INTRODUCTION
Sessile bacteria, protists, macroalgae, and invertebrates are widespread in the marine environment, especially on hard bottoms, where they colonize all substrates. Epibiosis is a direct consequence of surface limitation and results in spatially close associations between two or more living organisms belonging either to the same or to different species. These associations can be specifically guided by host chemistry, resulting in species-specific symbiotic or pathogenic assemblages (Harder, Reference Harder2008).
Several hydroid species have an epibiontic lifestyle, living associated with organisms of many phyla including Porifera, Cnidaria, Bryozoa, Mollusca and Chordata (Boero & Bouillon, Reference Boero, Bouillon and Rhode2005). The association of micro- with macro-organisms is a widespread phenomenon with profound impact on the physiology, ecology and evolution of both hosts and associated partners, but reports of interactions of microorganisms with Hydrozoa are scarce (Stabili et al., Reference Stabili, Gravili, Piraino, Boero and Alifano2006, Reference Stabili, Gravili, Tredici, Piraino, Talà, Boero and Alifano2008; Bavestrello et al., Reference Bavestrello, Cerrano, Di Camillo, Puce, Romagnoli, Tazioli and Totti2008). In particular, Bavestrello et al. (Reference Bavestrello, Cerrano, Di Camillo, Puce, Romagnoli, Tazioli and Totti2008) showed that, among protists epibiontic on marine hydroids, diatoms are the most abundant and diversified group, followed by foraminifera and sessile ciliata such as Vorticella and suctorians. Regarding the spatial distribution of epibionts, hydroid colonies represent a mosaic of different microhabitats, depending on their features as settling surfaces. A host specificity has also been observed: some epibionts are typical of only one or a group of species, such as Vorticella living on the teeth of Aglaophenia thecae (Bavestrello et al., Reference Bavestrello, Cerrano, Di Camillo, Puce, Romagnoli, Tazioli and Totti2008) or coralline algae that cover mainly Aglaophenia and Sertularella colonies (Di Camillo et al., Reference Di Camillo, Puce, Romagnoli, Tazioli, Totti and Bavestrello2006). Stabili et al. (Reference Stabili, Gravili, Piraino, Boero and Alifano2006) described a previously unknown association between Vibrio sp. AO1, a luminous bacterium related to the species V. harveyi, and the benthic hydrozoan Aglaophenia octodonta. Scanning electron microscopy analysis and culture-based and culture-independent approaches led to establish that luminous vibrios represent major constituents of the bacterial community inhabiting the A. octodonta surface, suggesting that the interaction between Vibrio sp. AO1 and the examined hydrozoan species is highly specific and that this could be explained by the feeding activity of this microorganism on the hydroid chitinous structures. The observed association supports the original hypothesis of Hood & Meyers (Reference Hood and Meyers1977) that a primary role of vibrios could be the colonization and initiation of degradation of chitinous material in aquatic ecosystems.
In the present study several Hydrozoa species were collected along the cost of the northern Ionian Sea to ascertain whether benthic hydroids are, as a group, a favourable microhabitat for both proliferation and persistence of luminous bacteria in marine biota. Luminous bacteria were searched for in twenty species of both thecatae and athecatae hydrozoans, characterized by the presence of chitinous structures in different portions of the colony. Thecate hydroids (subclass Leptomedusae) are covered by an outer rigid tubular skeleton of species-specific shape, the perisarc, composed of a layer of polysaccharides, including chitin, which overlies lamellae of quinone-tanned proteins (Knight, Reference Knight1968, Reference Knight1970a, Reference Knightb; Chapman, Reference Chapman, Muscatine and Lenhoff1974). By contrast, athecatae hydranths (subclass Anthomedusae), are usually never surrounded by perisarc, thus lacking a proper hydrotheca, but the rest of the colony, just as in thecates, is usually wrapped by a rigid structure, whose chitinization, thickening and hardening increase with age. The selected species are commonly found at shallow depths on the rocky coasts of the Mediterranean Sea (Riedl, Reference Riedl1970; Bouillon et al., Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004).
MATERIALS AND METHODS
Sampling
Batches of colonies of 20 hydrozoan species were collected by SCUBA diving (direct picking) during six surveys carried out along the Ionian coast of Apulia, Italy: Torre del Serpe (40.140696N 18.508229E) and Santa Caterina (40.141517N 17.98177E) (Figure 1) at 0–15 m of depth from February to March 2009. They were transported in the laboratory under controlled temperature, conserved in a thermostatic chamber, and processed for the isolation of luminous bacteria within 4 hours from collection.

