. Females oviposit single eggs at a time (Gabel & Thiéry, Reference Gabel and Thiéry1996) and several larvae can develop on one bunch of grapes, with the number limited by bunch size. All of our experiments were performed on third-generation individuals during August, because our initial pheromone trap monitoring in the experimental vineyard indicated small numbers of the first two generations (spring and early summer), but an abundant third generation. Before oviposition started, 25 grape bunches of similar size and phenology (based on visual examination) from each variety (one bunch per stock) were packaged in tube gauze bags (25 cm length, 12 cm diameter, closed at the two ends) on August 1 to prevent undated egg laying. During the peak of captures in the pheromone traps (Thiéry, Reference Thiéry2011), the isolation bags were removed for 48 h, allowing for two consecutive nights of oviposition. Gauze bags were then put back until the harvest date (33 days after bag closure) to prevent additional egg laying. In order to prevent early adult losses, each grape bunch was harvested 1 week before the expected first date of emergence. This allowed for nearly complete larval development (five instars until pupation for the faster growers). Each collected bunch was individually packed in a 1-liter parallelepiped box that was aerated and placed in a climatic chamber. This chamber was maintained at 22±1 °C, 45±10% relative humidity, and with a photoperiod of 15 h light: 8 h dark+1 h of dusk. The first emergence occurred on September 10, 38–39 days after egg laying. Thus, the first emerging adults spent about 87% of their total development time in the vineyard. Our procedure ensured the complete and accurate collection of nearly all adult emergences.
After laboratory storage, all grape bunches were checked daily for collection of visible pupae. Pupae were isolated in glass tubes (70×9 mm diameter) that were closed with cotton plugs, labeled, and stored in the same climatic chamber (Thiéry & Moreau, Reference Thiéry and Moreau2005). Pupal weight was determined in a randomly chosen sub-sample of at least 50 pupae (range: 53–74) in each grape variety. Individual weight was recorded to within 0.01 mg. The number of pupae in each grape bunch was also recorded. Overall parasitism in this study for the six cultivar populations was less than 1%; so its effect on pupal development was disregarded.
The following variables were measured: (i) percentage of grape bunches that harbored pupae, (ii) pupal population size in each grape variety, (iii) emergence rate of all collected pupae (thereafter corresponding to pupal mortality), (iv) pupal weight, (v) total development time (egg+larvae+pupae), and (vi) adult sex ratio.
Statistical analysis
The percentage of grape bunches that harbored pupae in the different grape varieties were compared using Fisher's exact test. Pupal population sizes were analyzed using a Negative Binomial generalized linear model (GLM) to compare the number of isolated pupae on different grape varieties and between grape bunches within a variety. The statistical significance of each term was assessed using likelihood ratio-based Chi-square test associated with a pair-wise Wilcoxon multiple comparison test. A Negative Binomial GLM accounts for overdispersion bias associated with count data, and so it was preferred over a classical Poisson model (Sileshi, Reference Sileshi2006). The percentage of emerging moths, sex ratios in different grape varieties, and sex-ratio bias within varieties were tested using Pearson's Chi-square test. Pupal weight variation among grape varieties, bunches, sexes, and within each variety was tested using ANOVA, and the statistical significance of each parameter was assessed by an F-statistic associated with the Tukey HSD test.
The bimodality of the emergence pattern was tested using a bimodality coefficient (b) defined as:
where S represents the skewness (asymmetry) of the distribution, K represents the kurtosis (peakedness) of the distribution, and n represents the sample size. A b-value greater than 0.55 indicates a bimodal distribution (Vega & Grundy, Reference Vega and Grundy1993) and S and K values of 0 indicate a normal distribution. Deviation from a normal distribution was tested by comparing skewness (S<0: left-tailed, S>0: right-tailed) and kurtosis (K<0: platykurtic, K>0: leptokurtic) to the theoretical value from Student's t-test for infinite degrees of freedom with an α risk of 5% (t 0,05; ∞=1,96) as:
where s S =√[6n(n−1)/(n−2)(n+1)(n+3)] and s K =√ [24n(n−1)2/(n−3)(n−2)(n+3)(n+5)].
