Introduction
Dynamics of insect herbivore populations depend on trophic interactions with other species in their community, being controlled by their predators or host plants (Denno et al., Reference Denno, Gratton, Hartmut and Finke2003). Bottom-up controlled herbivores are mostly affected by the availability of their host plants (both quantity and quality of resources), while top-down controlled herbivores are limited due to predation or parasitism by their natural enemies. The relative strength of bottom-up and top-down forces can be altered by disturbance, invasive species and habitat structure components (Denno et al., Reference Denno, Gratton, Hartmut and Finke2003) like isolation (Chase et al., Reference Chase, Burgett and Biro2010). Another set of factors influencing strength of bottom-up and top-down forces on insect herbivores are their traits such as behavior, life history and strategies like dispersal, life cycle and environmental factors such as host plant density or patch size (Nylin, Reference Nylin2001).
Disturbance influences the abundance of species, the species composition (structure), and dynamics of communities. It may increase resource availability for herbivores (Hobbs & Huenneke, Reference Hobbs and Huenneke1992), but in turn disturbance may also cause high parasitism by reducing habitat complexity, thus making refuges of herbivores less common (Wilkinson & Feener, Reference Wilkinson and Feener2007). Disturbance also can make communities more prone to invasion of alien species (Cohen, Reference Cohen, Bullock, Kenward and Hails2002). Insect communities are good model systems to test responses to disturbance such as alterations or changes in habitat because of their short generation time and high variability (Osborne et al., Reference Osborne, Loxdale, Woiwod, Bullock, Kenward and Hails2002).
Spatial structure of patches is an important factor influencing population and trophic dynamics of insects (Denno et al., Reference Denno, Gratton, Hartmut and Finke2003). Isolation usually decreases population sizes and diversity of communities, mostly by its effect of reducing dispersal and/or colonization. Isolation can also influence trophic dynamics of patches; it weakens top-down control (Chase et al., Reference Chase, Burgett and Biro2010), as higher trophic levels are more limited by habitat fragmentation (Cronin & Haynes, Reference Cronin and Haynes2004) and dispersal than herbivores (Elzinga et al., Reference Elzinga, van Nouhuys, van Leeuwen and Biere2007). Herbivore species show various responses to host plant density without a general pattern (Kareiva, Reference Kareiva, Denno and McClure1983); with known examples of different herbivore species having highest herbivory at large, small (Kareiva, Reference Kareiva, Denno and McClure1983) or intermediate densities (Underwood & Halpern, Reference Underwood and Halpern2012), while some species are independent of host plant density (Kareiva, Reference Kareiva, Denno and McClure1983). Density of host plants influences herbivores indirectly through individual characteristics of trees like the size (height and trunk diameter) (Shea et al., Reference Shea, Smyth, Sheppard, Morton and Chalimbaud2000) or the amount of pods (Arista & Talavera, Reference Arista and Talavera1996).
