Introduction
Chronic rhinosinusitis is a common condition characterised by inflammation of the nose and paranasal sinuses.Reference Fokkens, Lund and Mullol1, Reference Benninger, Ferguson, Hadley, Hamilos, Jacobs and Kennedy2 Topical intranasal steroids are the mainstay of medical treatment,Reference Fokkens, Lund and Mullol1, Reference Scadding, Durham, Mirakian, Jones, Drake-Lee and Ryan3 with patients often receiving prolonged courses pre- and post-operatively. Contamination of medical therapy devices, such as Venturi-style atomiser bottles and high-volume saline irrigation bottles, has recently come under scrutiny,Reference Rizzi, Batra, Hall, Citardi and Lanza4–Reference Lewenza, Charron-Mazenod, Cho and Mechor9 with evidence suggesting that these devices can harbour significant bacterial contamination.
Staphylococcus aureus bacterial infection has been suggested to play a role in chronic rhinosinusitis.Reference Singhal, Foreman, Bardy and Wormald10 Patients undergoing endoscopic sinus surgery and from whom S aureus is cultured have significantly worse pre-operative symptomatology and a higher rate of post-surgical clinical relapse.Reference Singhal, Foreman, Bardy and Wormald10, Reference Singhal, Psaltis, Foreman and Wormald11 Intracellular host cell invasion and bacterial biofilm establishment have been suggested as explanations for the host's inability to clear S aureus.Reference Plouin-Gaudon, Clement, Huggler, Chaponnier, Francois and Lew12–Reference Tan, Foreman, Jardeleza, Douglas, Tran and Wormald14 However, little is known about the potential risk of cross-contamination of bacteria passing between the nose and sinuses and the metered-dose nasal spray bottles used to deliver intranasal steroids. It is not unusual for patients to use the same bottle for weeks or even months, and the manufacturer's guidelines often do not give clear instructions on bottle tip cleaning or sterilisation.
The purpose of this study was to identify whether cross-contamination occurs between the patient's nose and their small volume, metered-dose nasal steroid spray bottle, and also to assess various methods of bottle sterilisation using an in vitro model.
Methods
Study design, patient recruitment and ethical considerations
This study was performed in the tertiary referral practice of the senior author (P-JW). Ethical approval was granted by the local Human Research Ethics Committee (approval number 2011104).
Twenty-five patients with stable chronic rhinosinusitis were prospectively recruited from the out-patient clinic. All patients met the Rhinosinusitis Task Force criteriaReference Benninger, Ferguson, Hadley, Hamilos, Jacobs and Kennedy2, Reference Lanza and Kennedy15 for the diagnosis of chronic rhinosinusitis, and were receiving medical treatment in the form of intranasal steroids delivered via small volume, metered-dose spray bottles. Stable disease was defined in this study as no surgical intervention in the preceding six months as well as no recent infections requiring systemic antibiotics. Patients were eligible for inclusion in the study if they could confirm that they had been using their nasal steroid spray bottle once daily as prescribed, and had not changed to a new bottle within the last two weeks. Patients were contacted by telephone at least 24 hours prior to their out-patient appointment to discuss these criteria and to offer the opportunity for study inclusion. All patients gave informed, written consent to study inclusion on the day of their clinic appointment.
Patient assessment
During their out-patient appointment, patients underwent clinical examination including routine endoscopic evaluation of the nasal cavities and swabbing of the middle meatus, regardless of clinical status. Further swabs were taken from the patient's nasal vestibule and the tip of their nasal steroid spray bottle (Figure 1); another sample was taken by spraying a single spray of the bottle contents onto a swab.

Fig. 1 Photograph of nasal spray bottle indicating the area (arrow) both swabbed for bacteria and inoculated for in vitro experiments.
All swabs were then processed and cultured using standard microbiological techniques, by Adelaide Pathology Partners (Mile End, Adelaide, South Australia). Reported results include the bacterial genus and the relative abundance of bacterial growth (i.e. light, moderate or heavy).
