INTRODUCTION
Tropical dry forests (TDF) are greatly threatened by their conversion to agricultural land, making the future of this biome largely dependent on conservation of remaining old-growth forests and regeneration and restoration of secondary forests (Dirzo et al. Reference DIRZO, MOONEY, CEBALLOS and YOUNG2010, Sánchez-Azofeifa et al. Reference SÁNCHEZ-AZOFEIFA, QUESADA, CUEVAS-REYES, CASTILLO and SÁNCHEZ-MONTOYA2009). This posits a challenge because understanding the ecological factors, processes and mechanisms that allow TDF regeneration is far from being complete (Quesada et al. Reference QUESADA, SÁNCHEZ-AZOFEIFA, ÁLVAREZ-AÑORVE, STONER, ÁVILA-CABADILLA, CALVO-ALVARADO, CASTILLO, ESPÍRITO-SANTO, FAGUDES, FERNANDES, GAMON, LOPEZARAIZA-MIKEL, LAWRENCE, MORELLATO, POWERS, NEVES, ROSAS-GUERRERO, SAGAYO and SÁNCHEZ-MONTOYA2009). For example, currently no more than five published studies on TDF seed-bank communities in old-fields exist (González-Rivas et al. Reference GONZÁLEZ-RIVAS, TIGABU, CASTRO-MARÍN and ODÉN2009, Lemenih & Teketay Reference LEMENIH and TEKETAY2006, Miller Reference MILLER1999, Rico-Gray & García-Franco Reference RICO-GRAY and GARCÍA-FRANCO1992, Vieira & Scariot Reference VIEIRA and SCARIOT2006).
Colonizing herbaceous plants are highly dependent on light to produce abundant seeds, which usually are able to stay dormant in the soil for long periods (Fenner Reference FENNER1985). Seed bank density of such herbaceous plants is expected to decline as TDF succession advances and light resources reduce in the understorey. In contrast, seeds of most woody species lack prolonged dormancy and suffer high predation rates (Briones-Salas et al. Reference BRIONES-SALAS, SÁNCHEZ-CORDERO and SÁNCHEZ-ROJAS2006, Garwood Reference GARWOOD, Leck, Parker and Simpson1989, Janzen Reference JANZEN1981, Khurana & Singh Reference KHURANA and SINGH2001), which makes their abundance in the soil largely dependent on local seed rain (Álvarez-Buylla & Martínez-Ramos Reference ÁLVAREZ-BUYLLA and MARTÍNEZ-RAMOS1990, Dalling & Denslow Reference DALLING and DENSLOW1998). Thus, the abundance and diversity of seeds of woody species in the soil is expected to increase as more reproductive woody plants become established during succession.
The temporal changes undergone by seed-bank communities may allow the exploration of mechanisms underlying species replacement during succession (sensu Connell & Slatyer Reference CONNELL and SLATYER1977). Such analysis is possible if the abundance and composition of mature plant communities are mirrored in the characteristics of the seed banks. There is some evidence indicating that this is true for woody species (Ceccon et al. Reference CECCON, HUANTE and RINCÓN2006, Dalling & Denslow Reference DALLING and DENSLOW1998). Three possible patterns can be expected to occur in seed-bank samples taken along a chronosequence, depending on the mechanisms underlying plant succession. If there is a change in the species composition of the seed bank over the chronosequence, following a species-by-species replacement pattern, a facilitation mechanism would be inferred. In contrast, if seeds of several woody species are found in recently abandoned fields but they disappear sequentially with successional time, a tolerance mechanism would be indicated. Finally, if species richness and composition of the seed bank increase during succession after the disappearance of a dominant early-colonizer species, an inhibition mechanism would be inferred.
In this paper we analyse structural and compositional changes of seed-bank communities over a chronosequence of abandoned cattle pastures (with fallow ages of 0–12 y) and old-growth TDF sites in western Mexico. We hypothesize that: (1) density, diversity, and species composition of seed-bank communities change with fallow age and plant growth form during the first 12 y of succession, (2) seeds of herbaceous species are replaced by seeds of woody species over succession, (3) abundance of seed-banks of woody species mirror the abundance of the species in the established community and (4) successional mechanisms (i.e. facilitation, tolerance or inhibition) can be inferred from patterns of species replacement in the seed bank over the chronosequence.
METHODS
Study site
This study was carried out in the municipality of La Huerta and in the Biosphere Reserve of Chamela–Cuixmala (19o30′N, 105°03′W; Figure 1a), in Jalisco, Mexico. The annual average temperature is 22 °C and the mean annual precipitation is 788 mm. Most rain (c. 93%) falls between June and October and from November to May a long dry season occurs. The dominant vegetation is TDF which is characterized by low-stature trees (maximum 12 m) and shrubs, developed mostly on slopes and ridges of low hills (Segura et al. Reference SEGURA, BALVANERA, DURÁN and PÉREZ2004). The conversion of the TDF to cattle pastures is the main agriculture practice in the study region, representing about 60% of the total area affected by human activities (Burgos & Maass Reference BURGOS and MAASS2004). Slash-and-burn is a highly recurrent practice; after some years of use, the pastures are abandoned and plant communities, initially dominated by herbaceous species and shrubs, develop in the open sites (Burgos & Maass Reference BURGOS and MAASS2004).