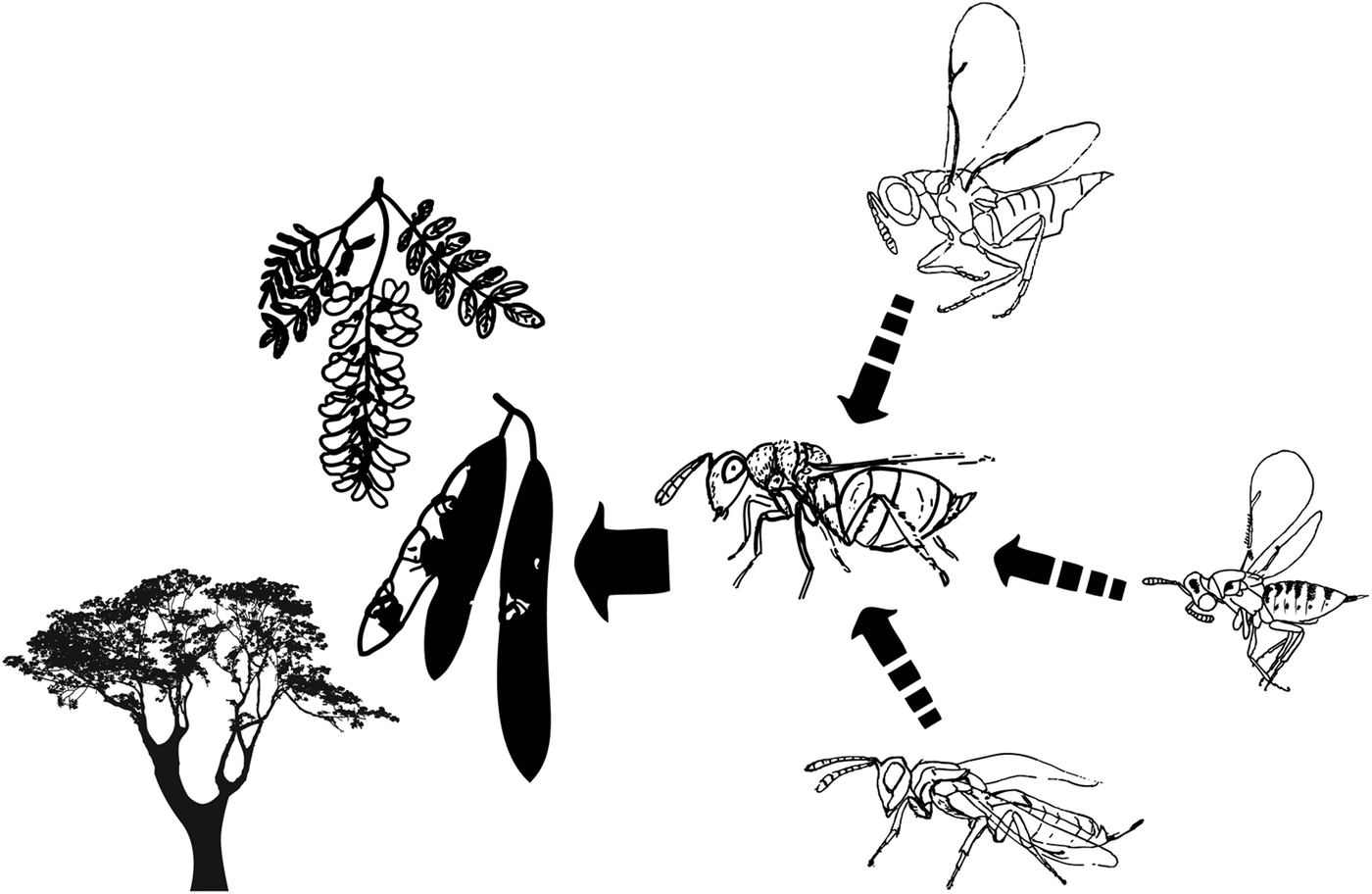
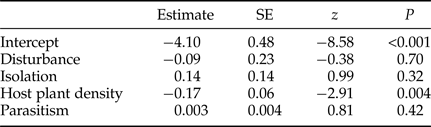
The tritrophic system of Robinia pseudoacacia provides an excellent model system for measuring effects of disturbances on insect populations. This system includes the pre-dispersal seed predator Bruchophagus robiniae Zerova, 1970 and its parasitoids. Seedpods can be regarded as a concentration of resources in time and space (Osborne et al., Reference Osborne, Loxdale, Woiwod, Bullock, Kenward and Hails2002). Prior to emergence seed predator and parasitoid individuals are closed inside the infested seed and seedpod in which they are developing (Batiste, Reference Batiste1967). Due to this isolation, insects are spatially separated so the total removal of individuals from selected patches is feasible by the removal of all the seedpods of black locust.
There is a need for studies investigating the dispersal and the population dynamics of insect communities in altered environments, and especially for those involving invasive species and their insect meta-communities (Cronin & Haynes, Reference Cronin and Haynes2004; Bezemer et al., Reference Bezemer, Harvey and Cronin2014). It is especially important to study several insect generations (Bezemer et al., Reference Bezemer, Harvey and Cronin2014) and higher trophic levels (Cronin & Haynes, Reference Cronin and Haynes2004) as host plants interact not only with their herbivores, but even with the natural enemies of their herbivore (Price et al., Reference Price, Bouton, Gross, Mcpheron, Thompson and Weis1980).
To simulate the effect of disturbance and to study the dispersal of insects we created experimentally vacated, and thus disturbed black locust patches. Our study provides a model ecosystem to analyze the effect of disturbance on the dynamics of a tritrophic system. The concept was to generate local extinctions in selected black locust patches by removing all pods, hereby also removing the insects hibernating inside them. We predict that vacated patches, which are close enough to a potential insect source, will be recolonized successfully. The seed predator and the parasitoid species may follow different patterns and dynamics recolonizing the vacated patches, due to their various responses to disturbance.
By rearing insects from the collected pod samples, we can get data about the initial occupation and diversity of patches, and abundance of species. In our field experiment it is particularly important that the host plant of the system is an invasive alien plant, with a host-shifted herbivore and parasitoids. Such communities provide the opportunity to study dynamics of systems, before they reach an equilibrium stage.