In vitro sterilisation techniques
To assess whether different sterilising techniques were adequate to clear bacteria from the nasal spray bottle tips, fresh bottle tips were inoculated with a S aureus reference strain (American Type Culture Collection 25923). Staphylococcus aureus was cultured overnight in cerebrospinal fluid broth (Oxoid Australia, Thebarton, South Australia) at 37°C in a shaking incubator. The culture was centrifuged at 8000 rpm for 8 minutes and the bacterial pellet resuspended in 0.45 per cent sodium chloride then diluted as necessary to create a 1 McFarland unit solution. This solution was streaked onto the sides of the bottle tip with a cotton tip applicator to create in vitro bacterial contamination. Following a 15 minute period of air drying, the bottle tip was swabbed and then streaked onto a blood agar plate (Oxoid Australia) in order to confirm bacterial infection.
Bottle tips inoculated in this fashion then underwent a variety of cleaning methods, namely: rinsing in boiling water for 5 seconds and wiping dry; rinsing in cold water for 5 seconds and wiping; cleaning with 70 per cent ethanol wipes; washing in diluted dishwashing liquid and wiping; or removing the bottle tip, rinsing and then microwaving in a beaker of water for 1 minute on high power. One tip went untreated to act as a control.
Bottle tips were left to air-dry and were then re-swabbed. Swabs were taken from the bottle tips and inoculated onto blood agar plates, which were then incubated at 37°C for 18–24 hours to identify the presence or absence of bacterial growth, assessed in a semi-quantitative manner.
All experiments were performed in triplicate.
Results
A total of 80 patients were contacted prior to their out-patient appointment. Of these, 25 patients (13 men and 12 women) fulfilled the study inclusion criteria. These patients had a mean age of 61.6 years (interquartile range, 55–67 years).
Twenty-three patients had undergone previous endoscopic sinus surgery, 10 reported a history of asthma and 1 was a current smoker. One patient reported sensitivity to salicylates which was not associated with asthma. No patients were taking systemic antibiotics at the time of recruitment, although five were subsequently prescribed antibiotics as a result of clinical findings at their out-patient appointment.
Twenty-four patients were using mometasone furoate (Nasonex; Schering, Kenilworth, New Jersey, USA) and one was using fluticasone furoate (Avamys; GlaxoSmithKline, London, UK). The estimated duration of use of each bottle prior to swabbing was not ascertained, but had to be a minimum of two weeks to justify study inclusion. No patient reported regularly cleaning their nasal spray bottle tip.
Microbiological results
Swab results for the cohort are shown in Table I. Staphylococcus aureus was cultured from the nasal vestibule of 7 of the 25 (28 per cent) patients; 5 of these 7 (71 per cent) patients also demonstrating S aureus in the middle meatus. For four of the five patients (80 per cent) with both vestibular and middle meatal S aureus, the same bacteria was cultured from the nasal bottle tip. Overall, there were 5 patients with S aureus growth from both the nasal cavity and the bottle tip, giving a cross-contamination rate of 5 of 7 (71 per cent) S aureus infected patients and 5 of 25 (20 per cent) total patients. There was no clear correlation between the extent of bacterial growth from bottle tip versus nasal swabs, with two patients demonstrating heavier bottle tip growth, two lighter growth and one equivalent growth, compared with nasal swab growth.
Table I Microbiological swab results

Pt no = patient number; CNS = coagulase-negative staphylococci; +ve = positive
In patients who cultured coagulase-negative staphylococci only, 11 of 18 (61 per cent) demonstrated concomitant bacterial growth from both nasal and bottle tip swabs.
One hundred per cent of patients grew either S aureus or coagulase-negative staphylococci from nasal vestibule or middle meatus swabs, with 18 of 25 (72 per cent) showing cross-contamination onto the nasal steroid bottle tip.
There was no bacterial growth from the bottle contents, for any patient.
In vitro assessment of sterilisation technique
Bottles were negative for bacterial growth before inoculation, and all were positive after inoculation. Negative controls demonstrated no growth of bacteria, and positive controls receiving no cleaning were always positive (Table II). Bottle tip sterilisation was achieved (i.e. no growth was observed following swabbing immediately after cleaning) with boiling water, ethanol swabs and microwaving (Figure 2). However, cleaning with cold water and with dishwashing liquid was unsuccessful in sterilising the bottle tips, with post-cleaning swabs growing S aureus colonies on blood agar plates.
