Cytology-based cervical cancer screening programs have significantly reduced the detection of precancerous cervical lesions in developed countries (Reference Varicella, Lortet-Tieulent and Plummer1;Reference Vaccarella, Franceschi and Engholm2). The human papilloma virus (HPV) test as primary screening or co-screening is increasingly more common (Reference Kitchener, Canfell and Gilham3;Reference Abraham and Stenger4), as infection with HPV is the essential cause for the development of cervical cancer (Reference Bosch, Lorincz and Muñoz5). Women with cytological diagnoses such as atypical squamous cells of undetermined significance (ASCUS), low-grade squamous intraepithelial lesion (LSIL), and women with high grade squamous intraepithelial lesion (HSIL) and/or who are HPV positive are commonly referred for a colposcopy examination for visualizing the cervix with strong illumination and magnification (Reference Underwood, Arbyn and Parry-Smith6). Colposcopy directed biopsies are taken from suspected lesions and sent for histopathological examination (Reference Underwood, Arbyn and Parry-Smith6). Active colposcopic management of low-grade cervical disease has been associated with a greater sensitivity of high-grade disease than the strategy of repeat cytology, as well as lower hospital and patient costs (Reference El-Sayed, Al-Daraji and Finnegan7;Reference Shireman, Tsevat and Goldie8). Also, in a randomized controlled trial, initial colposcopy caused less anxiety than initial cytological surveillance but with no long-term difference in psychosocial outcomes and costs (Reference Sharp, Cotton and Little9), and similar results have been reported in another recent study in women cytological abnormalities (Reference Korfage, Essink-Bot and Westenberg10), suggesting a reassuring effect of colposcopy management. In a trial from the United Kingdom, HPV testing as the primary strategy, with referring HPV 16/18 positive women to colposcopy, was the most cost-effective scheme (Reference Kitchener, Canfell and Gilham11). However, the HPV 16/18 screening strategy is predicted to result in a 20 percent increase in the number of colposcopies in the United Kingdom (Reference Kitchener, Canfell and Gilham11). In Northern Ireland, a recent study found that HPV triaging increased colposcopy referrals by 42 percent (Reference McKenna and McMenamin12).
Colposcopy is often limited to specialized outpatient or hospital based clinics. Optical colposcopes are often costly to purchase and usually confined to one office due to size and weight. Common barriers to failure of colposcopy follow-up of cytological abnormalities are geographical distances and the absence of on-site colposcopy, especially in the underserved women (Reference Hitt, Low and Bird13;Reference Hoover, Koumans and Montaño14). Thus, the projected increased need of colposcopies (Reference Kitchener, Canfell and Gilham11) may be difficult to provide. However, a newly developed low-cost, small, and portable colposcope, the Gynocular, may provide access to colposcopy in any economical or geographical setting (Reference Ngonzi, Bajunirwe and Wistrand15;Reference Ashrafun, Wistrand and Akter Begum16), thus facilitating active colposcopy management and follow-up of women with positive HPV test or cytological abnormalities.
The aim of this study was to estimate diagnostic accuracy by sensitivity and specificity of cervical lesions by the Gynocular, compared with a stationary colposcope, in women referred for colposcopy due to abnormal cytology in a high resource setting.
METHODS
We performed a randomized cross-over clinical trial and included 123 women for evaluating diagnostic accuracy of sensitivity and specificity of cervical lesions by the Gynocular and stationary colposcope in women with cytological abnormalities referred for colposcopy. In Sweden, all women are invited for cervical cytology every third year from 23 years of age and every fifth year in ages 51–60.