Our study hypothesis was that, disturbance affects the spread of black locust. Study predictions: when all seedpods are removed from selected black locust patches: (i) Recolonization patterns of seed predators and their parasitoids in vacated (disturbed) patches will be different, both will recolonize, but parasitoids will appear in larger amounts because they are less adapted to black locust than seed predators e.g. they also can be found on the plants surrounding black locusts. (ii) Quantities of seed predators and their parasitoids are significantly affected by isolation of vacated patches. (iii) Host plant density also has a significant effect (bottom-up control) on the quantities of the seed predators and their parasitoids. (iv) Parasitoids will have a significant effect (top-down control) on the quantities of seed predators.
Material and methods
Study system
The host plant of the analyzed tritrophic system (fig. 1) is a North American leguminous plant, the black locust Robinia pseudoacacia Linnaeus, 1753. Since its introduction to Europe in the 17th century (Rédei et al., Reference Rédei, Csiha, Keserû, Kamandiné Végh and Győri2011), black locust became one of the most widely distributed alien plants (Lambdon et al., Reference Lambdon, Pyšek, Basnou, Hejda, Arianoutsou, Essl, Jarošik, Pergl, Winter, Anastasiu, Andriopoulos, Bazos, Brundu, Celesti-Grapow, Chassot, Delipetrou, Josefsson, Kark, Klotz, Kokkoris, Kühn, Marchante, Perglová, Pino, Vilá, Zikos, Roy and Hulme2008). Black locust is often planted for agricultural and commercial uses, sand fixing and soil improvement (Rédei et al., Reference Rédei, Csiha, Keserû, Kamandiné Végh and Győri2011). Seedpods of black locust contain 1–15 dark brown, kidney-shaped seeds. The species produces huge amount of hard-coated seeds, with long viability, accumulating in soil for many years (Rédei et al., Reference Rédei, Csiha, Keserû, Kamandiné Végh and Győri2011).
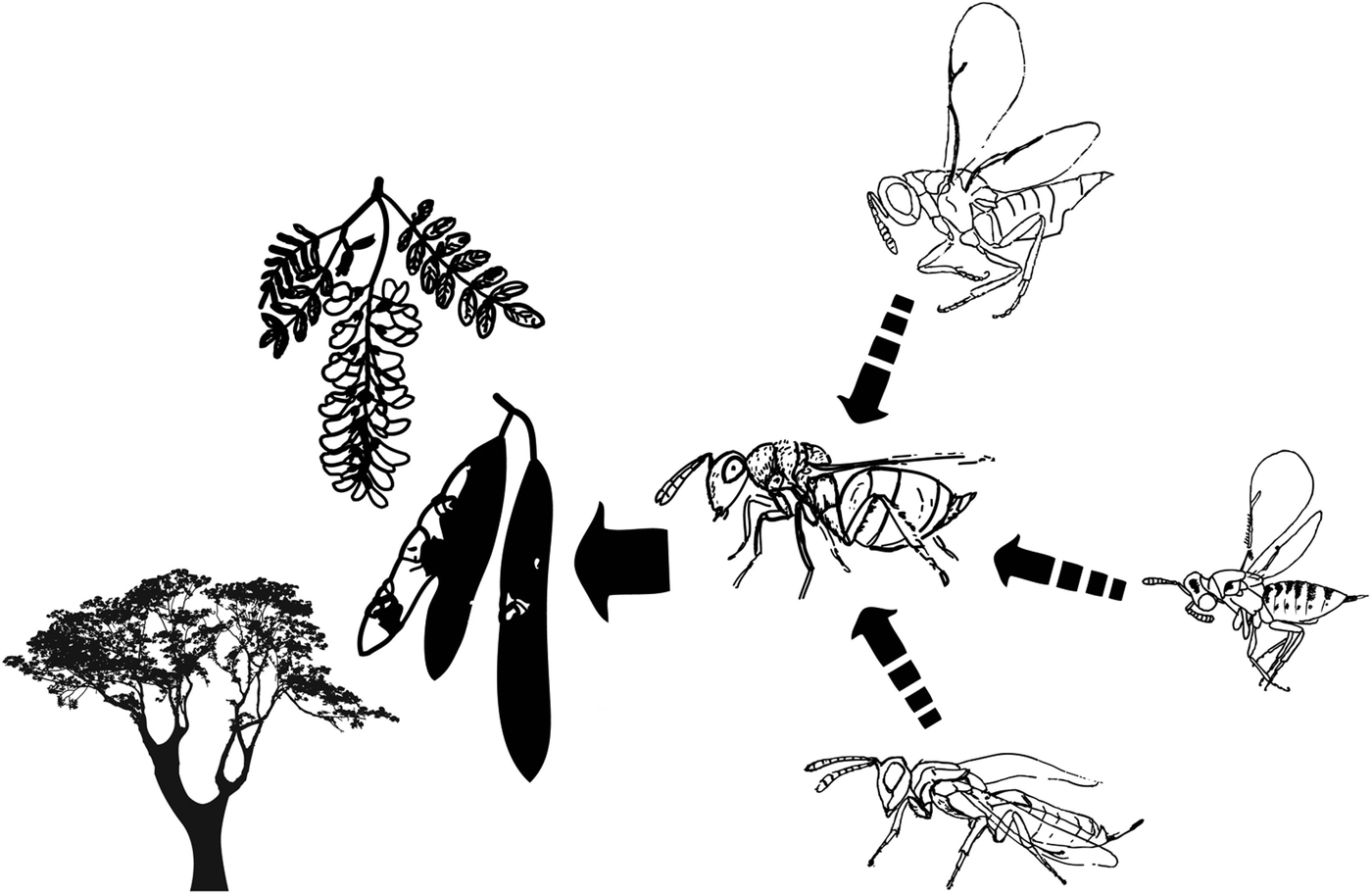
Fig. 1. The studied system: the host plant Robinia pseudoacacia, the seed predator Bruchophagus robiniae and its frequent parasitoids.