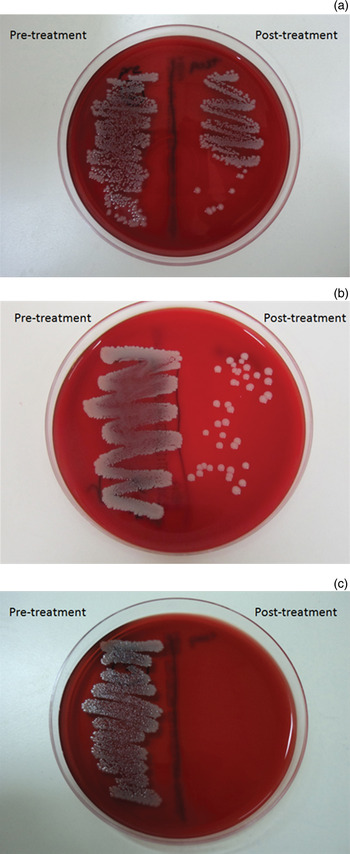
Fig. 2 Horse blood agar plate bacterial cultures demonstrating: (a) heavy growth after cold water washing; (b) light growth after cleaning with dishwashing liquid; and (c) no growth after 1 minute microwaving.
Table II Effect of cleaning methods on bacterial growth

*No inoculation. †No cleaning. –ve = negative; – = no growth; +ve = positive; ++ = heavy growth; += light growth
Discussion
These study findings indicate that, in patients with chronic rhinosinusitis treated with intranasal steroids delivered via a small volume, metered-dose spray bottle, cross-contamination can occur between the nasal cavity and the bottle tip. Maintaining clean, sterile working conditions is an important part of clinical care, and this has been emphasised within the hospital and primary care settings.Reference Rosenfeld16, Reference Boyce and Pittet17 However, the discovery of bacterial growth not only in the nasal vestibule and middle meatus but also on the tips of nasal steroid bottles raises the concern that patients may be persistently reinfecting themselves with bacteria when using their prescribed medication in the community. Our results indicate that over two-thirds of nasal steroid bottles can become contaminated with the same bacteria found within the nasal vestibule or middle meatus. This is a worrying finding in light of the fact that S aureus is a significant factor in chronic rhinosinusitis refractory to both medical and surgical treatment.Reference Jervis-Bardy, Foreman, Boase, Valentine and Wormald13, Reference Foreman, Jervis-Bardy and Wormald18, Reference Jervis-Bardy, Foreman, Field and Wormald19
Cross-contamination of adjunctive medical devices used in the treatment of chronic rhinosinusitis has been previously investigated. A number of studies have demonstrated, using standard culture techniques, that high-volume saline irrigation bottles can become contaminated with numerous bacterial strains after regular use.Reference Keen, Foreman and Wormald7, Reference Foreman and Wormald20 One recent study, using molecular techniques, identified an alarming 32 strains of bacteria from the saline irrigation bottles of 11 patients.Reference Lewenza, Charron-Mazenod, Cho and Mechor9 Re-usable Venturi-type atomiser bottles, as used in the out-patient clinic, have also been assessed for bacterial contamination. Dubin et al. Reference Dubin, White, Melroy, Gergan, Rutala and Senior5 found that the contents of lidocaine or tetrahydrozoline spray bottles were often contaminated with bacteria. However, a conflicting report by Rizzi et al. Reference Rizzi, Batra, Hall, Citardi and Lanza4 suggested that reusable bottles did not harbour significant amounts of bacteria. These studies, and others,Reference Spraggs, Hanekom, Mochloulis, Joseph and Kelsey21 have focussed on the contents of reusable bottles utilised within the out-patient clinic, and have not considered prescribed nasal steroid sprays. The one previous studyReference Bhattacharyya and Kepnes22 which did assess the presence of bacteria on nasal steroid bottles noted contamination of bottle tips with coagulase-negative staphylococci and oral flora. However, the authors did not establish whether these bacteria were environmental contaminants or present as a result of direct cross-contamination from the patients themselves. Furthermore, no in vitro experimental data were reported on appropriate sterilisation techniques.