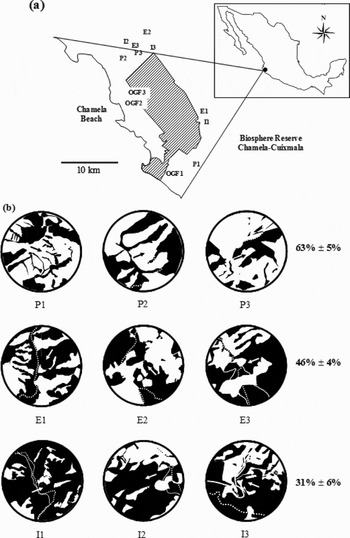
Figure 1. Geographic location of studied sites and matrix configuration surrounding each abandoned studied pastures at La Huerta Municipality (Jalisco), western Mexico. Map of location of 12 studied sites, three for each following successional categories; P: ‘pasture’ (0–1 y since abandonment), E: ‘early’ (3–5 y since abandonment), I: ‘intermediate’ (10–12 y since abandonment) and OGF: old-growth forest (a). Shaded area corresponds to the MAB Biosphere Reserve Chamela–Cuixmala. Matrix configuration around (in a circular area with a radius of 0.5 km) each studied site belonging to pasture, early and intermediate successional categories (b); in black are indicated areas covered with vegetation (secondary or old-growth forest) and in white open areas (active pastures, other agricultural fields, and roads), drawn from geo-referenced Google Earth® satellite images. Old-growth-forest sites were 100% covered by forest vegetation. Within the circular area, the position of the 20 × 50-m study plot is indicated by the tip of the black or white arrow. To the right, the mean and 1 SE of the percentage represented by open areas in each successional category is indicated.
Study system
We used a chronosequence as a study system. Increasing evidence indicates that the substitution of time by space implicit in chronosequences does not necessarily predict the temporal trajectories that would follow the succession in a given site over time (Chazdon et al. Reference CHAZDON, LETCHER, BREUGEL, MARTÍNEZ-RAMOS, BONGERS and FINEGAN2007, Johnson & Miyanishi Reference JOHNSON and MIYANISHI2008). Accordingly, with the aim of reducing predictive uncertainty associated with chronosequences, we considered four successional categories with three replicates each: ‘pasture’ (pastures of 0–1 y since abandonment), ‘early’ (pastures 3–5 y since abandonment), ‘intermediate’ (pastures 10–12 y since abandonment), and old-growth forest without any sign of human disturbance. The percentage of land in open conditions (active pasture and other agricultural land uses, and roads) in the matrix surrounded the studied sites diminished, on average, from 63% in pasture to 31% in intermediate successional categories (Figure 1b).
At each study site, 20 cylindrical soil samples (10 cm diameter, 15 cm depth) were randomly obtained within a plot of 20 × 50 m. This sampling effort (equivalent to 200 samples ha−1) exceeded the recommendation of Butler & Chazdon (Reference BUTLER and CHAZDON1998) in order to properly represent the seed bank in a given tropical forest area. Soil sampling was conducted in May 2005, towards the end of the dry season. Other studies have found that density and diversity of seed banks in TDFs peak during the dry season (Grombone-Guaratini & Rodrigues Reference GROMBONE-GUARATINI and RODRIGUES2002, Martins & Engel Reference MARTINS and ENGEL2007). Many woody species tend to produce seeds in the dry season (Bullock & Solís-Magallanes Reference BULLOCK and SOLÍS-MAGALLANES1990, Foster Reference FOSTER1986), while germination, seed predation, and/or damage by fungi are higher in the rainy season compared with the dry season.
Each soil sample was sifted using metallic sieves of different aperture size to remove soil and debris. Sifted material was inspected visually and with stereoscopic microscopes to separate all seeds of at least 1 mm diameter. Seeds were separated by unequivocal morphospecies that were identified to the lowest taxonomic level whenever possible. Samples of seed morphospecies were germinated in a greenhouse to obtain seedlings/plants that could be identified. Herbarium reference collections, specialized literature and specialists were consulted for taxonomic identification. All morphospecies were assigned to following plant growth forms: tree, shrub, woody climber, herbaceous climber, terrestrial herb and grass. Dispersal mode (animals, wind and gravity) was also assigned to each identified morphospecies. Because of the lack of tests on seed viability, density and diversity values of the studied seed banks could be overestimated (Simpson et al. Reference SIMPSON, LECK, PARKER, Leck, Parker and Simpson1989).
Data analysis
At the community level and for each growth form, values of seed density (seeds per area unit), species density (species per area unit) and species diversity (number and relative abundance of species, only for the whole community) were obtained. To do this, the program EstimateS 8.2 was used to generate a single trait value per plot, obtained from randomized accumulation curves of seeds and species, based on a matrix of species × samples for each plot; each cell of the matrix contained the number of seeds per species. Using the same program, two non-parametric estimators of the real number of species were also obtained for each plot. These estimators calculate the expected total number of species in a given site to determine, in a probabilistic way, the number of species missed by the sampling (Chao et al. Reference CHAO, CHAZDON, COLWELL and TSUNG-JEN2005). ACE (based on species abundance) and ICE (based on species incidence) estimators were obtained, as they have been proved to be effective for plant communities (Magurran Reference MAGURRAN2004). We used Fishers's α, which is insensitive to sample size (Magurran Reference MAGURRAN2004), to quantify species diversity. Because of the small sample size for several morphospecies in most sites, it was not possible to calculate species diversity at the growth-form level.