The native seed predators of black locust (in Appalachian Mountains, N-America) are Spermophagus hoffmannseggi (Bruchidae) and Apion spp. (Curculionidae) (Hargrove, Reference Hargrove1986), but these species were not introduced to Europe. The known European black locust seed predators are Etiella zinckenella, Aphis craccivora, Bruchophagus robiniae (Perju, Reference Perju1998) and Bruchidius cisti (Bartha et al., Reference Bartha, Csiszár, Zsigmond, Botta-Dukát and Balogh2008). These species cause loss in seed production of black locust populations.
Bruchophagus robiniae (Hymenoptera: Chalcidoidea: Eurytomidae) is a pre-dispersal seed predator, monophagous on black locust seeds (Perju, Reference Perju1998). As other Bruchophagus species, each Bruchophagus robiniae individual feed and develop inside one infested seed so each seed wasp consumes only one seed (Batiste, Reference Batiste1967) and each seed can house only one seed wasp (Traveset, Reference Traveset1995). Each larva overwinters hibernating inside the infested seed; the next spring it pupates and adults emerge (Traveset, Reference Traveset1995). B. robiniae has one generation per year. Oviposition may occur in early stages of pod development, on green pods containing immature seeds (Batiste, Reference Batiste1967). Most species of the genus Bruchophagus are univoltine and to date most species seem to be highly host-specific (Traveset, Reference Traveset1995; Neser & Prinsloo, Reference Neser and Prinsloo2004).
The number of seed predators is moderated by parasitoids from the superfamilies Chalcidoidea and Ichneumonoidea. Chalcid species parasitize larval or pupal stage of the herbivorous host, controlling the population dynamics of seed predators. In our previous study we reared two parasitoid species of B. robiniae from black locust pods: Eupelmus urozonus and Mesopolobus sp. (Lakatos et al., Reference Lakatos, László and Tóthmérész2016), with low parasitism (<10%).
Study area and data collection
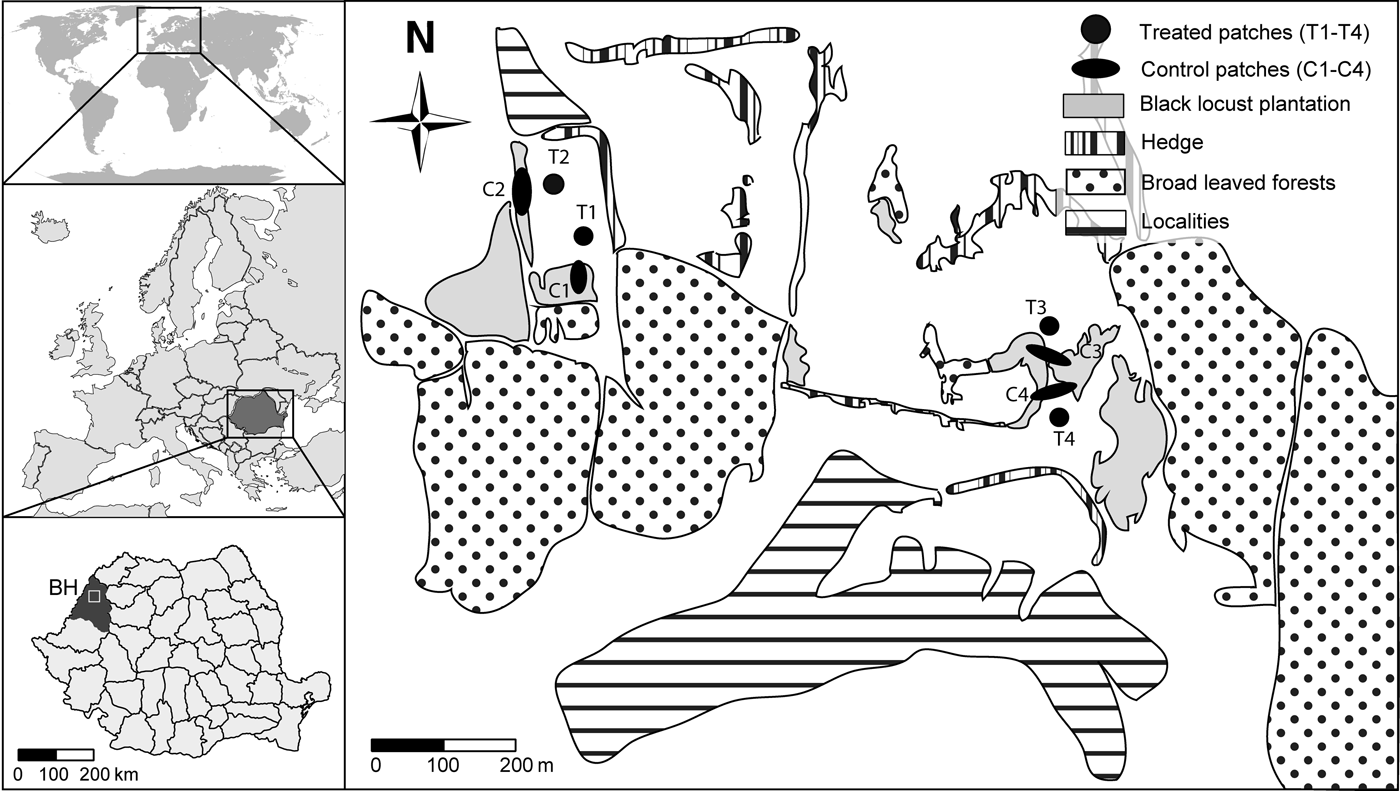
Experimentally vacated black locust patches were created near plantations to investigate the effect of disturbance on the colonization abilities and dynamics of the insect species (fig. 2). Choosing distant patches from plantations we ensured treatment effectiveness and simulation of escaped invasive plant patches. In November 2010 we monitored black locust plantations to find suitable patches for the treatment near Oradea (Bihor County, Romania). Using a global positioning system (GPS) device we marked the edges of black locust patches. Based on the collected information, four patches (as spatial replicates) were selected where the removal of all pods from trees and from the soil was possible. This resulted in four vacated patches and four control patches nearby vacated ones (fig. 2, table 1).
