Introduction
Attention-deficit/hyperactivity disorder (ADHD), the most common childhood-onset neurobehavioral disorder (Wolraich et al., Reference Wolraich, Brown, Brown, DuPaul, Earls, Feldman, Ganiats, Kaplanek, Meyer, Perrin, Pierce, Reiff, Stein and Visser2011), is characterized by age-inappropriate inattention, hyperactivity and impulsivity (American Psychiatric Association, 2013). According to a latest meta-analysis of 175 studies, the worldwide-pooled prevalence of ADHD is 7.2% in children and adolescents (Thomas et al., Reference Thomas, Sanders, Doust, Beller and Glasziou2015), and approximately 60% of cases demonstrate symptom persistence in adulthood (Sibley et al., Reference Sibley, Swanson, Arnold, Hechtman, Owens, Stehli, Abikoff, Hinshaw, Molina, Mitchell, Jensen, Howard, Lakes, Pelham and Group2017). Although numerous studies have been conducted on ADHD, the psychopathology of the disorder remains incompletely understood. In recent years, ADHD has been increasingly viewed as impairments among distributed functional networks or circuits. The functional network, which consists of brain regions that are correlated during a resting state or specific tasks, can be defined as a synchronized network of brain function (Fox et al., Reference Fox, Snyder, Vincent, Corbetta, Van Essen and Raichle2005) and is believed to reflect distinct mental states or processes (Shirer et al., Reference Shirer, Ryali, Rykhlevskaia, Menon and Greicius2012; Buckner and Krienen, Reference Buckner and Krienen2013). Aberrant communication between or within functional networks may underlie deficits in cognitive and affective functioning (Zang et al., Reference Zang, He, Zhu, Cao, Sui, Liang, Tian, Jiang and Wang2007; Kessler et al., Reference Kessler, Angstadt, Welsh and Sripada2014).
Resting-state functional connectivity (rsFC) techniques, which measure the correlations of brain activity across anatomically separated brain areas at rest, have been extensively applied to reveal abnormalities of large-scale intrinsic functional networks and the interaction between them in ADHD. Two recent narrative reviews of rsFC suggest that altered connectivity in ADHD is noted not only in the default mode network (DMN), frontoparietal network (FPN) and attention networks but also in reward-related and affective circuits (Castellanos and Aoki, Reference Castellanos and Aoki2016; Posner et al., Reference Posner, Park and Wang2014a). However, these findings remain inconsistent across different studies. For instance, although weaker functional connectivity within the DMN has been widely observed in individuals with ADHD (Castellanos et al., Reference Castellanos, Margulies, Kelly, Uddin, Ghaffari, Kirsch, Shaw, Shehzad, Di Martino, Biswal, Sonuga-Barke, Rotrosen, Adler and Milham2008; Fair et al., Reference Fair, Posner, Nagel, Bathula, Dias, Mills, Blythe, Giwa, Schmitt and Nigg2010; Choi et al., Reference Choi, Jeong, Lee and Go2013; Sripada et al., Reference Sripada, Kessler, Fang, Welsh, Prem Kumar and Angstadt2014), some studies have also reported stronger within-DMN functional connectivity in ADHD patients (McCarthy et al., Reference McCarthy, Skokauskas, Mulligan, Donohoe, Mullins, Kelly, Johnson, Fagan, Gill, Meaney and Frodl2013; Barber et al., Reference Barber, Jacobson, Wexler, Nebel, Caffo, Pekar and Mostofsky2015). Moreover, altered connectivity in the FPN and attention networks has been more divergent as both increased and decreased functional connectivity (Lin and Gau, Reference Lin and Gau2016; Mostert et al., Reference Mostert, Shumskaya, Mennes, Onnink, Hoogman, Kan, Arias Vasquez, Buitelaar, Franke and Norris2016; Sidlauskaite et al., Reference Sidlauskaite, Sonuga-Barke, Roeyers and Wiersema2016) have been found in ADHD. Comparing these results can be problematic because of the different analytic methods used and variations in the nomenclature and boundaries of functional networks.
At the analysis stage, seed-based analysis is the most basic analytic approach for rsFC, which measures correlations of time series between a seed region-of-interest (ROI) and the rest voxels of the brain (Biswal et al., Reference Biswal, Yetkin, Haughton and Hyde1995; Fox and Raichle, Reference Fox and Raichle2007). This analytic approach has become the most commonly used method in rsFC studies of ADHD due to its simplicity in terms of calculation and interpretation and can directly locate the effect regions that show functional connectivity with the seed regions. However, the locations and sizes of seed ROIs vary considerably across studies (Fox and Greicius, Reference Fox and Greicius2010; Power et al., Reference Power, Barnes, Snyder, Schlaggar and Petersen2012), which make it a challenge to organize functional connectivity alterations. In addition, small sample sizes, non-uniform recruitment criteria and heterogenous results in current connectivity studies hamper our understanding of the neurobiological dysfunction in ADHD. A novel meta-analytic strategy proposed by Kaiser et al., can help overcome these challenges (Kaiser et al., Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015). This method allows unification of diverse findings in seed-based rsFC studies by categorizing seeds and corresponding effect regions into a priori functional network parcellations based on their locations. Based on this categorization, a meta-analysis is then performed for each seed-network to statistically synthesize the results and leverage large sample sizes to identify reliable and homogeneous patterns of functional connectivity alterations across existing studies. This strategy has been applied to major depressive disorder, schizophrenia and obsessive-compulsive disorder (Kaiser et al., Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015; Dong et al., Reference Dong, Wang, Chang, Luo and Yao2018; Gursel et al., Reference Gursel, Avram, Sorg, Brandl and Koch2018). The three studies investigating the above disorders with this strategy all used the multilevel kernel density analysis (MKDA) methodology, which allows the inclusion of only positive findings in a meta-analysis. As negative findings (i.e. null findings) may cause a decrease of meta-analytic estimates (Radua et al., Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012), inclusion of only positive findings may increase the bias in the results.
Therefore, in this study, we conduct a meta-analysis using the anisotropic effect-size version of seed-based d mapping (AES-SDM), which considers both positive and negative findings (Radua and Mataix-Cols, Reference Radua and Mataix-Cols2012) to unify seed-based rsFC findings into consistent patterns of impairments among intrinsic functional networks in ADHD. We analyzed all networks from Yeo's 7 parcellations (Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead, Roffman, Smoller, Zollei, Polimeni, Fischl, Liu and Buckner2011), including the DMN, FPN, ventral attention network (VAN), dorsal attention network (DAN), affective network (AN), somatosensory network (SSN) and visual network, since all these networks have been reported to be altered in individuals with ADHD (Castellanos and Aoki, Reference Castellanos and Aoki2016).
Methods
Search strategy
The online search was conducted in the PubMed, Web of Science and EMBASE databases to retrieve studies published before 29 November 2018, using the keywords ‘attention deficit hyperactivity disorder’ or ‘attention-deficit/hyperactivity disorder’ or ‘ADHD’ or ‘hyperkinetic’ plus ‘rest*’ plus ‘connect*’ or ‘fMRI’. In addition, the references of retrieved studies and pertinent review articles were manually searched.