One-way analyses of variance (ANOVA) were used to assess differences in seed density, species density and species diversity among the four successional categories. To fulfil the parametric criteria of ANOVA, the count response variables (seed density and species density) were log(x+1)-transformed. Bonferroni multiple comparison tests were used a posteriori to detect significant (P ≤ 0.05) differences. Additionally, by lumping together sites of the same successional category, we constructed rank-abundance curves per successional category by plotting the seed density (seeds per m2) of each species (logarithmic scale) as a function of species abundance rank (Magurran Reference MAGURRAN2004). Log-log (potential) regression models, which approximate the Zipf–Mandelbrot model (Izák Reference IZÁK2006), were fitted to each rank-abundance curve to calculate the ordinate (an estimator of species dominance) and the slope of the curve. An analysis of covariance (ANCOVA) was applied to test differences in community evenness (slope of the rank-abundance curve) among successional categories.
To assess the hypothesis of replacement of seeds from plants with different growth forms during succession, graphs of the percentage of total seeds represented by different growth forms as a function of successional category were constructed. To assess possible mechanisms of species replacement during succession (sensu Connell & Slatyer Reference CONNELL and SLATYER1977), we did the following analyses. First, a Bray–Curtis similarity matrix (Austin Reference AUSTIN2005) was constructed considering values of seed density per species at each of the 12 studied sites. Second, a non-metric multi-dimensional scaling analysis (NMDS, Primer v.5) was used to ordinate the sites based on the Bray–Curtis similarity matrix. Multivariate analysis of variance (MANOVA) and a posteriori Bonferroni tests were performed to assess significant differences among successional categories over the ordination dimensions generated by the NMDS analysis. Finally, for each one of the 17 most-abundant species (with at least 15 seeds in all studied sites), we constructed graphs of the relative abundance of seeds as a function of successional category. The relative abundance of species was calculated by dividing the number of seeds of species i recorded in the three sites of a given successional category by the total number seeds of that species recorded in all studied sites. In the same way, to explore whether seed bank mirrored the abundance of mature plants, we calculated relative abundance of stems (diameter at breast height, dbh ≥ 1 cm) per successional category for the most abundant woody species recorded in the seed banks, based on data obtained in the same plots of our chronosequence (P. Balvanera, unpubl. data).