Fig. 2. Map of data collection sites near Oradea, Romania. Vacated black locust patches are marked as black circles while control patches are elliptic-shaped patches near vacated ones. All neighboring patches that contained any black locusts were treated as black locust patches. Native broadleaf forests (mainly oak and hornbeam woodlands) and shrub belts without black locust are also presented.
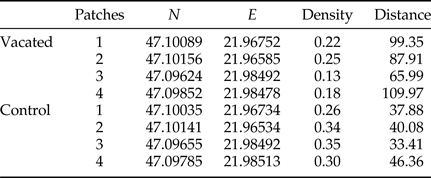
Table 1. Coordinates of vacated and control patch centroids (decimal degrees in WGS84) with host plant densities (per m2) and distances (m) between patch centroids (vacated versus control, control versus nearby black locust plantations).

In March 2011 we collected black locust pod samples from the eight selected patches. These samples represent the initial stage of the system. After sample collection, we experimentally vacated four selected patches by removing all the available pods from trees. Also, by using rakes we collected all of the litter from vacated patches, containing fallen pods. Sampling of pods was repeated in March 2012, 2013, 2014 and 2015 to gain data about the recolonization and dynamics of insect species.
To calculate the tree density in the patches (number of trees/area of patches) we registered the tree specimens with GPS and calculated the area of patches using Google Earth Pro™ version 7.1.2.2041 (http://earth.google.com/; ©2015 Google). The distances of patches from the surrounding black locust plantations were calculated with the determination of the centroids of each vacated patch using QGIS (QGIS Development Team, 2015).
To rear insects collected pod samples were placed into plastic containers, containing an average of 230 pods of the same tree. To assure the ventilation of samples, the containers were covered with punched plastic wrap. We counted the monthly emerged insects from the samples. At each census pod samples were poured out from plastic containers individually on a blank sheet of paper. Living specimens were caught with a brush dipped in ethanol. Then we searched for dead insect specimens between pods, and placed seedpods back into their container. The collected insects were stored in Eppendorf tubes with 70% ethanol or mounted on cards for identification.
Data analysis
Outcome variables were the seed predation by B. robiniae and the parasitism of B. robiniae. Seed predation was defined as the ratio of consumed seeds and seed numbers per samples. Parasitism was the ratio between abundance of parasitoid specimens and abundance of all emerged specimens per samples.