Study eligibility criteria
Original fMRI studies were included if they (1) had a current ADHD patient group diagnosed according to the DSM-IV, DSM-5, or ICD-10 criteria; (2) had a typically developing (TD) comparison group; (3) directly compared whole-brain seed-based rsFC between ADHD and TD subjects; and (4) reported results as coordinates in stereotactic space. Authors were contacted if the coordinates of seeds or between-group effects were not provided in the study. Moreover, studies with negative findings were also included.
The exclusion criteria for the studies were as follows: (1) not seed-based rsFC approach; (2) no whole-brain analyses (restricted to predefined ROIs); (3) coordinates of seed ROIs or between-group effects could not be retrieved; or (4) overlapping samples with the same seeds reported elsewhere. Studies on the same samples but with selection of different seeds were considered separate datasets; studies in which distinct ADHD groups were compared with a single TD group were coded as distinct datasets.
Data extraction
Data were extracted as follows. First, the coordinates of the center of mass of each seed ROI and the peaks of each between-group effect exhibiting significance at the whole-brain level were extracted. For seed ROIs that were anatomical regions from atlases or prior results, representative coordinates were obtained by calculating the center of mass of seed ROIs using ‘fslstats’ commands from the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl). Then, we categorized seed ROIs based on their coordinates for the location of the center of mass into previously defined functional networks. These functional networks were defined by a previous whole-brain rsFC network parcellation from 1000 healthy participants and included the DMN, FPN, VAN, DAN, AN, SSN and visual network (Buckner et al., Reference Buckner, Krienen, Castellanos, Diaz and Yeo2011; Yeo et al., Reference Yeo, Krienen, Sepulcre, Sabuncu, Lashkari, Hollinshead, Roffman, Smoller, Zollei, Polimeni, Fischl, Liu and Buckner2011; Choi et al., Reference Choi, Yeo and Buckner2012). Finally, effects were categorized according to the direction of the actual values of the effects into hyperconnectivity (increased positive or decreased negative rsFC in ADHD patients compared with TD controls; that is, ADHD > TDC) or hypoconnectivity (increased negative or decreased positive rsFC in ADHD patients compared with TD controls; that is, ADHD < TDC).
In addition, negative results (i.e. those that did not survive statistical correction or showed no group differences in rsFC) were also included as another category of effect direction.
Meta-analysis
The voxel-wise meta-analysis was performed using the AES-SDM software package (version 5.15, http://www.sdmproject.com/software), which is a statistical technique for meta-analyzing studies on differences in brain activity (Radua and Mataix-Cols, Reference Radua and Mataix-Cols2012). First, we selected coordinates of cluster peaks (the voxels where the rsFC differences between ADHD and TD subjects were highest) and statistics (T-scores, Z-scores or p values, if available) according to AES-SDM inclusion criteria. Second, the effect-size maps of differences in rsFC between ADHD and TD subjects were recreated separately for each study using an anisotropic unnormalized Gaussian kernel, which assigned a higher value to the voxels closer to the peak coordinates (Radua et al., Reference Radua, Rubia, Canales-Rodriguez, Pomarol-Clotet, Fusar-Poli and Mataix-Cols2014). Both positive and negative coordinates were reconstructed in the same map, which is important for preventing a particular voxel erroneously appearing to be indicating opposite directions at the same time (Radua and Mataix-Cols, Reference Radua and Mataix-Cols2009). Finally, individual maps were combined using meta-analytic calculations, which were weighted by the intrastudy variance and interstudy heterogeneity. Statistical significance was determined using standard permutation tests with an uncorrected p < 0.005 and |Z| > 1 as the main threshold. This threshold is thought to be approximately equivalent to a corrected p < 0.05 in AES-SDM based on empirical comparisons (Radua et al., Reference Radua, Mataix-Cols, Phillips, El-Hage, Kronhaus, Cardoner and Surguladze2012). To further reduce false positive errors, we reported only clusters with 100 or more voxels as recommended in a recent published meta-analysis (Tang et al., Reference Tang, Lu, Zhang, Hu, Bu, Li, Hu, Gao, Zeng, Gong and Huang2018).
Complementary analysis
Five kinds of complementary analyses were performed for each seed-network as follows. (1) To control for age differences between studies, a subgroup analysis of nonadult studies was conducted. Therefore, the mean analysis was repeated including only samples of children and adolescents. Subgroup analyses of medication-naïve samples or medicated samples were not possible because of insufficient studies; (2) Interstudy heterogeneity was examined using the heterogeneity Q statistic. Clusters were considered heterogeneous among studies if they showed significant heterogeneity and overlapped with the main results. The sources of heterogeneity were further explored through meta-regression analyses; (3) The potential effects of several relevant demographic and clinical variables were examined using simple linear regression. Independent variables explored by meta-regression were sex, the mean age and the percentage of medication-naïve patients. The percentage of comorbidity-free patients could not be analyzed due to insufficient data; (4) A jackknife sensitivity analysis was conducted for both the main and subgroup analyses to evaluate the robustness and reliability of the results. The statistical analysis was repeated several times, excluding one different study each time. If a previously significant finding remained significant in all combinations of studies or with one or two exceptions, then it can be regarded as highly replicable; (5) Funnel plots and Egger's test in AES-SDM were used to visualize and quantitatively test the possibility of publication bias for each cluster, respectively (Egger et al., Reference Egger, Davey Smith, Schneider and Minder1997; Ioannidis et al., Reference Ioannidis, Munafo, Fusar-Poli, Nosek and David2014). The results showing p < 0.05 in Egger's test were considered to reflect a significant publication bias.