RESULTS
Overall, 2941 seeds in a total sampled area of 1.88 m2 were recorded, corresponding to a mean density of 1560 seeds m−2 across all studied sites. In total, 102 unequivocal morphospecies were recorded (Appendix 1) from which 52% were identified to species, 23% to genus and 25% to family. Only 8.3% of total seeds were not taxonomically identified. From the identified taxa, 12 were trees (23.1%), five shrubs (9.6%), two woody climbers (3.8%), 14 herbaceous climbers (27%), 10 terrestrial herbs (19.2%) and six grasses (11.5%); three taxa (5.8%), identified at the family level, could not be associated to any growth form. Hereafter, we will refer to morphospecies as species.
Successional patterns and replacement among plant growth forms
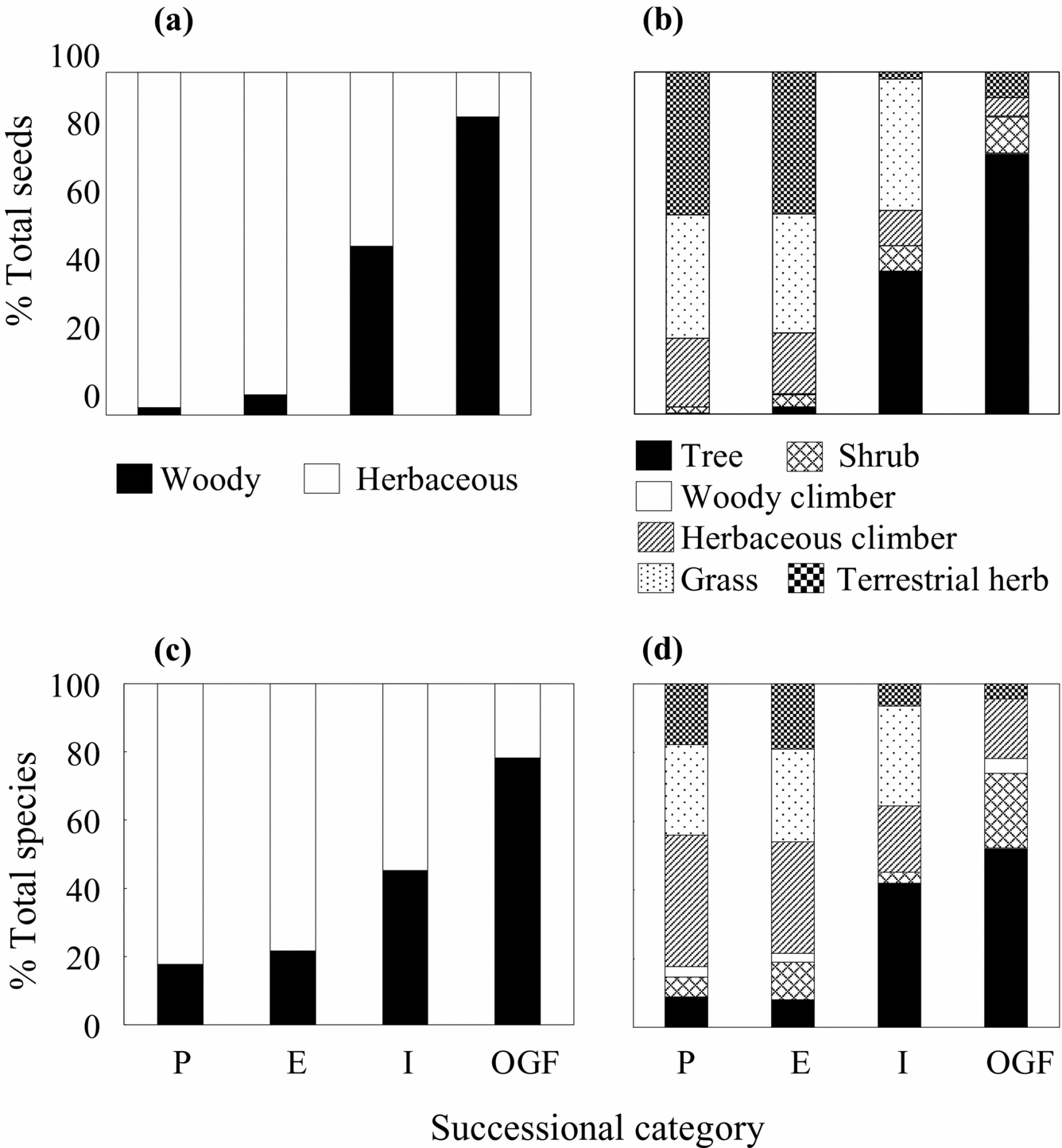
Seed-bank density. Overall, seed density decreased from the younger to older successional categories (F 3,8 = 4.1, P = 0.05; Figure 2a). However, this trend differed depending on plant growth form (Table 1). While seed density of herbaceous plants decreased (F3,8 = 4.84, P = 0.03), that of woody plants did tend to increase, although this trend was not significant (F3,8 = 2.9, P = 0.09). These patterns were mostly due to reductions in seed density of terrestrial herbs (F3,8 = 7.24, P = 0.01) and grasses (F3,8 = 5.57, P = 0.02), and an increase in the seed density of trees (F3,8 = 10.4, P = 0.004). Clearly, in proportional terms, seeds of herbaceous plants (mostly terrestrial herbs and grasses) were replaced by those of woody plants (mostly trees; Figure 3a, b).

Figure 2. Seed-bank structural changes across four tropical dry-forest successional categories (as indicated in Figure 1) at La Huerta (Jalisco), eastern Mexico. Seed density (a), Species density (b), Fisher's α species diversity (c). Vertical lines indicate ±1 SE. Successional categories that do not share same letters are statistically different (P ≤ 0.05).
Table 1. Seed-bank changes in density, species density and species diversity over a chronosequence of abandoned pastures and old-growth-forest (OGF) sites at Chamela, Mexico. Pasture: 0–1 y since abandonment, Early: 3–5 y since abandonment, Intermediate: 10–12 y since abandonment, OGF: old-growth forest. Values are mean ± 1 SE for different growth forms. Only seeds of species classified by growth forms are included. For each case, successional categories not sharing the same superscript letter differ significantly (P ≤ 0.05).

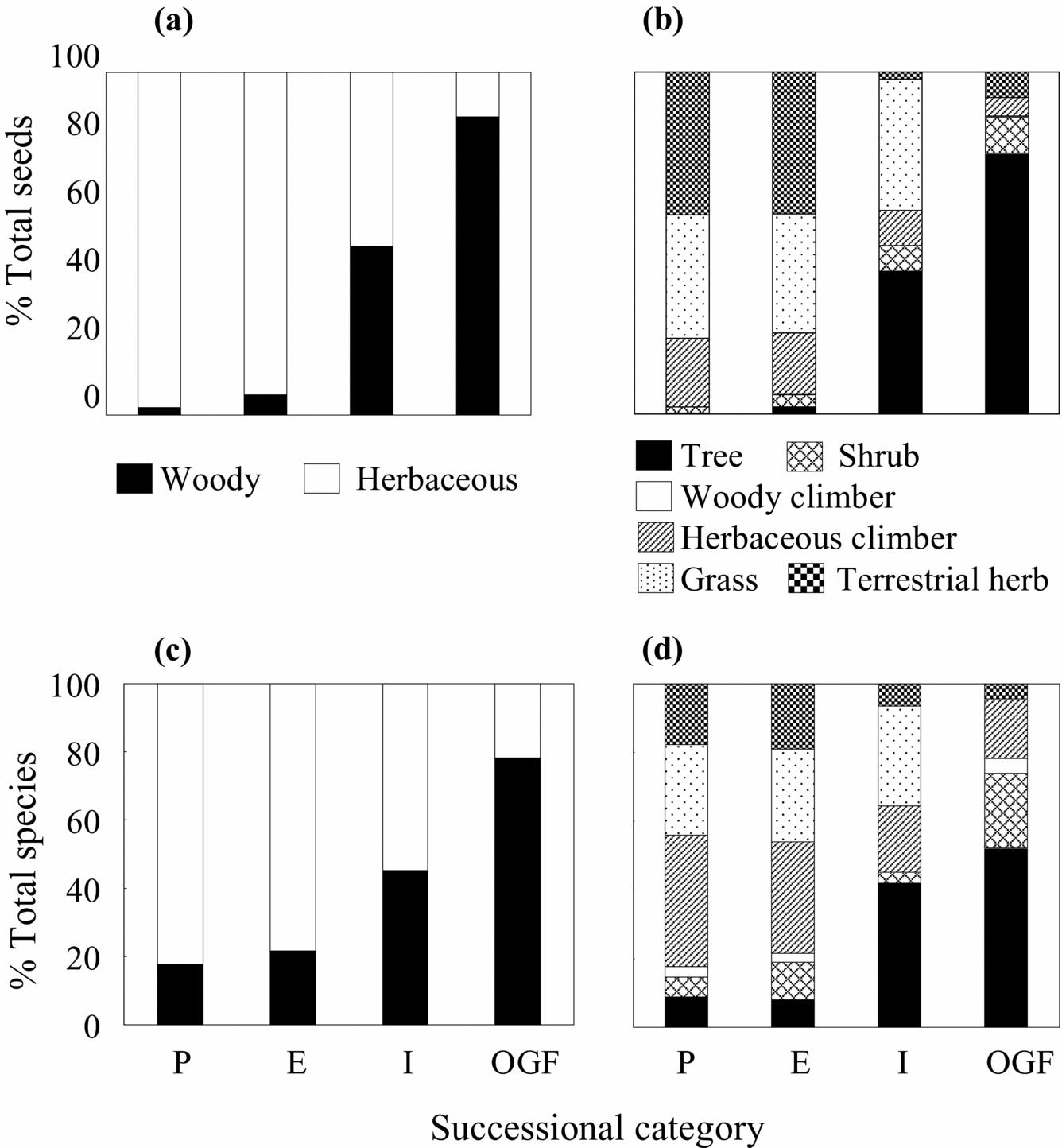