The Swede score systematic colposcopy system was used to grade the cervical lesions (Reference Ngonzi, Bajunirwe and Wistrand15–Reference Bowring, Strander and Young18). Directed punch biopsy and excisional cone biopsy were used as the gold-standard. All the participating women were examined in a randomized order by both the Gynocular and the stationary colposcope in a cross-over design. The inclusion criteria were: (i) women with ASC-US (atypical cells of undetermined significance) or LSIL (low-grade squamous intraepithelial lesion) and high risk human papilloma virus (HPV) and HR HPV positivity, or any high grade HSIL (high-grade squamous intraepithelial lesion) of CIN 2 (cervical intraepithelial neoplasia) or more, regardless of HPV status, referred for colposcopy to the Department of Obstetrics and Gynecology, Danderyd Hospital, Stockholm, Sweden, during June 1, 2012 to June 30, 2013. (ii) Ability to understand written and oral information in Swedish. (iii) Willingness to sign the consent form to take part in the study after oral and written information was given. Exclusion criteria were (i) on-going vaginal bleeding, (ii) any previous gynecological examinations within a week before the examination, (iii) pregnancy. If the woman chose not to take part in the study, she had a standard colposcopy examination. Participating women were examined by one of six specialists in Obstetrics and Gynecology, trained in practical colposcopy, cone excisional procedures, and colposcopy courses as well as having regular colposcopy clinics at the Department of Obstetrics and Gynecology, Danderyd Hospital, Stockholm, Sweden. In Sweden, colposcopy training is part of the Obstetrics and Gynecology training and a specialist of Obstetrics and Gynecology is considered qualified to practice colposcopy.
A standard colposcope (Carl Zeiss Colposcope 150 FC, Carl Zeiss Surgical GmbH, Oberkochen, Germany) and the Gynocular (Gynius AB, Stockholm, Sweden) were used for examination colposcopy. The Gynocular was attached to a camera tripod during the examination. The clinic nurse gave written study information in the waiting room to the woman and the doctor gave oral information and enrolled the patient. Women were randomly allocated a clinic day in groups by the study nurse coordinator to start the examination with either the stationary colposcope or the Gynocular. The cross-over design was carefully chosen to lessen possible observer variability (Reference Cox19).
When carrying out the Swede score examination, each of five colposcopic variables (aceto-whiteness, margins plus surface, vessel pattern, lesion size, and iodine staining) was given a score of 0, 1, or 2 points (Reference Ngonzi, Bajunirwe and Wistrand15–Reference Strander, Ellström-Andersson and Franzén17;Reference Cox19). A non-lubricated self-holding speculum was placed in the vagina and the cervix was visualized. The examination began with a review of cervical vessel patterns with the colposcope or the Gynocular as randomized, using the red-free (green filter) mode. Due to the nature of the exam, the instruments or the results of the exam could not be hidden from the doctors. All the women also had a new liquid based cytology specimen taken at the time of examination using a plastic spatula on the cervix and a cervix brush in the cervical canal (Reference Arbyn, Herbert and Schenck20;Reference Richart21). The cervix was then dabbed with 5 percent acetic acid, and after 1 minute was followed by the completion of the first colposcopic examination. During each examination, the four Swede score variables (aceto-whiteness, margins plus surface, vessel pattern, lesion size) were scored by the colposcopist and immediately documented by the assistant nurse. The colposcopist then changed instruments and repeated the examination and then again directly reported the new Swede score to the assistant nurse. Next, the cervix was swabbed with 5 percent Lugol's iodine solution and the colposcopist scored the Swede score's fifth variable (iodine staining) with both instruments as randomized and reported the scores to the assistant nurse. The examination was finalized with one or more biopsies taken from areas of colposcopy visualized suspected cervical lesions when Swede score >0 (Reference Hoover, Koumans and Montaño14–Reference Strander, Ellström-Andersson and Franzén17). Women above 40 years with CIN 1 lesions and with no wish for future pregnancies were offered excisional cone biopsy. Women under 40 years of age were given the choice of being treated with excisional cone biopsy or follow-up with renewed colposcopy after 12 months. Women with lesions grade CIN 2 or higher underwent an excisional cone biopsy by laser or LLETZ/LEEP technique at the discretion of the gynecologist performing the surgery. Women with invasive cancers were referred to the Department of Gyneoncology, Karolinska University Hospital, Stockholm, Sweden. The study was performed according to CONSORT 2010 and STARD checklists (checklist on request).
The histopathology diagnoses were graded according to the CIN classification system (Reference Arbyn, Herbert and Schenck20) and were considered as gold-standard. The cervical biopsies were analyzed at the Laboratory of Clinical Pathology and Cytology, Danderyd Hospital, Karolinska Institutet, Stockholm, Sweden. The Thinprep (Hologic Inc. Bedford, MA) tests were analyzed at the Laboratory of Clinical Pathology and Cytology, Karolinska University Hospital, Karolinska Institutet, Stockholm, Sweden.