The emergence patterns of the two genders and in the different grape varieties were compared using a Cox proportional hazards regression model and the statistical significance of each parameter was assessed with likelihood ratio-based Chi-square test. The relationship between mean emergence delay (emergence delay=adult birth date−oviposition date) and mean pupal mass for males and females was assessed separately using Pearson's product–moment correlation test.
All statistical analysis was performed with R software (v. 2.10.1 R Development Core Team, 2008) implemented with the e1071 package for skewness and kurtosis computation and the survival package for the Cox proportional hazards regression model.
Results
Population characteristics of moths from different grape varieties
The percentage of grape bunches that harbored pupae was similar in all six grape varieties (Chardonnay: 95%, Chasselas: 80%, Merlot: 90%, Noah: 90%, Pinot: 100%, and Riesling: 100%; Fisher's exact test: P=0.15), but pairwise comparisons indicated significant differences in the total number of pupae in the different grape varieties (GLM: χ5 2=62.27, P<0.0001, fig. 1). Moreover, the distribution was not homogenous in grape bunches within a variety (range: 0–25 pupae per bunch, χ114 2=442.92, P<0.0001). Wilcoxon pairwise multiple comparison tests indicated significantly more pupae on Merlot and Chardonnay than on Riesling and Chasselas (fig. 1). The percentage of emerging moths was significantly different in the six grape varieties (Chardonnay: 71%, Chasselas: 91%, Merlot: 85%, Noah: 89%, Pinot: 88% and Riesling: 92%; Pearson's Chi-square test: χ5 2=35.84, P<0.0001), and this difference was mainly due to the low percentage on Chardonnay. The adult sex ratio was similar in the six grape varieties (Pearson's Chi-square test: χ5 2=2.39, P=0.79) and there was an approximately equal ratio of males and females on each variety (Pearson's Chi-square test: all P-values>0.05).
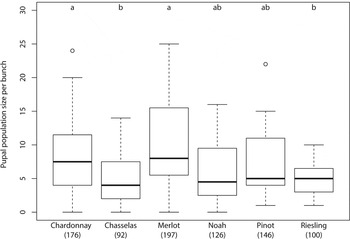
Fig. 1. Number of L. botrana pupae per bunch in different grape varieties. Bold line, median; box: middle two quartiles; dashed lines, 1.5×interquartile range; open circle, extreme value. The numbers inside parentheses indicate number of pupae per 20 bunches. Columns with the same letter are not significantly different (P>0.05) based on a nonparametric pairwise Wilcoxon multiple comparison test.
Pupal weight of moths from different grape varieties
The weight of pupae differed significantly in the six grape varieties (ANOVA, variety effect: F 5, 281=2.53, P=0.03, fig. 2). This difference can be explained by the low weight of pupae on Merlot and the high weight of pupae on Pinot (Tukey HSD test: P=0.01). Pairwise tests indicated that none of the other comparisons of weight were significant (P>0.26). Overall, female pupae were heavier than male pupae (sex effect: F 1, 281=534.75, P<0.0001) in all grape varieties (interaction sex×variety: F 5, 281=0.77, P=0.57) and there was no significant difference in bunches within each grape variety (nested effect of bunches within varieties: F 94, 281=1.15, P=0.19). The pupal population size was not correlated to the pupal weight (r Pearson=−0.32, P=0.53).

Fig. 2. Weight of male and female L. botrana pupae in different grape varieties. Bold line, median; box, middle two quartiles; solid circle, mean; dashed lines, 1.5×interquartile range; open circle, extreme value. The numbers in parentheses indicate number of pupae that were weighed.
Timing of emergence of moths from different grape varieties
Among the six grape varieties, the emergence of adults lasted up to 45 days (Table 1, fig. 3), and faster growers needed 39 days to complete their development. Nevertheless, for both genders within each grape variety, the bimodality coefficient was less than 0.55 (range indicating bimodal distribution: 0.30–0.52), indicating that each emergence occurred as a single wave. The distribution of female emergence deviated from normality in Chardonnay, Merlot, and Pinot grapes and the distribution of male emergence deviated from normality in Chasselas and Merlot grapes. In all cases, deviations were due to early massive emergence (S>0) in a short time (K>0), except for females on Chardonnay (no excess of kurtosis).