Explanatory variables were the disturbance (factorial variable with two levels: CONTROL and VACATED), the isolation of patches (continuous variable containing distances (m) between patch centroids: vacated versus control, control versus nearby black locust plantations), and the density of black locusts in patches (continuous variable: number of black locusts in the area of analyzed patches). The abundance of parasitoids was used as explanatory variable in the models of seed predation, while the abundance of B. robiniae was used as an explanatory variable in the model of parasitism. The variance inflation factors for all explanatory variables were between 1.13 and 2.40. Therefore, there was no multicollinearity, and we could retain all variables in the models.
The number of collected pods multiplied by the median seed number was used as a covariate in the models, because numbers of pods and seeds differed between host plants. We used years as a random factor (factorial variable with five levels: 2011, 2012, 2013, 2014, and 2015). We also used a blocking factor because the spatial arrangement of the patch pairs (factorial variable with two levels: block1 and block 2) (fig. 2). We used patch id as a random factor to control for the different number of collected samples per patches. Patches were nested in blocks and blocks were nested in years, therefore we used a nested design for random factors.
To investigate the effect of disturbance on seed predation and parasitism of black locust we used logistic generalized linear mixed models (GLMMs) with a repeated measures and also an Analysis of covariance (ANCOVA) designs using the statistical computing environment R version 3.2.2 (R Development Core Team, 2015). Logistic GLMMs were performed with the function glmer, using Laplace approximation of the maximum likelihood and logit link function from the package lme4 (Bates et al., Reference Bates, Maechler and Bolker2012). For multiple comparisons we used the function glht from the package multcomp (Hothorn et al., Reference Hothorn, Bretz and Westfall2008). For the GLMMs the outcome variables were prepared as a two column data frame containing the successes and total observations (successes and failures) (Crawley, Reference Crawley2012).
Results
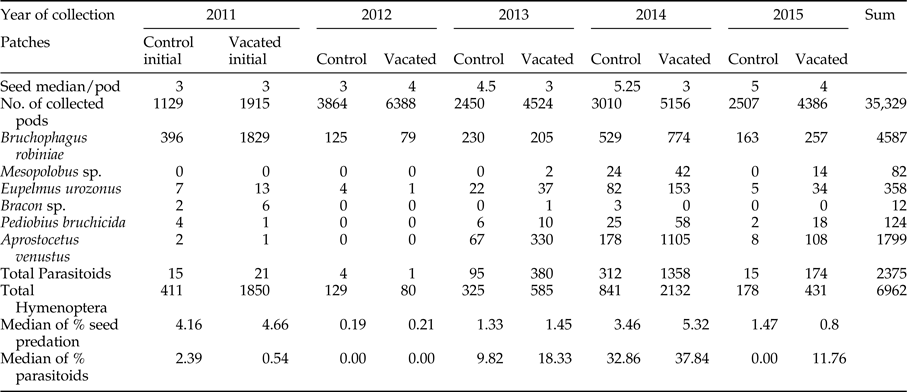
In the initial stage herbivores were abundant, while parasitoid species were present in a low number in both type of patches (vacated and control). The number of emerged individuals suggests that at the initial stage vacated patches were better habitats for the seed predators, while control patches were better habitats for parasitoids (table 2).
Table 2. Number of collected seedpods and number of species in the five study years.
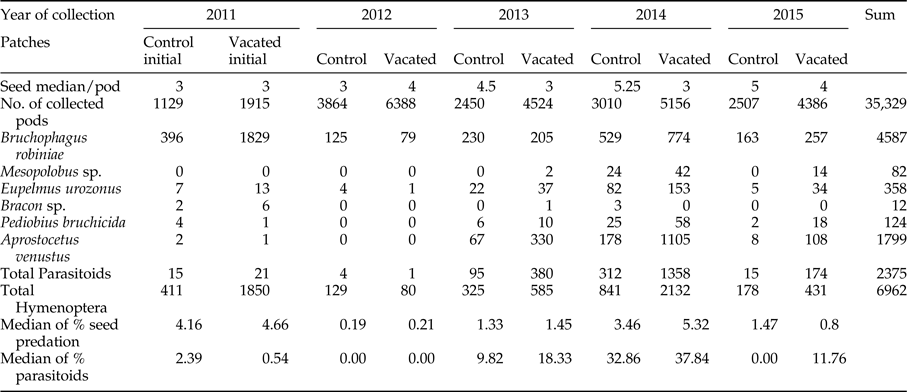
We found that the seed predator and its parasitoids dispersed into vacated patches of black locust and recolonized them (fig. 3). In the first year after the disturbance, the decrease in seed predation and parasitism was almost 100% in both vacated and control patches, after which the seed predation slowly increased to 3–5% and also the parasitism to 30–40% (from now on we will consider this period the abundance growth phase), than dropped in the last year (fourth year after disturbance) to the size of seed predation measured in the first year after the disturbance, but parasitism of vacated patches remained much higher than at the initial stage (table 2).