The study was approved by the Stockholm Regional Ethical Review Board, Dnr: 2013/1855–3, 2009/2032–31/1, 2013/1855–3, as well as by The Medical Products Agency of Sweden, Uppsala, Sweden, Dnr: 461:2010/502414. The study was registered as ISRCTN72259107 at www.controlled-trials.com after the enrolment of the participants started, due to the publishing restraints of the parallel patenting process of the Gynocular.
The Gynocular (Gynius AB, Stockholm, Sweden) has optical and light specifications comparable to a stationary colposcope (Supplementary Table 1). The Gynocular is a monocular with 300 mm focal distance and 3 magnifications: 5×, 8×, and 12× and measuring 50 × 33 × 166 mm. The Gynocular is light-weight and may be hand-held, but also comes with a tripod-mounting clip that screws into a standard tripod enabling the medical professional to perform colposcopy in a hands-free mode for ease of biopsy. The Gynocular has high intensity LEDs for warm-white illumination, green filter, and is powered by a rechargeable lithium-ion battery. A smartphone can be attached to the Gynocular by using a smartphone adapter, for image capturing and video colposcopy through a colposcopy application enabling secure cloud based distant consultation and review of cervical lesions (Supplementary Figure 1). Figure 1 shows a cervical image of a normal HPV positive cervix and a HPV positive cervix with a high grade lesion captured by as Samsung Galaxy 4 and Samsung Galaxy 5 smartphone. The Gynocular is a patented, CE marked, and an approved colposcope by the US Food and Drug Administration (FDA).
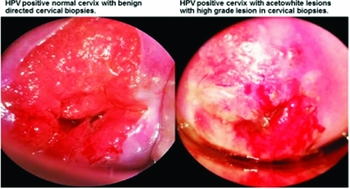
Figure 1. Cervical images of a normal cervix and cervix with high grade lesion captured during colposcopy with the Gynocular and a Samsung Galaxy 4 and Samsung Galaxy 5 smartphones.
Statistical Methods
For sample size estimation, it was expected that 70 percent of the women entering the study, would have a CIN2+ cervical biopsy result. It was further expected that the sensitivity for identifying pathology was 86 percent using the traditional colposcope and 91 percent using the Gynocular. The non-inferiority margin for the difference between the devices (Gynocular-Colposcope) was defined as 5 percent. A total of 230 subjects were needed for 80 percent power to show that the lower limit of the 95 percent confidence interval for the difference was greater than -5 percent. However, due to recruitment problems, an unplanned interim analysis was performed when 123 patients were included in the study. Based on the overall results from the interim analysis it was decided to end the study.
An application to shorten the clinical study was approved by the Stockholm Regional Ethical Review Board.
All statistical analyses have been performed using R version 2.14 (22). The baseline patient characteristics of the women were summarized using means (SD) and frequencies (percentage) (Supplementary Table 2). To test the level of agreement between the Gynocular and the stationary colposcope, we calculated the percentage agreement and the weighted kappa statistic (Reference Landis and Koch23). Cervical lesions were classified by the Swede scores system (Reference Ngonzi, Bajunirwe and Wistrand15–Reference Bowring, Strander and Young18) using the Gynocular and a stationary colposcope. We calculated detection rates of CIN 1, CIN 2, CIN 3, CIN 3+ (invasive cancer), AIS (adenocarcinoma in situ) and benign punch and cone cervical biopsies. A positive biopsy result was defined as CIN 1, CIN 2, CIN 3, CIN 3+, and we calculated the Swede score's sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV) using biopsy as a gold-standard for all cutoff levels of Swede score between 1 and 10. The results are presented in tables and as receiver operating characteristic (ROC) curves. All data were included in the analysis.
RESULTS
Supplementary Figure 2 shows the study flow-chart. The women's baseline characteristics are presented in Supplementary Table 2. The mean age was 33.4 (±9.9) years, with a median age of 31. Liquid based cytology was benign in thirty-three (27.7 percent) women, and detected twelve (10.1 percent) with ASCUS (Atypical Squamous Cells of Unknown Significance), thirty-nine (32.8 percent) with cervical intraepithelial neoplasia 1 (CIN1), twenty-three (19.3 percent) with CIN2, ten (8.4 percent) with CIN3, and zero (0 percent) with CIN3+ (invasive cancer). One (0.8 percent) woman had AIS on cytology.