Figure 3. The relative contribution of different plant growth forms to the total number of seeds and species recorded in the seed-bank at four TDF successional categories (as indicated in Figure 1) at La Huerta (Jalisco), eastern Mexico. Proportion of total seeds represented by woody (trees, shrubs and woody climbers) and herbaceous (terrestrial herbs, herbaceous climbers and grasses) plants (a); proportion of total seeds represented by different growth-forms (b); proportion of total species density represented by woody and herbaceous plants (c); proportion of total number of species represented by different growth-forms (d).
Species density. Overall, species density did not vary among successional categories (Figure 2b). On average (±SE), 18 ± 2 species per 0.16 m2 per site were recorded. The expected species density calculated using non-parametric estimators did not vary among successional categories either. These estimators indicated the existence of twice as many species per site (ACE = 43 ± 14, ICE = 41 ± 7), when compared with our sampling effort. Species density, however, changed over the chronosequence depending on growth-form categories (Table 1). Species density of herbaceous plants declined (F 3,8 = 7.2, P = 0.01), but increased for tree species (F 3,8 = 4.6, P = 0.04). Species density of shrubs and woody climbers remained similar over the chronosequence. The relative frequency of herbaceous species was clearly replaced by that of woody species (mainly trees) over the chronosequence (Figure 3c, d).
Species diversity. For the whole seed-bank community (F 3,8 = 0.67, P = 0.59; Figure 2c), as well as for herbaceous and woody plants separately, Fisher's α diversity index exhibited a maximum in the intermediate successional category, but this peak was not significant (Table 1). The slope of the rank-abundance curve, however, differed significantly among successional categories (ANCOVA successional category × species rank-abundance interaction: F 3,153 = 61.8, P < 0.001), showing a diversity maximum in the intermediate category (Figure 4). In the pasture category, the slope of the rank-abundance curve was very pronounced (b = −2.12) with the three most abundant species representing 83% of all seeds. In the early category, the slope was less pronounced (b = −1.68), and percentage of three most abundant species reduced to 67%. The intermediate category showed the highest evenness (flattest slope, b = −1.12) and lowest percentage of three most abundant species (48%). In the old-growth forest, the slope (b = −1.57) was steeper when compared with the intermediate category, and three species represented 76% of total seeds. While in the pasture and early successional categories the dominant species were herbaceous, in the intermediate and old-growth forest woody species were dominant (Figure 4). The second most abundant species in the old-growth forest was a herbaceous Amaranthaceae species which was not found in earlier successional categories.