Fig. 3. Proportions of seed predation (a, c) and parasitism (b, d) in the seed predator community of black locust (R. pseudoacacia). Dark boxplots denote control patches, while grey ones denote vacated patches. Boxplots show the medians, interquartile-ranges, minimum and maximum values of the seed predation and parasitism. Open circles are outliers.
Black locust pods collected in 2011 (initial stage) yielded 32.47% of the total hymenopterans (table 2). Pods collected in 2012 (first year after the disturbance) yielded only 3% of the total hymenopterans. Pods collected in 2013 (second year after the disturbance) yielded 13% of the total hymenopterans. Pods collected in 2014 (third year after the disturbance) yielded 42.7% of the total hymenopterans. In 2015 (fourth year after disturbance) collected pods yielded only 8.75% of the total hymenopterans.
Disturbance and dynamics
We found significant year effect in seed predation (logistic GLMM: χ2 = 87.42, df = 4, P < 0.001, fig. 3a and fig. 4b), and parasitism (logistic GLMM: χ2 = 130.81, df = 4, P < 0.001, figs 3b and 4d). After controlling with years we found insignificant changes in seed predation due to the disturbance (figs 3c, 4a, table 3). After controlling with years the parasitism was significantly higher in disturbed patches compared with control ones (figs 3d, 4c, table 4).

Fig. 4. Changes in seed predation and parasitism of the seed predator B. robiniae in the seed predator community of black locust (R. pseudoacacia). Changes are plotted against treatment (a, c) and years (b, d). Points present medians of seed predation and parasitism for the given treatment–year combination.
Table 3. Logistic GLMM for the seed predation by Bruchophagus robiniae in the pods of black locust (R. pseudoacacia) (N = 164) in four replicates of vacated and control patches.
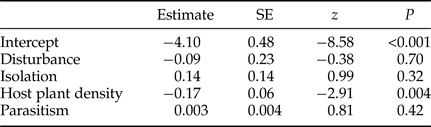
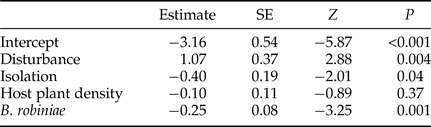
Table 4. Logistic GLMM for the proportion of parasitoids in the seed predator insect community in the pods of black locust (R. pseudoacacia) (N = 164) in four replicates of vacated and control patches.
Isolation, host plant density and parasitoid effect
Seed predation was not affected by the isolation of patches (table 3). The parasitism of this seed feeding insect community was affected negatively by patch isolation (table 4). Seed predation decreased significantly with the increasing density of host plants in patches (table 3), while parasitism was not affected by the density of host plants in patches (table 4). The correlation between seed predation and parasitism was not significant (table 3), but the number of parasitoids had a significantly negative effect on the percentage of herbivores (logistic GLMM: estimate = −0.09, SE = 0.02, z = −5.3, P < 0.001). The number of herbivores correlated negatively and significantly with the parasitism (table 4).
Disturbance effect on the extent of change in seed predation and parasitism
The seed predation and parasitism showed significant changes in both vacated and control patches through the period of the experiment (year effect). But vacated patches showed larger changes than control ones: both in the case of seed predation (repeated measures ANCOVA: estimate = 0.31, SE = 0.1, z = 3.24, P = 0.01), and parasitism (repeated measures ANCOVA: estimate = 0.58, SE = 0.24, z = 2.4, P = 0.016).
Discussion
In vacated patches we found that the abundance of insects was lower in most of the years following the disturbance (2012, 2013, 2015, except in 2014), than in 2011. In 2014 the abundance exceeded the initial values found in 2011, which suggest that there is a periodicity in the found changes, and in 2014 the system showed a local maximum. Species of this tritrophic system dispersed into vacated patches and recolonized them.
We found that the disturbance affected only the levels of parasitism (fig. 3d, tables 3 and 4). After the disturbance, parasitism in vacated patches was significantly larger compared with control patches. Seed predation in vacated and control patches did not differ significantly.