Figure 2. ROC curves for predicting a positive biopsy result, defined as CIN2, CIN3, or CIN3+. For patients with both cone and punch biopsy the worst results are used. The thinner lines represent the 95 percent confidence intervals for the ROC curves
Punch biopsy was benign in thirty-six (29.8 percent). It showed CIN1 in thirty-three (27.3 percent) and CIN2 in thrity-one (25.6 percent). Eleven (9.1 percent) women had CIN3, and one (0.8 percent) had invasive cervical cancer (CIN3+). One (0.8 percent) woman biopsy showed AIS. Thus, punch biopsy diagnosed CIN2+ (CIN2, CIN3 and invasive cancer) in forty-four (35.7 percent) of the women while cytology detected CIN2+ in thirty-four (27.6 percent) of the women.
A total of sixty-two (50 percent) of the women were treated with excisional cone biopsy and 88 percent of these women had the exact same histological diagnosis in punch and cone biopsy with a Kappa coefficient of 0.70. Eleven (8.9 percent) of the cone biopsies were benign, seven (5.7 percent) were CIN1, twenty (16.3 percent) CIN2, twenty-two (17.9 percent) were CIN3, and two (1.6 percent) were CIN3+. When comparing Swede score and directed punch biopsies to the final diagnosis of cone biopsy, punch biopsy had a sensitivity of 88.7 percent (75.9–96.3 percent) and a specificity of 86.7 percent (59.5–98.3 percent), with no significant differences using the stationary colposcope or the Gynocular (Figure 2). Observed overall sensitivity for detecting CIN2+ in biopsy was 79.2 percent for Gynocular and 81.6 percent for the stationary colposcope. The observed difference between the paired proportions was -2.1 percent with a 95 percent confidence interval ranging from -11.8 percent to 4.8 percent. As the lower limit was below -5 percent, statistical non-inferiority could not be claimed, based on the included 123 patients. Furthermore, there were no significant differences between the Swede scores of the Gynocular and the stationary colposcope in predicting a positive biopsy result, defined as CIN 2, CIN 3, or CIN 3 + (Figure 3 and Table 1). The sensitivity and positive predictive value decreased while specificity and negative predictive value increased with increased Swede score (Table 1), both for the Gynocular and for the colposcope, and additional analysis of each individual item of the Swede score, showed no significant differences between the different colposcopes (data not shown).

Figure 3. Cross-tabulation of Swede score of Gynocular and Colposcope with Kappa coefficient of 0.947 and p-value < .001.
Table 1. Sensitivity, Specificity, Positive Predictive Value (PPV) and Negative Predictive Value (NPV) for Different Cutoff Levels for Swede Score by Gynocular and a Stationary Colposcope

To further evaluate if the cross-over design had influenced the scoring of the second instrument, we compared the Swede score and CIN diagnosis of only the first instruments used and found no differences between the instruments in detecting CIN2+ (data not shown).
Swede scores were obtained by cervical examination with a standard colposcope and the Gynocular. A cross tabulation of Swede scores by stationary colposcope versus the Gynocular showed a Kappa coefficient of 0.947 and p-value < .001 (Figure 3). There were no adverse events using the Gynocular or stationary colposcope.
DISCUSSION
In this cross-over randomized study evaluating the Gynocular to stationary colposcope for diagnostic accuracy of cervical lesions, we showed that there were no significant differences in sensitivity or specificity in detecting cervical lesions by either instruments. Moreover, there was a high correlation for both instruments with regard to Swede score, directed punch biopsy and the final histopathological diagnosis of the excisional cone biopsy, confirming that the Gynocular is as accurate as a stationary colposcope in diagnosing cervical lesions.
The main strength of our study is its randomized cross-over design in women referred with abnormal cytology and thus enabling pathological cervical disease to be diagnosed by both instruments. The cross-over randomized design was used to reduce the risk of intra-observer variability (Reference Bowring, Strander and Young18). Another strength is that all women with Swede score >0 had at least one biopsy, which also increases the strength of the study and the validity of the accuracy of the diagnosis. In addition, all the cytology and biopsies were analyzed in single-site accredited laboratories.