Results
Included studies
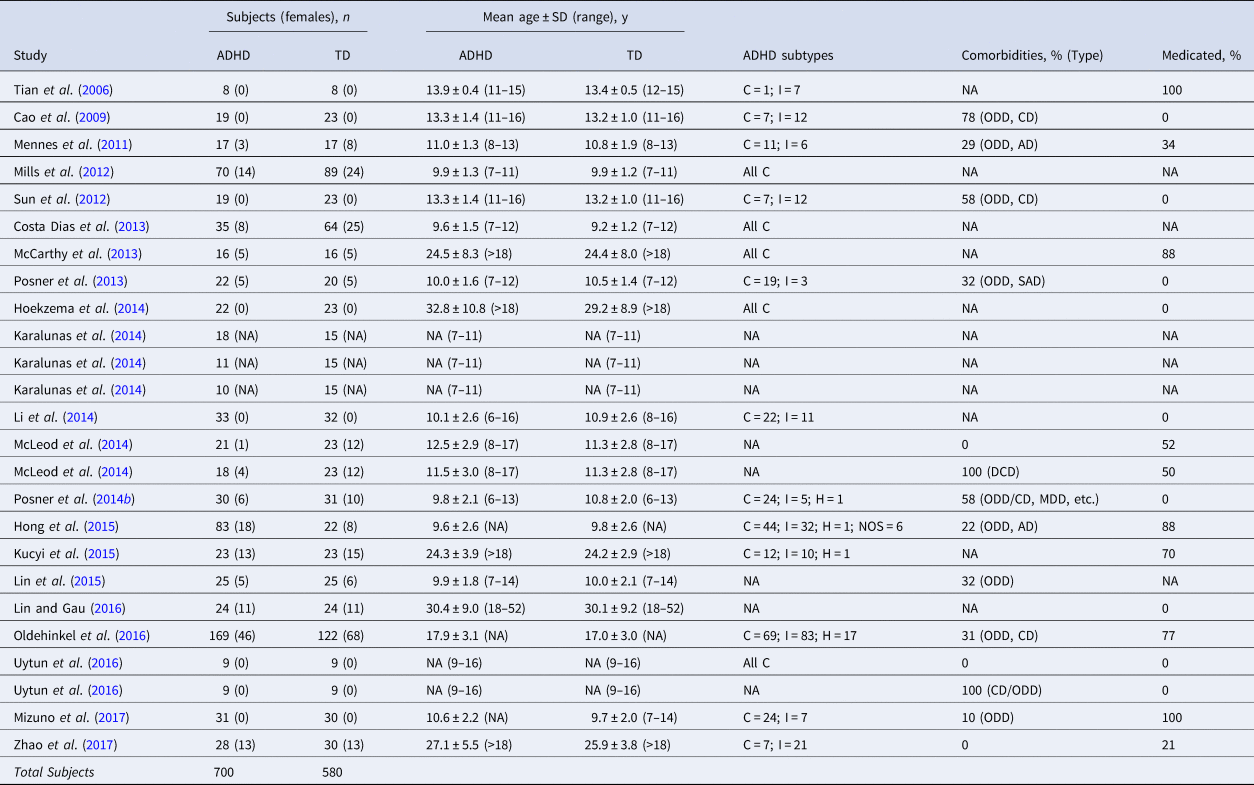
A total of 700 ADHD patients, including 497 children and adolescents (<18 years) and 203 adults (>18 years), 580 TD controls (464 children and adolescents, 116 adults), 49 seed ROIs, 235 peak coordinates and 8 negative results extracted from 25 datasets of 21 studies were analyzed. The demographic and clinical characteristics of the included studies are summarized in Table 1, and methodological information is also available (online Supplementary Table S1). Figure 1 shows the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gotzsche, Ioannidis, Clarke, Devereaux, Kleijnen and Moher2009) of the search strategy and selected studies. After categorizing seed ROIs into seven seed-networks, 10 studies for the DMN, 11 studies for the FPN and 8 studies for the AN were included in the meta-analysis (online Supplementary Table S2). Given that the number of studies for the DAN, VAN, SSN and visual network separately was too small to draw reliable conclusions, pooled meta-analyses of those seed-networks were not conducted.
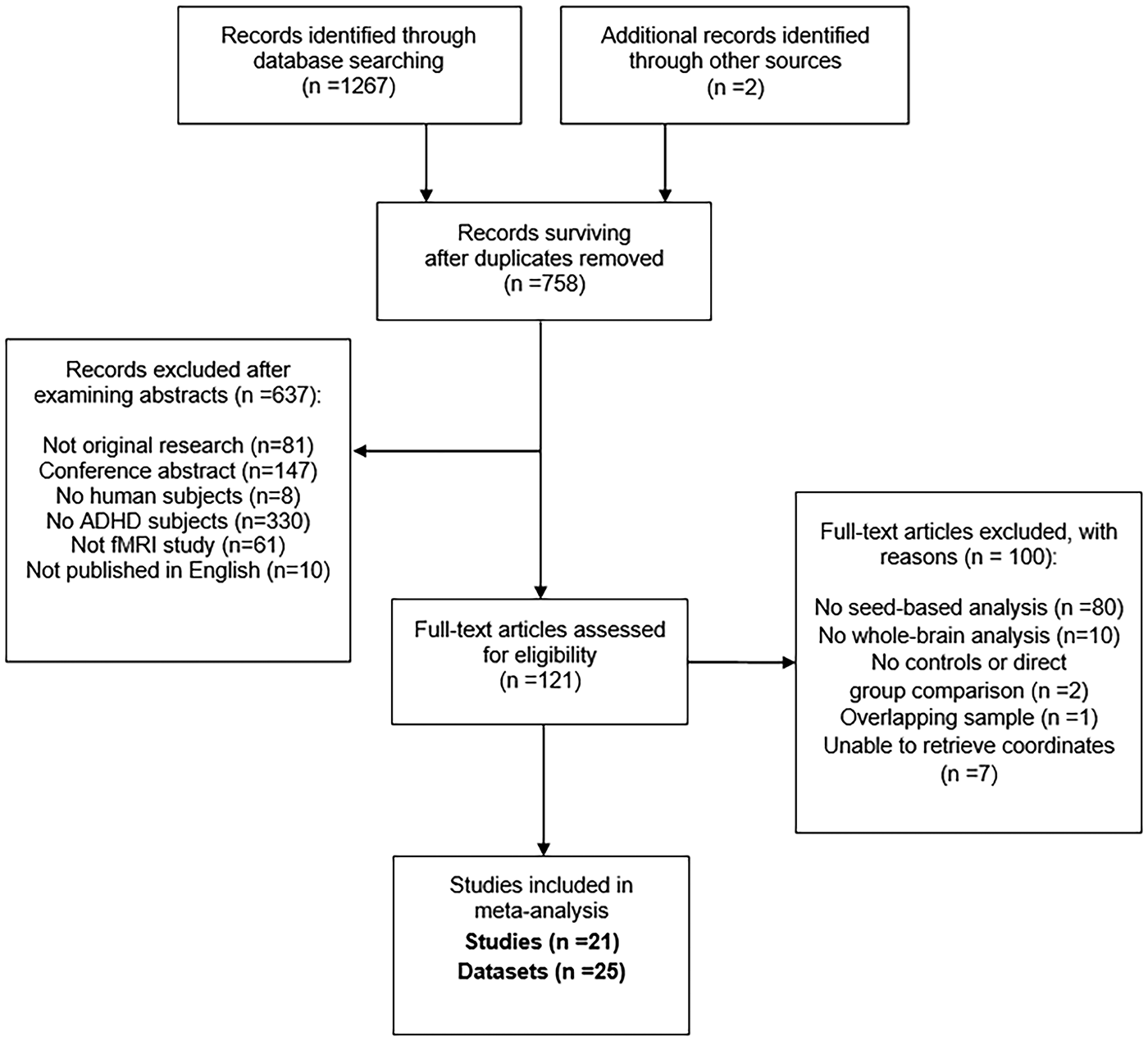
Fig. 1. PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flow diagram of the search strategy and retrieved studies. ADHD, attention-deficit/hyperactivity disorder; fMRI, functional magnetic resonance imaging.