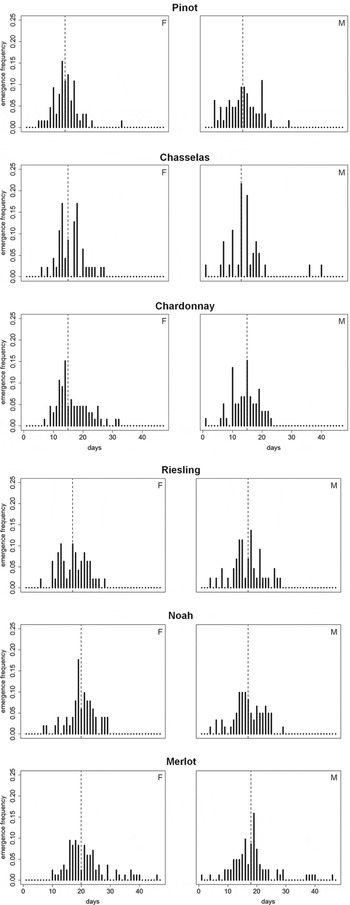
Fig. 3. Emergence of L. botrana females (F) and males (M) in different grape varieties. Grape varieties are listed in the order of pupal emergence, from earliest to latest. Vertical dashed line indicates mean emergence time.
Table 1. Analysis of the variation in emergence phenology of male and female L. botrana on six different host grape plants.

N, sample size; W, Shapiro–Wilk test; P w, P-value associated with Shapiro–Wilk test; S, skewness; P s, P-value associated with t-test for skewness bias; K, kurtosis; P k , P-value associated with t-test for kurtosis bias; b, bimodality coefficient (see the text for details).
Analysis of emergence delay indicated that gender and grape variety had significant effects on emergence time, but that there was no interaction of these variables (Cox proportional hazards regression model, sex effect: χ1 2=11.15, P<0.001; variety effect: χ5 2=96.34, P<0.0001; interaction sex×variety: χ5 2=7.13, P=0.21, fig. 4). Males emerged an average of two days before females (emergence delay after the first emergence [mean±SD males: 15.94±6.25 days, females 17.77±6.06 days, fig. 4). Emergence occurred first on Pinot grapes and last on Merlot grapes (fig. 3).

Fig. 4. Variations in emergence delay according to the varieties and sexes, from the earliest (Pinot) to the latest (Merlot). Bold line, median; box, middle two quartiles; solid circle, mean; dashed lines, 1.5×interquartile range; open circle, extreme value. The numbers in parentheses indicate the total number of emerging adults.
Relationship of emergence delay and pupal mass
Finally, our results indicate that there was no significant relationship between emergence delay and pupal weight in females (Pearson's product–moment correlation, r=−0.78, P=0.07, fig. 5a) or in males (r=−0.54, P=0.26, fig. 5b).

Fig. 5. Relationship between mean emergence delay and pupal mass of (a) female moths and (b) male moths on each of the six grape varieties.
Discussion
The present study of the emergence phenology of L. botrana on six varieties of grape hosts had four major findings: (i) more adult moths develop on certain grape varieties; (ii) the host plant for the larvae affects adult emergence phenology; (iii) male moths emerged before female moths in all grape varieties; and (iv) the grape variety that hosted larvae affected the phenology of emergence, and had different effects on males and females. It is well-established that abiotic and biotic factors affect the timing of many phenological characteristics (Forrest & Miller-Rushing, Reference Forrest and Miller-Rushing2010). Our results clearly show that the characteristics of the larval host plant influence the timing of emergence of L. botrana adults.