Figure 4. Rank-abundance curves for seed-bank communities of four TDF successional categories at Chamela, Mexico. Successional categories as indicate in Figure 1. White circles correspond to herbaceous terrestrial, white squares to grass, white triangles to herbaceous climber, black circles to tree, black squares to shrub and black triangles to woody climber species. Species acronyms for the six most abundant species at each successional category as follows: Amart = Amaranthaceae, Anlep = Antigonon cf. leptopus, Coele = Cordia elaeagnoides, Clvis = Cleome viscosa, Crsub = Croton suberosus, Desp = Desmodium sp., Eupho = Euphorbiaceae, Hepal = Heliocarpus pallidus, Hysua = Hyptis suaveolens, Kasp = Kallstroemia sp., Micua = Mimosa quadrivalvis, Miare = Mimosa arenosa, Pani1 = Panicum sp. 1, Pani2 = Panicum sp. 2, Phsp = Phaseolus sp. 1, Picon = Piptadenia constricta, Poace1 = Poaceae sp. 1, Poace2 = Poaceae sp. 2. Note y-axis is scaled logarithmically.
Species replacement over the chronosequence
NMDS minimized stress (0.05) with three dimensions in the ordination of the sites. Successional categories differed only along dimension-1, following a sequence of younger to older categories (F3,8 = 22.1, P = 0.0003; Figure 5). Pasture differed significantly (P < 0.01) from the intermediate and old-growth forest categories; the early category was not different from pasture but differed from intermediate and old-growth forest categories. The intermediate category was different from the old-growth forest. Groups of species showed maximum relative densities at different successional categories. Eight species (seven herbaceous and one shrub) had their greatest relative abundance in the pasture and early categories (Figure 6a-h), four herbaceous species in the early category (Figure 6i-l), two tree species in the intermediate category (Figure 6m, n), and three (two woody and one herbaceous) species in the old-growth forest categories (Figure 6o-q).

Figure 5. NMDS ordination of abandoned pasture and old-growth TDF sites from Chamela, Mexico. Sites are designated by successional category as indicated in Figure 1. Dashed ellipses include sites of same successional category. See text for further details.

Figure 6. Chronosequence changes in the relative density of the 17 most abundant species in the seed-bank of abandoned pastures and old-growth TDF sites at the Municipality of La Huerta, western Mexico. Successional categories as indicated in Figure 1. After the species name, in parentheses the species growth-form is indicated: T = tree, S = shrub, TH = terrestrial herb, HC = herbaceous climber and G = grass. In (a), (m), (n), (o) and (q) lines connected by open circles indicates changes among successional categories of the relative abundance of stems with dbh ≥1 cm.
Seed bank mirrored the abundance of established individuals in four of five woody species analysed (Figure 6m, n, o, q). The exception was the shrub Mimosa arenosa, which seeds were only found in the pasture category while its stem abundance was highest in the early successional category (Figure 6a).
DISCUSSION
The fact that a third of the total recorded species in our chronosequence were represented by a single seed (singletons), concurs with the finding that rare species are typical of seed banks in tropical forests (Garwood Reference GARWOOD, Leck, Parker and Simpson1989) and suggests that our sampling effort missed even more rare species. According to ACE and ICE estimators, our sampling effort potentially overlooked approximately half of the possible extant species. The aggregated pattern of spatial distribution of the seeds, common in TDFs (Condit et al. Reference CONDIT, ASHTON, BAKER, BUNYAVEJCHEWIN, GUNATILLEKE, GUNATILLEKE, HUBBELL, FOSTER, ITOH, LAFRANKIE, LEE, LOSOS, MANOKARAN, SUKUMAR and YAMAKURA2000, Hubbell Reference HUBBELL1979), could also contribute to increasing the probability of missing species with our random sampling design (Butler & Chazdon Reference BUTLER and CHAZDON1998). Despite these sampling limitations, we were able to detect clear successional patterns. These patterns support the hypothesis that herbaceous plants are replaced by woody plants during succession and suggest that a facilitation mechanism drives species replacement in the studied TDF successional system, as discussed below.
Successional patterns and replacement among plant growth forms
Seed bank density. Reduction in the seed bank density along the chronosequence parallels successional trends described in TDFs (González-Rivas et al. Reference GONZÁLEZ-RIVAS, TIGABU, CASTRO-MARÍN and ODÉN2009, Rico-Gray & García-Franco Reference RICO-GRAY and GARCÍA-FRANCO1992) and temperate deciduous forests (Hyatt & Casper Reference HYATT and CASPER2000, Roberts & Vankat Reference ROBERTS and VANKAT1991). For example, in north Yucatan, Mexico, the density of the seed bank in a cornfield 1 y after abandonment was five times greater than in cornfields with fallow ages of more than 30 y (Rico-Gray & García-Franco Reference RICO-GRAY and GARCÍA-FRANCO1992). In Nicaragua, the seed bank density in a 4-y-old secondary TDF was doubled when compared to a 14-y-old secondary forest (González-Rivas et al. Reference GONZÁLEZ-RIVAS, TIGABU, CASTRO-MARÍN and ODÉN2009).
As predicted, we observed a progressive replacement of seeds of herbaceous plants by those of woody plants over the chronosequence. This pattern is concurrent with the general successional patterns observed in the secondary succession of forest communities (Bazzaz Reference BAZZAZ1996, Bekker et al. Reference BEKKER, VERWEIJ, BAKKER and FRESCO2001, Lemenih & Teketay Reference LEMENIH and TEKETAY2006, Lyaruu et al. Reference LYARUU, ELIAPENDA and BACKÉUS2000, Roberts & Vankat Reference ROBERTS and VANKAT1991). The reduction of reproductive populations of heliophilic forbs may explain the rapid decrease of seeds of herbaceous plants over the chronosequence. As succession advances the forest canopy closes, reducing light resources in the understorey. This effect is particularly marked in the rainy season, when TDF leaf area index maximizes (Barradas Reference BARRADAS1991). In this season, forest canopy openness at 1.30 m above ground at our intermediate successional sites was similar to that in the old-growth-forest sites (15% vs 12%, S. Maza-Villalobos, pers. obs.).