Table 1. Summary of the demographic and clinical characteristics of the studies included in the meta-analysis
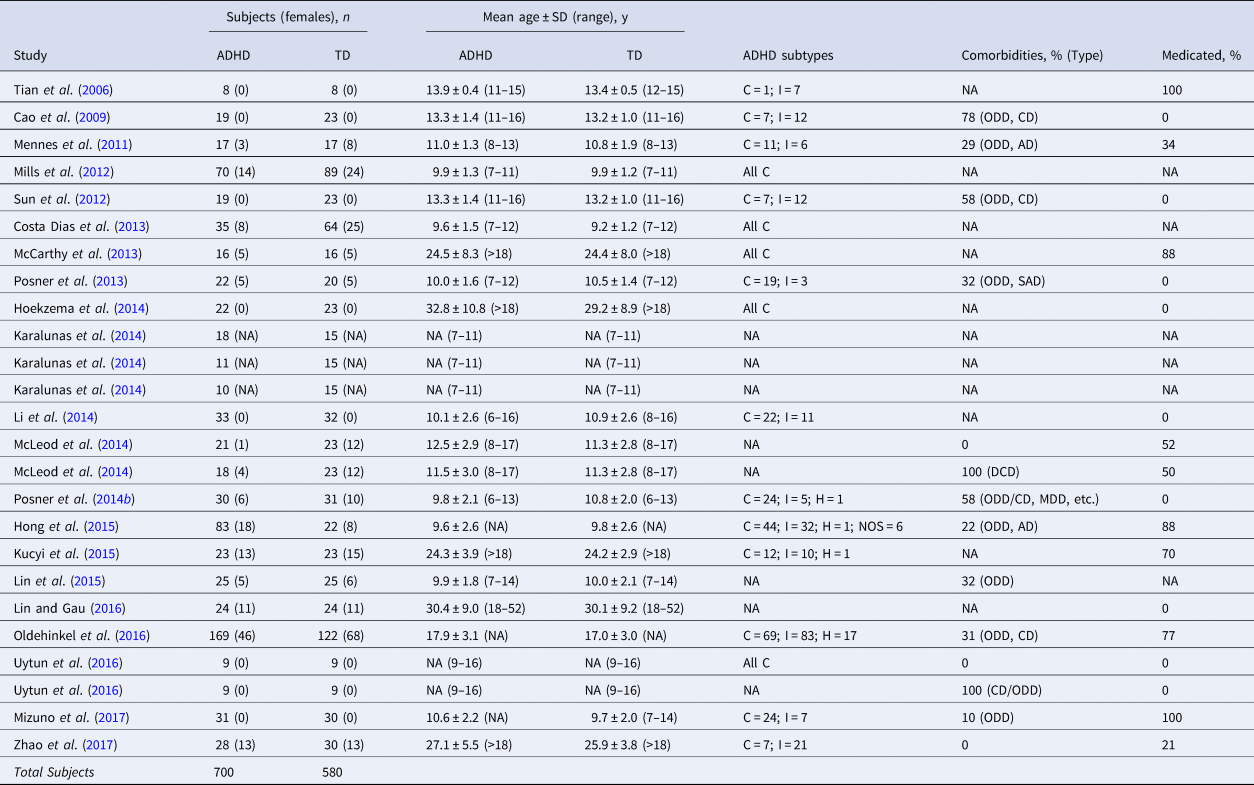
C, combined type; I, inattentive type; H, hyperactive-impulsive type; NOS, not otherwise specified; DCD, developmental coordination disorder; ODD, oppositional defiant disorder; CD, conduct disorder; MDD, major depressive disorder; AD, anxiety disorder; SAD, separation anxiety disorder; SD, standard deviation; NA, not available.
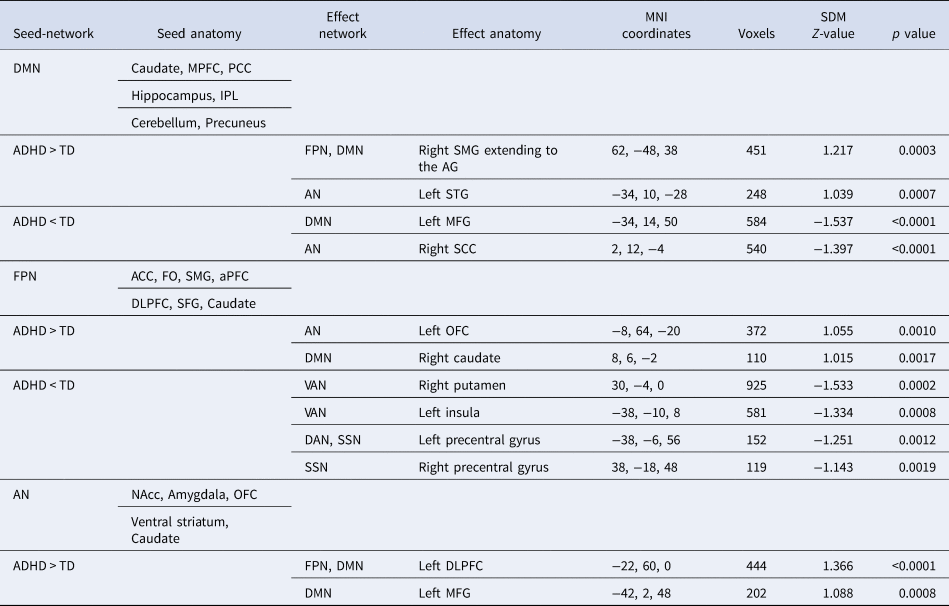
Table 2. Meta-analysis of abnormal resting-state functional connectivity in ADHD subjects compared with TD controls

MNI, Montreal Neurological Institute; SDM, seed-based d mapping; DMN, default mode network; VAN, ventral attention network; DAN, dorsal attention network; FPN, frontoparietal network; SSN, somatosensory network; AN, affective network; ACC, anterior cingulate cortex; FO, frontal operculum; SMG, supramarginal gyrus; AG, angular gyrus; NAcc, nucleus accumbens; DLPFC, dorsolateral prefrontal cortex; SFG, superior frontal gyrus; OFC, orbitofrontal cortex; aPFC, anterior prefrontal cortex; PCC, posterior cingulate cortex; MPFC, medial prefrontal cortex; IPL, inferior parietal lobe; MFG, middle frontal gyrus; STG, superior temporal gyrus; SCC, subcallosal cingulate cortex; DLPFC, dorsolateral prefrontal cortex
Within-network dysfunction
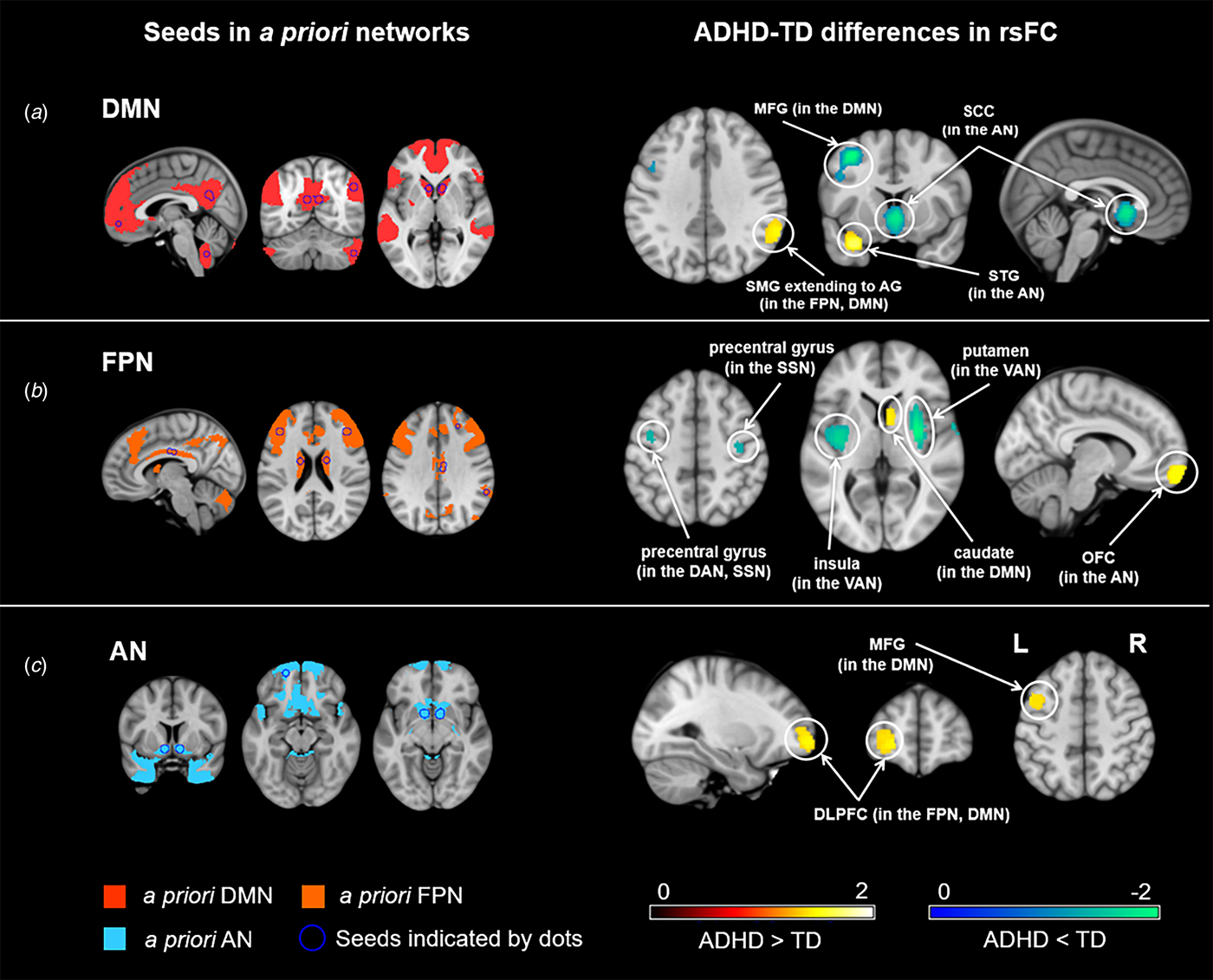
ADHD was associated with hypoconnectivity within the DMN. We found hypoconnectivity between the DMN seeds and the left middle frontal gyrus (MFG) (Table 2, Fig. 2a). This altered connectivity was also observed between the DMN seeds and areas of the medial prefrontal cortex (MPFC) in nonadult samples (online Supplementary Fig. S1A).

Fig. 2. Meta-analysis of abnormal resting-state functional connectivity (rsFC) for three seed-networks in attention-deficit/hyperactivity disorder (ADHD). The left 3 columns illustrate the seed regions of interest indicated by dots and categorized by a priori functional networks. The right 3 columns illustrate abnormal rsFC in ADHD subjects compared to typically developing (TD) individuals. DMN, default mode network; FPN, frontoparietal network; AN, affective network; DAN, dorsal attention network; VAN, ventral attention network; SSN, somatosensory network; MFG, middle frontal gyrus; STG, superior temporal gyrus; OFC, orbitofrontal cortex; SCC, subcallosal cingulate cortex; SMG, supramarginal gyrus; AG, angular gyrus; DLPFC, dorsolateral prefrontal cortex; L, left; R, right.
In addition, hyperconnectivity was found within the FPN in the nonadult samples, which peaked in the dorsal anterior cingulate cortex (dACC) (online Supplementary Fig. S1B).
Between-network dysfunction
Hyperconnectivity between the DMN and the FPN
ADHD was characterized by hyperconnectivity between the DMN seeds and the right supramarginal gyrus extending to the right angular gyrus that spread across the FPN and the DMN (Fig. 2a). Meanwhile, hyperconnectivity was also found between the FPN seeds and areas of the caudate in the DMN (Fig. 2b).
Hyperconnectivity and hypoconnectivity between the DMN and regions of the AN