Two of our most important results are: (i) the number of pupae varied significantly between grape bunches and (ii) some grape varieties harbored more pupae than others. This second point is consistent with the findings of a previous study (Sharon et al., Reference Sharon, Zahavi, Soroker and Harari2009). The difference in pupal population size among grape varieties could be due to differences in egg laying and/or differences in larval mortality (Maher et al., Reference Maher, Jolivet and Thiéry2001; Moreau et al., Reference Moreau, Thiéry, Troussard and Benrey2007, Reference Moreau, Rahme, Benrey and Thiéry2008). The differential acceptability of a grape variety is controlled by stimulants and deterrents perceived by ovipositing females (Thiéry et al., Reference Thiéry, Gabel, Farkas and Pronier1992; Maher et al., Reference Maher, Jolivet and Thiéry2001, Reference Maher, Thiéry and Städler2006; Maher & Thiéry, Reference Maher and Thiéry2004). Our results indicate that females: probably oviposited more on Merlot and Chardonnay, suggesting a preference for these varieties. Previous research documented different larval mortalities earlier in the season (before or at bunch closure) among different grape varieties (Gabel & Roehrich, Reference Gabel and Roehrich1995). These authors attributed this to host plant resistance, but a part of their observed differential mortality may have been due to larvae dropping from the bunches. However, recent laboratory studies, in which ripened dried berries were offered to L. botrana, did not confirm the presence of different mortality in different grape varieties (Moreau et al., Reference Moreau, Arruego, Benrey and Thiéry2006a,Reference Moreau, Benrey and Thiéryb). Thus, the variation in the number of adults in the different grape varieties that we observed most likely results from differences in oviposition preference.
Female pupae were heavier than male pupae in each of the six grape varieties. This occurs in many Lepidoptera because the females store nutrients for egg production (Raven, Reference Raven1961; Slansky & Scriber, Reference Slansky, Scriber, Kerkut and Gilbert1985). Females may become larger by feeding for a longer time, resulting in slower development (Thiéry & Moreau, Reference Thiéry and Moreau2005; Moreau et al., Reference Moreau, Arruego, Benrey and Thiéry2006a,Reference Moreau, Benrey and Thiéryb,Reference Moreau, Benrey and Thiéryc). Consequently, males tend to emerge before females. This protandry is common in fruit tortricids and several other Lepidoptera (Rodriguez-del-Bosque et al., Reference Rodriguez-del-Bosque, Smith and Browning1989; Roehrich & Boller, Reference Roehrich, Boller, Van der Gesst and Evenhuis1991; Wiklund et al., Reference Wiklund, Kaitala, Lindfors and Abenius1993). Protandry appears to provide at least two advantages: (i) maximization of copulation opportunities for males (reviewed in Wiklund & Fagerström, Reference Wiklund and Fagerström1977; Bulmer, Reference Bulmer1983), and (ii) minimization of the pre-reproductive period of females, so that males are available upon females’ emergence (Fagerström & Wiklund, Reference Fagerström and Wiklund1982). Consistent with our results, Torres-Vila et al. (Reference Torres-Vila, Stockel and Roehrich1995) showed that 3-day-old L. botrana males were the most efficient at mating.
We also found that grape variety affected the duration of larval development and pupal weight. Size and rate of development are typically inversely related (Danks, Reference Danks1994), but our results seem to contradict this pattern. Although the correlation that we observed was only marginally significant (possibly due to the small number of tested varieties), our results indicated that larvae developed faster and were heavier on Pinot grapes, and developed slower and were lighter on Merlot grapes. It should be noted that the presence of high quality or quantity of food can disable the trade-off of size and rate of development (Reznick et al., Reference Reznick, Nunney and Tessier2000; Wissinger et al., Reference Wissinger, Steinmetz, Alexander and Brown2004). Prolongation of development is potentially an important component of fitness, because it can affect larval exposure to predators and parasites (Benrey & Denno, Reference Benrey and Denno1997).