In the first year after treatment parasitoid species recolonized vacated patches in a low number. Although many parasitoid species have good dispersal and flight abilities (Yu et al., Reference Yu, Zhang, Wu, Wyckhuys and Guo2009), the recolonization by parasitoids may be slower than that of their hosts (Elzinga et al., Reference Elzinga, van Nouhuys, van Leeuwen and Biere2007), because higher trophic levels need the conditions to be favorable for multiple (lower) trophic levels (Cronin & Haynes, Reference Cronin and Haynes2004). Parasitoids need their herbivorous host to recolonize vacated host plant patches, and host plant patches have to be suitable for that (Elzinga et al., Reference Elzinga, van Nouhuys, van Leeuwen and Biere2007). After the successful recolonization of vacated patches by the seed predators the accumulation of parasitoid species takes more growing seasons.
An interesting result is how parasitoid species responded to disturbance (table 2, fig. 3). After the setback due to treatment, in the second and third year after treatment (2013 and 2014) the number of parasitoids was increasing. This high consumption may have been the main cause of the second drop in seed predator abundance. This pattern of recolonization looks like a host–parasitoid dynamic. In many occasions disturbance causes high parasitism by its effect of ruining refuges of herbivores (Wilkinson & Feener, Reference Wilkinson and Feener2007). Bruchophagus species are known to suffer from various levels of parasitism between 3.2 and 98% (Soroka & Otani, Reference Soroka, Otani and Floate2011).
With the isolation of vacated patches seed predation showed no changes, while parasitism was decreased significantly by isolation. This suggests that herbivores have better dispersal or resource finding abilities, and that isolation can weaken top-down control (Chase et al., Reference Chase, Burgett and Biro2010), or that parasitoid species are less adapted to this host-shifted community, and are still using their previous hosts as alternatives.
Regarding the host plant R. pseudoacacia density we found, that denser patches had significantly lower seed predation than less dense patches (table 3). A possible cause of this result is a negative correlation between tree density and the availability of seedpods. In dense patches trees are more resource and space limited, with decreased leaf area per tree for photosynthesis, bearing smaller amount of fruit then trees in sparse patches (Arista & Talavera, Reference Arista and Talavera1996). During fieldwork we observed, that relatively dense patches of black locust carry fewer seedpods (pers. obs.). So bottom-up control has an important impact on seed predation, but not on the parasitism. We found that the host plant density of patches had no influence on the proportion of parasitoids. Our results suggest that the seed predator is not only affected by bottom-up effect of its host plant density, but the herbivore of this community is also the subject of top-down control by its parasitoids, especially in disturbed (vacated) patches.
The recolonization of vacated patches, and probably the decline in control patches, suggests that vacated and control patches are connected. Albeit, this connectivity was not too strong as vacated, and control patches differed in abundances of species even in the initial stage (Cronin & Haynes, Reference Cronin and Haynes2004). If insects in a patchy habitat form a patchily distributed population than the dynamics of patches are linked together by the dispersal of insects (Pannell & Obbard, Reference Pannell and Obbard2003) and thus populational changes may be also linked. Active dispersal of small insects is a short-term movement from a few meters up to a kilometer (Elzinga et al., Reference Elzinga, van Nouhuys, van Leeuwen and Biere2007). Most Bruchophagus seed predator species feed on herbaceous legume plants (Soroka & Otani, Reference Soroka, Otani and Floate2011), so they do not need to disperse for long distances to find another host plant. Although the dispersal of small insects can be enhanced by the air flow, this passive dispersal is not a directional movement (Compton, Reference Compton, Bullock, Kenward and Hails2002). The monophagy of Bruchophagus species (Kamm, Reference Kamm1989) makes their recolonization and dispersal more difficult. Population dynamics of Bruchophagus species are known to fluctuate between years resulting variable seed consumption and parasitism (Soroka & Otani, Reference Soroka, Otani and Floate2011).
Conclusions
The top-down control in the studied system was fortified by the disturbance. Since the host plant of the system is an invasive species, partial disturbances of habitat patches of such species (as part of invasive species management) may contribute to the severity of the top-down control (high parasitism), which may lower the effects of seed predators. On the contrary to the expected, the total removal of seedpods from black locust patches may increase the amount of black locust seeds as less of them might be consumed, so more seeds can accumulate in the soil facilitating its spread. This effect of disturbance on black locust may be useful in designing a biological control against invasive species.
Acknowledgements
The authors thank Stephen B. Heard and two anonymous reviewers for the valuable and helpful comments on the manuscript. The work of Z. László was supported by a grant from the Romanian Ministry of Education, CNCS – UEFISCDI, project number PN-II-RU-PD-2012-3–0065 (45/30.04.2013). The study was supported by the SROP-4.2.2.B-15/1/KONV20150001 project. Authors were supported by the OTKA K 116639 research grant.
Disclosure
The authors declare that they have no conflict of interests.
Author contributions
L.T. and L.Z. designed the study. L.T. collected the data, participated in the statistical evaluation and paper writing. L.Z. and T.B. participated in the statistical evaluation of the data and paper writing.