Although our study identified the same species of bacteria both within the nose and on nasal spray bottle tips, it would not be possible to ascertain whether these were the same strain or clonal type unless molecular clonal identification was performed, which was not possible within the scope of the present study. However, future studies investigating nasal spray and nasal irrigation bottle contamination would benefit from such detailed investigation. In the current study, S aureus was detected in the nasal vestibule in 7 of 25 (28 per cent) patients, and it is reasonable to question how this compares to colonisation rates previously reported in normal individuals.Reference Kuehnert, Kruszon-Moran, Hill, McQuillan, McAllister and Fosheim23 However, of these seven patients, five (71 per cent) demonstrated coexisting S aureus in the middle meatus. We believe that these results reflect the clearance of S aureus harbouring mucus from the sinonasal cavity into the nasal vestibule. In only one case did we find nasal vestibular infection with cross-contamination to the bottle tip but no bacterial growth in the middle meatus.
We found a high prevalence of coagulase-negative staphylococcal growth, with 22 of 25 (88 per cent) patients' swabs producing such growth alone or in combination with another bacterial strain. This result correlates well with a previous study which reported a coagulase-negative staphylococci detection rate of 75 per cent when using standard culture techniques and 100 per cent when using culture-independent molecular identification techniques.Reference Feazel, Robertson, Ramakrishnan and Frank24 Furthermore, the presence of coagulase-negative staphylococci in swabs both from the nose and from steroid bottle tips substantiates the occurrence of bacterial cross-contamination between nose and bottle, and supports the theory that other, more pathogenic organisms (e.g. S aureus) may utilise the same mechanism to cause patient re-infection.
Reassuringly, no cultures of nasal spray bottle contents grew any pathogenic bacteria. This suggests that benzyl chloride, the bactericidal agent added to all steroid sprays, was functioning as expected.
As with all research, we accept that our study had certain methodological limitations. We performed a single, ‘snapshot’ assessment of microbiological incidence, and did not examine the temporal relationship between nasal infection and subsequent steroid bottle tip contamination, or vice versa. Therefore, the true pathogenic potential of steroid bottle tip contamination to cause clinical reinfection cannot be assessed by the present study. Furthermore, our study did not assess the correlation between culture positivity and patient outcome, and thus we cannot comment on the clinical significance of our culture results. A longer-term, longitudinal study should be undertaken to address both these research questions. However, despite these limitations we believe that our study findings give a valuable overview of the cross-contamination that occurs in this patient group, and identify issues requiring further investigation.
• Nasal steroid spray bottle tips can become contaminated with nasal vestibule bacteria
• Tips can be sterilised with simple cleaning methods
• The importance of this should be emphasised to every patient
The results of our in vitro sterilisation experiments indicate that patients must be educated on the importance of cleaning and sterilising their nasal steroid spray bottles. The fact that no patient included in this study regularly cleaned their spray bottle suggests that this is an issue which must be addressed. We did not perform the in vitro sterilisation experiments using patient-contaminated bottles; however, the fact that each experimentally inoculated bottle tip demonstrated heavy bacterial growth on swab culture, prior to cleaning, indicates the effectiveness of the successful cleaning techniques in eliminating the bacterial load. The different cleaning methods assessed were chosen because we would expect them to be readily available to any patient within the household setting. Cleaning techniques such as the use of dishwashing liquid or cold water alone were unsuitable for bottle tip sterilisation. However, boiling water, ethanol wipes and microwaving were effective, and these should be the preferred choice. Of these, washing in boiling water or microwaving would seem to be the most accessible for patients.
A further interesting observation from this study was that patients were generally unaware that the spray tip could be detached from the steroid spray bottle, facilitating easier cleaning.
Conclusion
The tips of small volume, metered-dose spray bottles, such as those regularly employed to deliver intranasal steroids, can become contaminated with the same bacteria found within the nasal cavity. Boiling water, ethanol wipes or 1 minute microwaving are suitable sterilisation methods that can be employed by the patient to ensure that there is no risk of reinfection or cross-contamination. Whether these bottles represent a focus of reinfection with pathogenic bacteria such as S aureus remains unknown; further research should focus on the risk of reinfection from contaminated bottles, and also investigate the temporal relationship between bottle use, contamination and clinical reinfection.
Nevertheless, the importance of sterilisation of nasal steroid bottle tips should be emphasised to every patient receiving a prescription for such medication.
Acknowledgement
This study received funding from the University of Adelaide and the European Rhinologic Society.