One of our most interesting results is that the emergence phenology of L. botrana was different on different grape varieties. Many abiotic factors, such as temperature and humidity, influence adult emergence (Curry & Feldman, Reference Curry and Feldman1987; Weiss et al., Reference Weiss, Murphy, Ehrlich and Metzler1993; Chuche & Thiéry, Reference Chuche and Thiéry2012). Nevertheless, the different grape varieties in our study were close together, grew on similar soil, and were exposed to similar climate; hence we believe that environmental differences had only a minor role in causing differences in emergence. Genotypic variation among different populations that are locally adapted to their hosts may also cause variability in adult emergence (Wood et al., Reference Wood, Olmstead and Guttman1990; Feder et al., Reference Feder, Hunt and Bush1993; Feder, Reference Feder1995; Groman & Pellmyr, Reference Groman and Pellmyr2000; Thomas et al., Reference Thomas, Bethenod, Pelozuelo, Frérot and Bourguet2003). However, the moths in our small experimental vineyard were from a single population, so genotypic variation is also an unlikely cause of the variation in emergence that we observed. Thus, grape variety seemed to play the major role in determining the phenology of adult emergence of L. botrana in our study. Interestingly, for each grape variety, emergence occurred as a single wave. Furthermore, in Chardonnay, Chasselas and Pinot, the emergence phenology was different for males and females. This suggests that males and females have different food requirements and/or assimilation abilities. Male and female moths both had an extended period of emergence on Merlot. Merlot was also associated with prolonged larval development and lighter pupae. A complete understanding of factors that affect emergence phenology is not yet available, but nutrients or secondary compounds present in grapes, which can vary significantly in different grapes (Zhu et al., Reference Zhu, Zhang, Deng, Li and Lu2012), undoubtedly play a role. The rootstock and soil characteristics could also affect emergence of moths. Merlot contains high levels of flavan-3-ols (flavanols), including dihydroquercitin and stilbenes (Zhu et al., Reference Zhu, Zhang, Deng, Li and Lu2012), and such molecules could potentially affect emergence phenology. We suggest further study of this issue.
The variation in adult emergence phenology that we observed agrees with previous studies which reported that quantitative plant defenses should limit the annual number of insect generations by extending the larval development time (see Cizek et al., Reference Cizek, Fric and Konvicka2006 for an example). It is possible that the size and shape of the grape bunches in the different varieties, as well as other characteristics of the larval micro-habitat, may influence development time. For instance, different larval micro-distributions of the Bay Checkerspot butterfly (Euphydryas editha bayensis) vary in development time by up to 11 days (Weiss et al., Reference Weiss, Murphy, Ehrlich and Metzler1993; Weiss & Weiss, Reference Weiss and Weiss1998). Humidity and solar radiation are the most important micro-climatic parameters for larval development, because development time depends on temperature due to the small size of larvae (Willmer, Reference Willmer1982; Weiss et al., Reference Weiss, Murphy, Ehrlich and Metzler1993). In the case of grapes, the compactness of clusters differs among varieties, leading to differences in sunlight exposure and temperature (Fermaud, Reference Fermaud1998; Pieri & Fermaud, Reference Pieri and Fermaud2005), but we cannot exclude the possibility that there is a genetic basis for increased variation of emergence, a ‘bet-hedging strategy’ of L. botrana (Forrest & Miller-Rushing, Reference Forrest and Miller-Rushing2010 and references therein). Further experiments that employ fine temperature measurements inside grape bunches are needed to establish the contribution of such factors.
The mechanism(s) underlying the variation of emergence of L. botrana is unknown. Nonetheless, the greater variation in emergence of larvae that feed on Merlot could be advantageous for individuals of temperate regions, in which there is greater variation of environmental factors such as temperature and precipitation. In the last L. botrana generation (at the end of summer), the emergence of all adults within a narrow window of time could be risky, because inclement weather at this time could affect flight and oviposition (Goulson, Reference Goulson1993). Thus, it might be expected that increased variation in emergence would improve overall survival of the final generation. In other words, an extended period of adult emergence could be a ‘bet-hedging strategy’ (Danforth, Reference Danforth1999; Hopper, Reference Hopper1999), even if such a strategy depends on population genetic diversity. Increased variation of adult emergence might allow some individuals to escape detrimental conditions and contribute to long-term persistence of populations in a vineyard.
Large-scale field experiments are clearly needed to evaluate the fitness of early- and late-emerging adults under controlled conditions. In addition, a field survey analyzing the reproductive success of subsequent generations of moths that have the most extreme phenologies (those that emerged on Merlot and Chasselas) should provide important information regarding the contribution of the reproductive capacity of individuals to the foundation of the following year's population. Optimally, such a survey should also consider the dispersive capacities of adults that have rapid and slow larval development, and the effect of parasitoids on the rate of development (Xuéreb & Thiéry, Reference Xuéreb and Thiéry2006). This last point should receive attention because it is important to have population-level data on this significant vineyard pest. The data provided here on adult emergence phenology of L. botrana could also be useful for the development of age-structured mathematical models of vineyard infestation (Ainseba et al., Reference Ainseba, Picart and Thiéry2011). Such knowledge should improve the assessment of pest outbreaks in subsequent generations.