Despite there being a clear increasing trend in the representation of seeds of woody plants over the chronosequence, seeds of grasses were still abundant in the 10–12-y-old secondary forest sites. Long-lasting seed dormancy (Baskin & Baskin Reference BASKIN and BASKIN1985), together with seed dispersal from nearby active pastures (Dupuy & Chazdon Reference DUPUY and CHAZDON1998), may explain such prevalence. Because such sites were mostly surrounded by secondary or old-growth forest vegetation, we believe that seed dormancy was the main determinant. Thus, our results indicate that a legacy of the agricultural activity, expressed in the high proportion of seeds of exotic species of grass (e.g. Panicum sp., Rhynchelytrum repens), persist for several years after field abandonment.
The increase of seeds from woody species over the chronosequence could result from an increase in the abundance of reproductive individuals (i.e. increasing local seed rain) and of seeds dispersed from nearby sources. The fact that abundance of seeds from common woody species paralleled that of stems of the same species over the chronosequence supports the former possibility. After some years of succession, the developing forest canopy can provide food, shelter, perching and nesting sites for seed-dispersing mammals and birds (Holl Reference HOLL1999, Janzen Reference JANZEN1988). At the same studied chronosequence, abundance of frugivorous bats (Avila-Cabadilla et al. Reference AVILA-CABADILLA, STONER, HENRY and ALVAREZ-AÑORVE2009) and birds (J. Schondube, pers. comm.) increased substantially in the pastures of more than 5 y since abandonment, which concurs with the evident increase in the abundance and species diversity in the seed bank of trees in the 10–12 y old secondary forests. Also, several TDF woody species are widely dispersed by wind (Bullock Reference BULLOCK, Bullock, Mooney and Medina1995, Gentry Reference GENTRY, Bullock, Mooney and Medina1995, Greene et al. Reference GREENE, QUESADA and Calogeropoulos2008). The fact that at early successional sites there were seeds but not stems of the wind-dispersed species C. suberosus (Figure 6o) support that exogenous seed dispersal is important for some species.
The dominance of seeds of woody plants in the old-growth forest was largely related to small seeds produced by a few species that fruit copiously during the dry season (e.g. H. pallidus and C. elaeagnoides) and to seeds of species that may remain dormant in the soil due to an impermeable seed coat (e.g. the legumes Acacia farnesiana, Caesalpinia sp., Senna atomaria). Large seeds were not found in the seed bank. This might be because large-seeded species produce fruits and disperse during the rainy season (Bullock & Solís-Magallanes Reference BULLOCK and SOLÍS-MAGALLANES1990, Garwood Reference GARWOOD, Leck, Parker and Simpson1989) when seed predation and germination occur (Janzen Reference JANZEN1981, Khurana & Singh Reference KHURANA and SINGH2001).
Species density. The uniform pattern shown by species density over the chronosequence is similar to that found in a successional chronosequence of burned sites (up to 26 y after abandonment) of Mediterranean vegetation in Australia (Wills & Read Reference WILLS and READ2007). In our case, this pattern resulted from a combination of the reduction of herbaceous and grass species and the increase of woody species over the chronosequence, as occurred in seed banks of secondary TDF in Nicaragua (González-Rivas et al. Reference GONZÁLEZ-RIVAS, TIGABU, CASTRO-MARÍN and ODÉN2009). Such replacement of species with different growth forms is consistent with the tendencies of secondary succession observed in different terrestrial plant systems (Lyaruu et al. Reference LYARUU, ELIAPENDA and BACKÉUS2000, Roberts & Vankat Reference ROBERTS and VANKAT1991).
Species diversity. The hump-like successional pattern in species diversity in the seed bank was parallel to the one observed in several tropical and temperate forest successional systems, where species diversity increases to an upper value to later decrease with successional time (Bazzaz Reference BAZZAZ1975, Bekker et al. Reference BEKKER, VERWEIJ, BAKKER and FRESCO2001). The peak of species diversity observed in the intermediate successional category resulted from a mixture of seeds from herbaceous species, which were dominant in pasture and early successional sites and seeds of woody species, which were abundant in the seed bank of old-growth forest. Furthermore, the highest number of rare species (with one or two seeds) was recorded at the intermediate successional category.
Species replacement and successional mechanisms
In our study system, we did not find any herbaceous or woody species present over all successional categories. This result suggests that species are not able to establish along the whole environmental gradient sampled by our chronosequence. The clear parallel between the abundance of seeds and stems of woody species over the chronosequence strongly supports this idea and concurs with the observation that seed banks of woody species are mostly determined by local seed rain (Ceccon et al. Reference CECCON, HUANTE and RINCÓN2006, Dalling & Denslow Reference DALLING and DENSLOW1998). This parallelism enables us to explore mechanisms of species replacement during succession (sensu Connell & Slatyer Reference CONNELL and SLATYER1977).