ADHD showed hyperconnectivity between the DMN seeds and the left superior temporal gyrus (STG) as well as hypoconnectivity between the DMN seeds and portions of the subcallosal cingulate cortex (SCC) (Fig. 2a). Both of these clusters were located in the AN but were related to diverse functions.
Hypoconnectivity between the FPN and regions of the VAN or SSN
Hypoconnectivity was found between the FPN seeds and regions of the VAN, including the right putamen and the left insula (Fig. 2b). Moreover, hypoconnectivity was also observed between the FPN seeds and the bilateral precentral gyrus, regions belonging to the SSN (Fig. 2b).
Hyperconnectivity between the FPN and regions of the AN
Hyperconnectivity was observed between the FPN seeds and areas of the left orbital frontal cortex (OFC), which is in the AN (Fig. 2b).
Hyperconnectivity between the AN and regions of the DMN or FPN
ADHD was linked to hyperconnectivity between the AN seeds and regions of the left MFG, which is located in the DMN (Fig. 1c). Hyperconnectivity was also found between the AN seeds and parts of the left dorsolateral prefrontal cortex (DLPFC), which has been implicated as a key region of the FPN (Fig. 1c).
Complementary analyses
Subgroup analyses
Subgroup analyses of 13 nonadult studies (7 for the DMN and 8 for the FPN) revealed that the above results remained largely unchanged or were even more significant in some clusters (online Supplementary Table S5 and Fig. S1). Moreover, five additional significant clusters emerged in this subanalysis of studies. We found hyperconnectivity between DMN seeds and regions of the bilateral insula, which were located in the VAN, along with the left STG in the SSN. In addition, within-DMN seeds exhibited hypoconnectivity with the right medial prefrontal cortex (MPFC). In addition, we also found hyperconnectivity within the FPN, which peaked in the anterior cingulate cortex (ACC). The analysis was not repeated for AN studies because all samples were children or adolescents.
Heterogeneity analyses and publication bias
No significant between-group heterogeneity was found in the results for the DMN and AN. For the FPN results, significant between-group heterogeneity was detected in the SFG and the putamen. None of the clusters reported above showed a significant publication bias based on Egger's test (p > 0.05). Funnel plots are presented in online Supplementary Fig. S2.
Meta-regression analyses
Regression analyses showed that the mean age (DMN: available in all studies but two; FPN: available in all studies but one), the percentage of male patients (available in all studies) and the percentage of medication-naïve patients (DMN: available in all studies; FPN: available in all studies but one) were not associated with ADHD-related rsFC changes, at least linearly. Meta-regression analysis for AN studies was not possible as the number of studies was insufficient.
Jackknife sensitivity analyses
For the results of the main analysis, jackknife sensitivity analyses revealed that the result in the left MFG remained significant in all combinations of datasets, while the remaining resultant clusters remained significant in all but 1 combination of datasets (online Supplementary Table S4). The results of the jackknife sensitivity analyses for the subgroup analysis of nonadult samples are listed in online Supplementary Table S6.