Fig. 1. Map showing the sampling stations in the northern Ionian Sea off the coast of Otranto, Lecce (Italy): (A) Torre del Serpe; (B) Santa Caterina.
Taxonomic identification
Hydroids were examined and photographed both alive and mounted on slides by stereo and light microscopes and were identified by using recent literature (Svoboda, Reference Svoboda1979; Svoboda & Cornelius, Reference Svoboda and Cornelius1991; Bouillon et al., Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004, Reference Bouillon, Gravili, Pagés, Gili and Boero2006).
Microscope observation of hydroids and microalgae
Hydroids were also mounted on slides for epifluorescence microscope observations and photographed. Hydrozoan colonies were observed using a Zeiss Standard Axioplan microscope equipped with a halogen lamp (Hg 100) light. Blue light excitation with a BP 485/20 excitation filter, a FT 510 chromatic beam splitter and a LP 520 barrier filter were used to observe slides. The presence of both luminous bacteria and microalgae was detected.
Quantitative analysis of bacteria living on hydroids
For each examined species, five groups of colonies (~1 g), were gently washed in sterile seawater (0.2 µm pore filtered) to promote the detachment of epibiotic bacteria. The colonies were then suspended in sterile seawater and sonicated three times (Branson Sonifier 2200, 60 W, 47 kHz for 1 minute in an ice bath) to further optimize the detachment of surface bacteria. Sonication was interrupted for 30 seconds every minute, when samples were shaken manually (Danovaro et al., Reference Danovaro, Manini and Dell'Anno2002). One or 5 ml of each sonicated sample, and appropriate decimal dilutions, were plated onto Marine Agar 2216 (Beckton Dickinson and Company) and, after incubation for 2 days at 22°C, the total culturable bacteria, including luminous ones, were counted according to the colony-forming units (CFU) method. After the incubating period, luminous bacterial colonies were detected in a dark room by emission of visible light and counted.
RESULTS
Twenty hydroid species, referred to 16 genera and 10 families (Table 1) were collected.
Table 1. Taxonomic identification of the selected species.