Our results do not lend support to the existence of a tolerance mechanism of replacement of species. We did not find in the recently abandoned pastures seeds of an initial pool of species from which some species disappear and other remain over the chronosequence. In fact, our NMDS analysis indicates that species found in the younger successional categories were totally different from those found in later successional ones. Our results do not support the existence of a successional inhibition mechanism because there was not a single dominant species in the seed bank of earlier successional categories and because species density did not change over the chronosequence. Instead, our results show the existence of groups of species that have their maximum regenerative potential (via soil seeds) at different successional stages. Such segregation of species over succession therefore suggests the existence of a mechanism of facilitation in which stages of colonization, development, facilitation and replacement dictate the change in the structure and species composition in the successional community. In our chronosequence, the pioneer, colonizing, group would include short-lived forbs like Cleome viscosa, Kallstroemia sp., Desmodium sp. and grass species, as well as the shrub M. arenosa. In the seed bank of intermediate sites, most of these species were replaced by woody species like C. elaeagnoides, C. suberosus, Piptadenia constricta and H. pallidus. These species were also abundant in the old-growth forest.
In young TDF secondary vegetation in Mexico it is common to observe abundant populations of Mimosa species. It has been proposed that once established these shrub species inhibit secondary succession in old fields (Burgos & Maass Reference BURGOS and MAASS2004). However, in our chronosequence the seeds of M. arenosa were found only in the recently abandoned pastures, nonetheless abundance of stems of this species showed a maximum in the early successional category. This suggests that potential for regeneration of this species beyond early successional stages is quite poor. Recent studies indicate that Mimosa species could play a facilitating, instead of an inhibitory, role in succession (Lebrija-Trejos et al. Reference LEBRIJA-TREJOS, BONGERS, PÉREZ-GARCÍA and MEAVE2008). These shrubs are associated with nitrogen-fixing bacteria and their foliage can create favourable shady conditions which facilitate establishment and development of seedlings of tree species (Khurana & Singh Reference KHURANA and SINGH2001). Abundant and diverse communities of seedlings and saplings of woody species have been observed under the canopies of Mimosa in TDFs dominated by these shrubs (Romero-Duque et al. Reference ROMERO-DUQUE, JARAMILLO and PÉREZ-JIMÉNEZ2007). Furthermore, in the studied chronosequence, established populations of M. arenosa dominate in the 3–5-y-old secondary forest but its abundance diminishes remarkably in the intermediate successional category and was practically absent in the old-growth forest. Thus, M. arenosa in our system could be a facilitator more than an inhibitor species, supporting the idea that a facilitation mechanism operates during the studied old-field TDF succession.
CONCLUSION
Our chronosequence study indicates that the structure and composition of seed banks strongly change over the first 12 y of TDF succession in abandoned pastures. A critical breakpoint did occur at 10–12 y after pasture abandonment, when seeds of early forb and grass species were replaced in abundance and diversity by those of woody species. At this stage, diverse seeds of tree species were found, but the structure and composition of the seed bank was still distinguishable from that in the old-growth forest. A clear replacement of groups of seed species was observed over the chronosequence, suggesting that facilitation is a possible successional mechanism in our studied system. Future experimental and long-term studies are needed to test the chronosequence trends here documented, and assess the importance of such trends for the TDF regeneration in old fields.
ACKNOWLEDGEMENTS
Authors thanks to the Estación de Biología Chamela (Instituto de Biología, UNAM) for all facilities provided. Eloy Cisneros, Mariana Álvarez-Añorve, Luis Daniel Ávila-Cabadilla and Fernando Pineda-García helped us with field work. Patricia Balvanera kindly provided the data on stem abundance of woody species. Alfredo Pérez-Jimenez and Guillermo Ibarra-Manríquez assisted us with taxonomic identification. Ximena García, Eduardo Mendoza and two anonymous reviewers made comments that substantially improved the manuscript. This paper constitutes a partial fulfilment of the Graduate Program in Biological Science of the National Autonomous University of Mexico. Susana Maza-Villalobos-Méndez acknowledges CONACYT-Mexico and DGEP-UNAM for PhD fellowship grants. This study was supported by Grant SEMARNAT-CONACYT 2002-C01-0597 and SEP-CONACYT CB-2005-01-51043.
Appendix 1. Species recorded at seed-bank communities in a chronosequence of abandoned pastures and old-growth tropical dry forest sites at Chamela, Mexico. Where applicable, growth-form (GF; T: tree, S: shrub, WC: woody climber, TH: terrestrial herb, HC: herbaceous climber, U: unknown) and dispersal syndrome (DS; A = animal, both epizoochory and endozoochory, W = wind, G = gravity and U = unknown) are indicated. Unidentified species (morphospecies) are indicated by the prefix MSP. Successional categories: P = ‘pasture’ (0–1 y since abandonment), E = ‘early’ (3–5 y since abandonment), I = ‘intermediate’ (10–12 y since abandonment), OGF = old-growth forest. For each successional category there were three sites. Figures indicate the number of seeds recorded per species per 1.88 m2 at each site, per species in all sites (last column) and for all species at each site (last row).