The main weakness of our study was that hiding which instrument was being used was not possible due to the nature of the instruments. Also, the cross-over design might have influenced the scoring of the second instrument. We evaluated other study designs, but they would have been difficult to implement due to patient discomfort, time constraints and doctor availability. However, by using cross-over study design and block randomization, we reduced the risk of the second examination's possible influence of cervical impression to affect the statistical calculations. Furthermore, when we compared the Swede score and CIN diagnosis of only the first instruments used, there were no differences. It is also possible, if 230 patients would have been recruited, that statistical non-inferiority could have been shown.
Studies from the Swedish National Cancer Registry (Reference Silfverdal, Kemetli and Andrae24;Reference Silfverdal, Kemetli and Sparén25) show that women with low-grade and high-grade CIN lesions alike profit from additional investigation with biopsy rather than repeat cytology. Treatment reduced cancer risks even if the woman had a negative biopsy (Reference Silfverdal, Kemetli and Andrae24;Reference Silfverdal, Kemetli and Sparén25). In addition, in a multi-center randomized controlled trial, direct colposcopy identified more cervical lesions than repeat cytology (Reference Cruickshank, Murray, Parkin, Smart and Walker26). A recent meta-analysis concluded that the observed high sensitivity and low specificity of colposcopy directed punch biopsies could be the result of selection bias as most women in colposcopy studies were already selected for colposcopy, due to an abnormal cytology (Reference Underwood, Arbyn and Parry-Smith6). With the transfer to HPV primary screening, the emphasis is moving from the positive predictive value of colposcopy to the negative predictive value, that is, the effectiveness of colposcopy in excluding CIN2+ in women who test HPV positive and cytology negative or with HPV positive alone. To address this, colposcopy by the International Federation of cervical Pathology and Colposcopy (IFCPC) colposcopy categories and correlation to histological diagnosis in women screened HPV positive or VIA positive was recently studied by Ghosh et al. (Reference Ghosh, Mittal and Banerjee27). They found that the negative predictive value of colposcopy to detect high grade lesions in HPV positive women was 76.7 percent and in VIA positive women 23 percent (Reference Ghosh, Mittal and Banerjee27). This is an important finding and strengthens the value of colposcopy in HPV positive women, especially as colposcopy was the most cost-effective scheme in HPV positive women in the ARTISTIC trial (Reference Kitchener, Canfell and Gilham11). It is also reassuring that colposcopy identified HPV positive women's cervical lesions, as many HPV positive women do not develop cervical high-grade lesions and emphasize the important role of colposcopy to exclude cervical lesions and thereby avoid overtreatment in HPV positive women (Reference McKenna and McMenamin12).
With the development of the Gynocular, colposcopy is no longer restricted to specific examining rooms in colposcopy clinics or hospitals. The increased need of colposcopy following positive HPV screening could accurately be provided by the Gynocular in both high resource and low resource settings by doctors and nurses (Reference Ngonzi, Bajunirwe and Wistrand15;Reference Ashrafun, Wistrand and Akter Begum16;Reference Nessa, Roy and Chowdhury28). This approach could increase access for women to obtain and adhere to colposcopy surveillance, especially in remote and underserved areas.
In conclusion, a colposcopy examination by the Gynocular or stationary colposcope showed no significant differences in sensitivity, specificity, PPV, or NPV in detecting cervical lesions. With the Gynocular, gold-standard colposcopy can thus be provided anywhere and simplify access, treatment and adherence to follow-up colposcopy on cervical screening positive women and reduce the risks of overtreatment
SUPPLEMENTARY MATERIAL
Supplementary Tables 1 and 2 http://dx.doi.org/10.1017/S0266462315000252
Supplementary Figures 1 and 2 http://dx.doi.org/10.1017/S0266462315000252
CONFLICTS OF INTEREST
EAWS and IS initiated the project. EAWS, HKK, MP, MThor and DA designed the study. HKK, MP, MP, DA, MThor and EAWS provided gynaecological expertise. HKK and MP collected the data. MThur, IS and MThor provided statistical expertise. All authors performed literature searches and wrote and edited the article. All authors contributed to analysis of the data.
Funding
MThur received financial support for the submitted work from Gynius AB for statistical expertise.
Competing Interests
EAWS and IS are shareholders in Gynius. H&M Conscious Foundation and Gynius AB funded the research project. EAWS is the inventor of the Gynococular.