Discussion
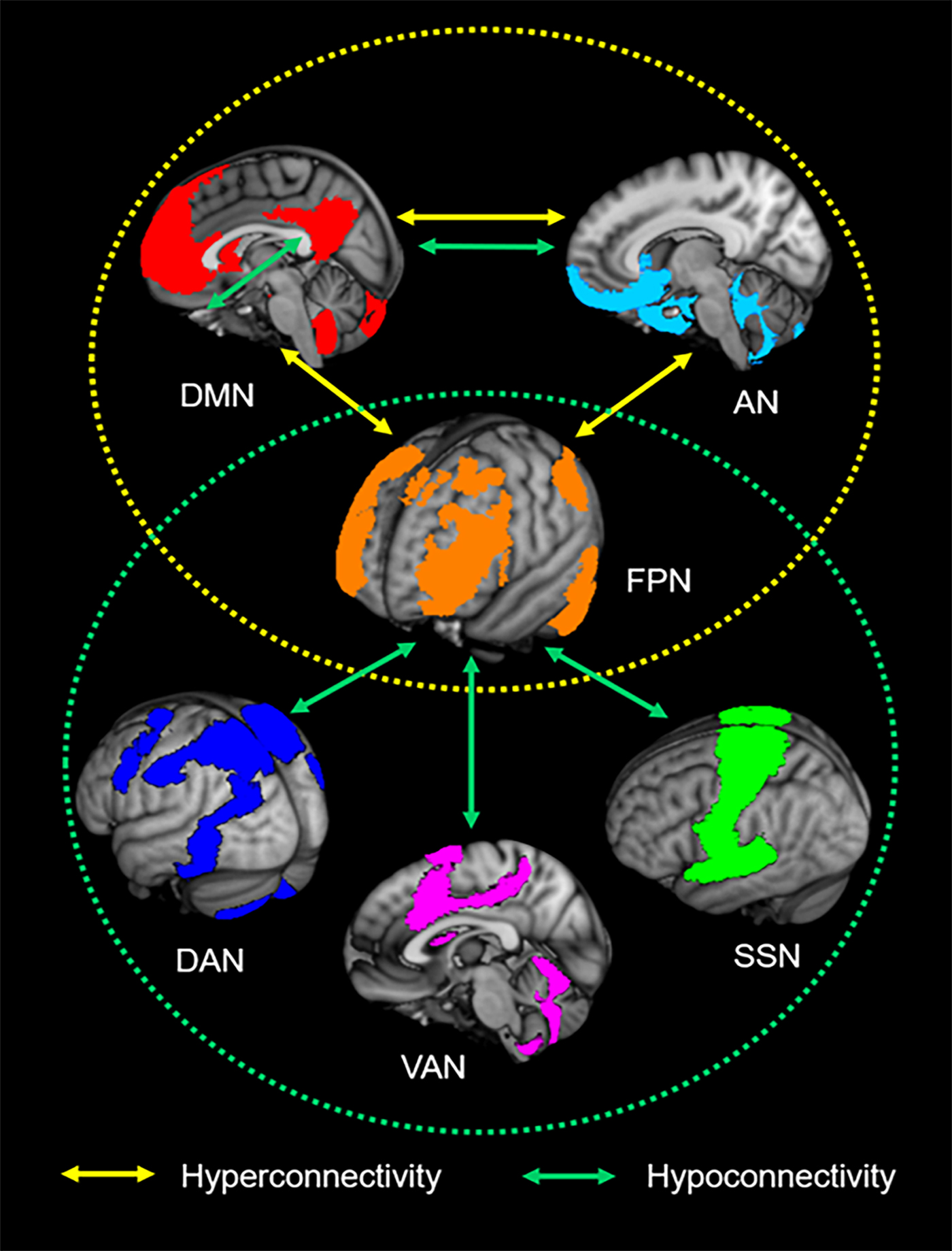
The present meta-analysis motivates a consistent pattern of large-scale brain network impairments in ADHD in which the FPN plays a key role in regulating the functions of other networks (Fig. 3). Our finding of imbalanced connectivity between the FPN and regions of the DMN and VAN (also referred to as ‘SN’) supports the well-known triple-network dysfunction model of pathophysiology associated with multiple psychiatric disorders (Menon, Reference Menon2011), including ADHD, and may underlie the symptoms of inattention that characterize ADHD. Beyond this model, we also found dysconnectivity between these three networks and two other functional networks: the somatosensory network (SSN) and the affective network (AN). These findings suggest the non-negligible roles of the SSN and AN in the abnormal network interactions that may account for ADHD-related motor hyperactivity and impulsive symptoms.
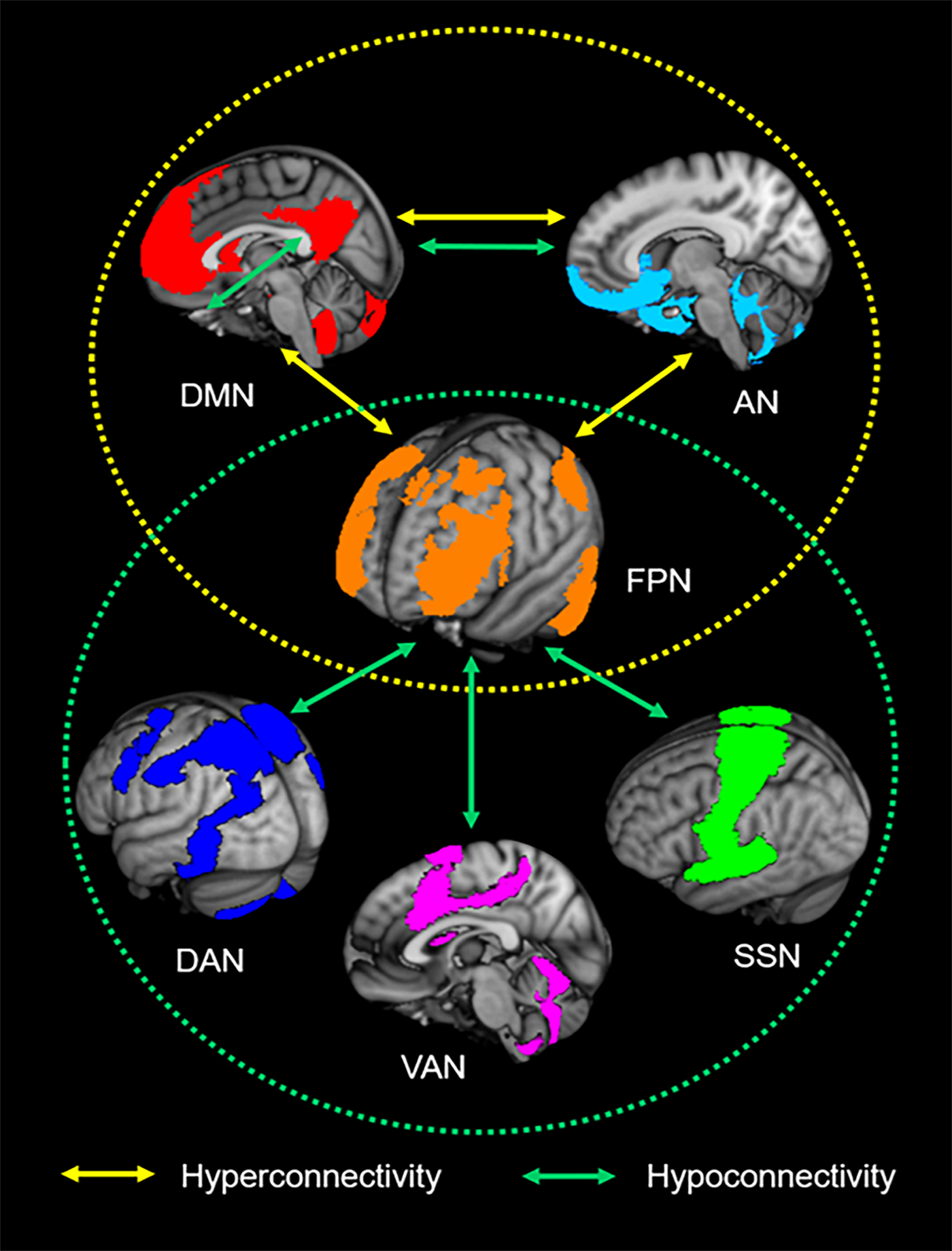
Fig. 3. Neurocognitive network dysfunction patterns of attention-deficit/hyperactivity disorder (ADHD). The yellow circle represents the aberrant interplay among the default mode network (DMN), frontoparietal network (FPN) and affective network (AN) in ADHD. The green circle represents ADHD-associated hypoconnectivity between the FPN and the dorsal attention network (DAN), ventral attention network (VAN) and somatosensory network (SSN). The overlap of the patterns suggests that the FPN is a core intrinsic network of ADHD pathophysiology.
Hyperconnectivity was observed in individuals with ADHD between the DMN and the FPN along with hypoconnectivity within the DMN itself, a network involved in self-related activity (Buckner et al., Reference Buckner, Andrews-Hanna and Schacter2008), especially spontaneous mind-wandering (Fox et al., Reference Fox, Spreng, Ellamil, Andrews-Hanna and Christoff2015; Bozhilova et al., Reference Bozhilova, Michelini, Kuntsi and Asherson2018). Weaker within-DMN connectivity has been a common finding in ADHD and may reflect weakness in the integration of inner activity. In original studies, DMN-FPN hyperconnectivity was reported to be associated with reduced negative functional connectivity in ADHD subjects compared to TDCs (Sun et al., Reference Sun, Cao, Long, Sui, Cao, Zhu, Zuo, An, Song, Zang and Wang2012; Lin and Gau, Reference Lin and Gau2016). This finding is consistent with that of an earlier resting-state fMRI study showing a reduced or absent anticorrelation between the DMN and the FPN in ADHD (Castellanos et al., Reference Castellanos, Margulies, Kelly, Uddin, Ghaffari, Kirsch, Shaw, Shehzad, Di Martino, Biswal, Sonuga-Barke, Rotrosen, Adler and Milham2008). The authors postulated that this functional alteration may underlie the attentional lapses in ADHD as mind-wandering mediated by the DMN may disrupt or interfere with the normal functioning of the frontoparietal control network. However, this hypothesis requires further verification from evidence of the behavioral correlates of DMN-FPN dysfunctional interactions. A recent study suggested that ADHD-related mind-wandering can be explained by deficient control of task-related cognition instead of excessive generation of task-unrelated thoughts, which may partly derive from dysfunction in the intrinsic architecture of the FPN rather than the DMN (Vatansever et al., Reference Vatansever, Bozhilova, Asherson and Smallwood2019). Therefore, the roles of the DMN and FPN in the neural correlates of mind-wandering in ADHD require further clarification.
The present study also revealed hypoconnectivity in ADHD between the FPN and the insula, which is a critical part of the salience network (‘VAN’ in this study) and implicated in the evaluation of motivational salience stimuli (Lopez-Larson et al., Reference Lopez-Larson, King, Terry, McGlade and Yurgelun-Todd2012; Vasic et al., Reference Vasic, Plichta, Wolf, Fallgatter, Sosic-Vasic and Gron2014). According to a network model of insula function, when a salient event is detected, the insula plays a role in switching between other functional networks to facilitate access to attention and working memory resources (Menon and Uddin, Reference Menon and Uddin2010). Therefore, impairments of connectivity between the insula and frontoparietal control network may lead to inappropriate behavioral responses to salient stimuli, which potentially cause the core ADHD symptoms of inattention. Meanwhile, the FPN also exhibited hypoconnectivity with the putamen. As one of the main structures composing the striatum, the putamen primarily regulates motor function via connections with cortical motor areas (Di Martino et al., Reference Di Martino, Scheres, Margulies, Kelly, Uddin, Shehzad, Biswal, Walters, Castellanos and Milham2008; Cai et al., Reference Cai, Ryali, Chen, Li and Menon2014) and supports high-level cognitive functions such as working memory (Chang et al., Reference Chang, Crottaz-Herbette and Menon2007) and language processing (Booth et al., Reference Booth, Wood, Lu, Houk and Bitan2007). Previous meta-analyses revealed hypoactivation in the DLPFC, insula and putamen during attention tasks (Hart et al., Reference Hart, Radua, Nakao, Mataix-Cols and Rubia2013) and inhibition tasks (Norman et al., Reference Norman, Carlisi, Lukito, Hart, Mataix-Cols, Radua and Rubia2016) in patients with ADHD relative to controls. Therefore, the abnormal connectivity between FPN regions and the insula as well as the putamen may underlie the deficits of attention and inhibition control that characterize ADHD.
Furthermore, hypoconnectivity was also observed between the FPN and the precentral gyrus, which is an important part of the sensorimotor system and is involved in multiple executive functions, such as sustained attention, response inhibition and task switching (Dibbets et al., Reference Dibbets, Evers, Hurks, Bakker and Jolles2010; Lei et al., Reference Lei, Du, Wu, Chen, Huang, Du, Bi, Kemp and Gong2015). Hyperactivation of the precentral gyrus has been shown to be associated with impaired motor inhibition in ADHD (Cortese et al., Reference Cortese, Kelly, Chabernaud, Proal, Di Martino, Milham and Castellanos2012). The abnormal connectivity between the precentral gyrus and the FPN may be related to deficits of motor inhibition control in ADHD.
We also found hyperconnectivity between the AN and prefrontal regions of the DMN and the FPN. In the original study, the ADHD-related increased connectivity between the nucleus accumbens (NAcc) and the prefrontal cortex (e.g. the anterior prefrontal cortex (aPFC) and MFG) was associated with greater impulsivity (Costa Dias et al., Reference Costa Dias, Wilson, Bathula, Iyer, Mills, Thurlow, Stevens, Musser, Carpenter, Grayson, Mitchell, Nigg and Fair2013). Meanwhile, the DMN and FPN both showed hyperconnectivity with regions of the AN, including the STG and the OFC. Previous findings suggested that dysfunction within a striato-amygdalo-prefrontal cortical network accounted for deficits in orienting toward, recognizing, and allocating attention to emotional stimuli, which may contribute to emotion dysregulation in ADHD (Shaw et al., Reference Shaw, De Rossi, Watson, Wharton, Greenstein, Raznahan, Sharp, Lerch and Chakravarty2014). Therefore, the hyperconnectivity among the default, frontoparietal and affective networks found in the current study may contribute to symptoms of emotion dysregulation and impulsivity in ADHD.
The lack of data related to the VAN, DAN, SSN and visual network suggests a bias in seed selection in existing rsFC studies. Considering the statistical power, meta-analyses of studies on these networks were not conducted, but we narratively reviewed the results of these studies. A relatively consistent finding that we identified was hypoconnectivity between the VAN seeds (e.g. the insula, VFC and putamen) and the regions of the SSN (e.g. the primary motor cortex and the precentral gyrus) in ADHD patients compared to TDCs. In addition, both hypoconnectivity and hyperconnectivity were observed between regions of the DAN and visual network. In detail, the DAN seeds (the FEF and IPS) showed hypoconnectivity with the right fusiform gyrus, while the FEF also exhibited hyperconnectivity with regions of the left occipital gyrus.
Although we did not find consistent functional connectivity alterations regarding the cerebellum in the current meta-analysis, original studies have reported that regions of the cerebellum showed dysconnectivity with the ACC, putamen, amygdala and hippocampus in ADHD (Tian et al., Reference Tian, Jiang, Wang, Zang, He, Liang, Sui, Cao, Hu, Peng and Zhuo2006; Cao et al., Reference Cao, Cao, Long, Sun, Sui, Zhu, Zuo, Zang and Wang2009; Karalunas et al., Reference Karalunas, Fair, Musser, Aykes, Iyer and Nigg2014; Posner et al., Reference Posner, Siciliano, Wang, Liu, Sonuga-Barke and Greenhill2014b). Two studies using lateral cerebellar areas (e.g. Crus I/II) as the seed demonstrated that the cerebellum exhibited greater functional connectivity with widespread regions in the DAN, VAN and SSN in ADHD adults (Kucyi et al., Reference Kucyi, Hove, Biederman, Van Dijk and Valera2015) and lower connectivity with the left DLPFC in ADHD children (Mizuno et al., Reference Mizuno, Jung, Fujisawa, Takiguchi, Shimada, Saito, Kosaka and Tomoda2017). These findings suggested the potential role of cerebro-cerebellar interactions in the neural mechanism of ADHD. More evidence is needed to verify these results and clarify their relations to cognitive and behavioral functions in ADHD.
In addition, mixed use of global signal regression (GSR) was noted in the studies included in current meta-analysis. Eleven of twenty-one original studies applied GSR in fMRI image preprocessing, while the other studies did not apply this method. Two studies reported results with and without applying GSR and found discrepancies between the results with GSR and those without GSR (Lin et al., Reference Lin, Tseng, Lai, Matsuo and Gau2015; Zhao et al., Reference Zhao, Li, Yu, Huang, Wang, Liu, Cao, Qian, Zang, Sun and Wang2017), suggesting that GSR may affect group comparisons and contribute to inconsistent results. However, the effects of GSR remain confusing as various sources are attributed to the global signal (Liu et al., Reference Liu, Nalci and Falahpour2017); therefore, the application of GSR in resting-state fMRI preprocessing remains controversial. Some studies have suggested that applying GSR can reduce the effects of motion (Yan et al., Reference Yan, Cheung, Kelly, Colcombe, Craddock, Di Martino, Li, Zuo, Castellanos and Milham2013; Power et al., Reference Power, Mitra, Laumann, Snyder, Schlaggar and Petersen2014) and increase connection specificity (Fox et al., Reference Fox, Zhang, Snyder and Raichle2009; Weissenbacher et al., Reference Weissenbacher, Kasess, Gerstl, Lanzenberger, Moser and Windischberger2009), while other studies indicate that the use of GSR may increase negative correlations (Murphy et al., Reference Murphy, Birn, Handwerker, Jones and Bandettini2009) and thus distort estimations of group differences in rsFC (Gotts et al., Reference Gotts, Saad, Jo, Wallace, Cox and Martin2013; Hahamy et al., Reference Hahamy, Calhoun, Pearlson, Harel, Stern, Attar, Malach and Salomon2014). We think that reporting results both with and without GSR may increase credibility and help enhance comprehension of the effects of GSR.
In summary, the current pattern of abnormal communication within and between large-scale functional networks highlighted the dysfunctional control of the FPN on internal thought and external stimuli, along with inhibition and motion, which may contribute to deficits in attention and inhibition control. Meanwhile, we found hyperconnectivity among the affective, default and frontoparietal networks, which may be associated with emotion dysregulation and impulsivity in ADHD. In addition, these network abnormalities seem to be rather stable and independent of sex, age or medication state as demonstrated by the complementary analyses. These findings support the triple-network dysfunction model but also suggest the involvement of sensorimotor and affective systems that may facilitate the development of pathophysiologic symptoms in ADHD. The intrinsic network dysfunction pattern provides a potentially mechanistic framework for understanding the neuropsychology and behavioral symptoms of ADHD. However, further study is needed to directly test and verify the functional and behavioral correlations of these network alterations.
Limitations
Several limitations need to be considered in this study while also suggest future directions. First, the number of included studies for each network was relatively small. Although we employed jackknife analyses to evaluate the robustness and reliability of the results, a cautious interpretation of the findings is necessary. Meanwhile, integrating the results for the VAN, DAN, SSN and visual network is difficult via meta-analysis due to insufficient studies. Future studies can select regions of these networks as seeds to further investigate the connectivity among these functional networks. Second, the small sample sizes in most of the included studies may bias the results. Therefore, future research should include large samples to validate these findings. Third, considerable variations exist in the data acquisition and imaging processing methods in the included studies. Different scanners, motion-correction methods and eye states all have potential effects on the results (Buckner et al., Reference Buckner, Krienen and Yeo2013). Unfortunately, due to the insufficient number of studies within methodologic categories, the moderating effects of these variables were impossible to evaluate in the current study and merit future investigation. Forth, the hemispheric distribution of the selected seed regions may be a potential source of bias; however, the current meta-analysis cannot analyze this potential bias since the number of studies is insufficient for a separate analysis. Fifth, in the absence of empirical data, conducting a meta-analysis of adult-only samples is impossible, resulting in insufficient knowledge of rsFC abnormalities in adult ADHD patients and reflecting the need for future investigations of age-related differences in rsFC in ADHD. Lastly, subgroup analyses of ADHD subtypes, comorbidities and medication use cannot be performed due to a lack of information. Although meta-regression analysis showed no relationship between the percentage of medication-naïve patients and ADHD-associated functional connectivity changes, the influence of medication should be further investigated by directly comparing medicated and medication-naïve patients.
Conclusions
To our knowledge, this is the first meta-analysis of seed-based rsFC fMRI studies to identify impairments of large-scale functional networks in ADHD. A consistent pattern of abnormal connectivity within and between intrinsic brain networks involved in spontaneous mind-wandering, attention, emotional and sensory processing and goal-directed regulation of these functions may underlie the core cognitive and affective deficits that characterize ADHD. Future studies should further explore and validate brain-behavior relationships in this disorder.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S003329171900237X.
Acknowledgements
The authors would like to thank their tutors and colleagues for their time and valuable help.
Financial support
This study is supported by grants from the National Natural Science Foundation of China (81671669) and Sichuan Provincial Youth Grant 2017JQ0001.
Conflict of interest
None.