Specimens of thecatae hydroids, namely Aglaophenia kirchenpaueri (Figure 2A), Aglaophenia octodonta (Figure 2C), Aglaophenia tubiformis (Figure 3A), Halopteris diaphana (Figure 3C), Plumularia setacea (Figure 4A) and Ventromma halecioides (Figure 4C), under blue light excitation, showed a strong green fluorescence on the external side of the perisarc (chitinous exoskeleton) around hydrocladia. In particular, fluorescence was concentrated in the folds along the hydrocaulus and at the base of the hydrothecae (Figures 2B, D, 3B, D & 4B, D).
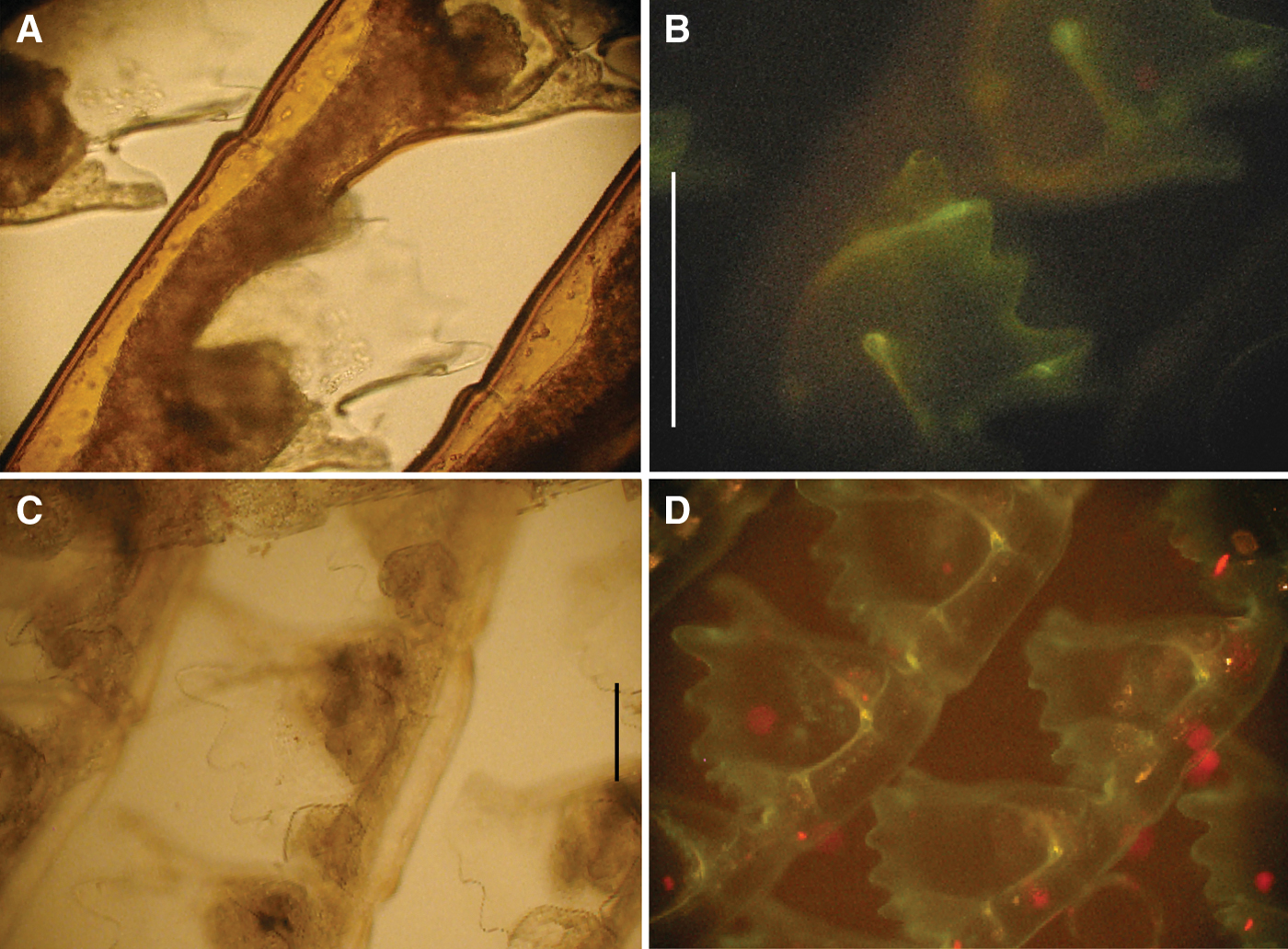
Fig. 2. Aglaophenia kirchenpaueri photomicrographs, living material: (A) hydrothecae at transmitted light; (B) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Aglaophenia octodonta photomicrographs, living material: (C) hydrothecae at transmitted light; (D) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Scale bars: (A & B) 500 µm; (C & D) 100 µm.

Fig. 3. Aglaophenia tubiformis photomicrographs, living material: (A) hydrothecae at transmitted light; (B) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Halopteris diaphana photomicrographs, living material: (C) hydrothecae at transmitted light; (D) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Scale bars: (A & B) 200 µm; (C & D) 250 µm.

Fig. 4. Plumularia setacea photomicrographs, living material: (A) hydrothecae at transmitted light; (B) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Ventromma halecioides photomicrographs, living material: (C) hydrothecae at transmitted light; (D) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Scale bars: (A & B) 100 µm; (C) 500 µm; (D) 250 µm.
Five examined Hydrozoa species, namely the athecate Amphinema dinema, and the thecates Antennella siliquosa, Dynamena disticha, Sertularella ellisii and Sertularia perpusilla (Figures 5A, C, E & 6A, C), showed a medium fluorescence on the external side of their perisarc (Figures 5B, D, F & 6B, D). In the athecatae A. dinema fluorescence was localized mainly on the hydrorhiza.

Fig. 5. Amphinema dinema photomicrographs, living material: (A) hydroid at transmitted light; (B) hydrorhiza at epifluorescence (green, luminous bacteria; red, microalgae). Antenella siliquosa photomicrographs, living material: (C) hydrothecae at transmitted light; (D) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Dynamena disticha photomicrographs, living material: (E) hydrothecae at transmitted light; (F) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Scale bars: (A) 500 µm; (B) 100 µm; (C & D) 300 µm; (E & F) 250 µm.
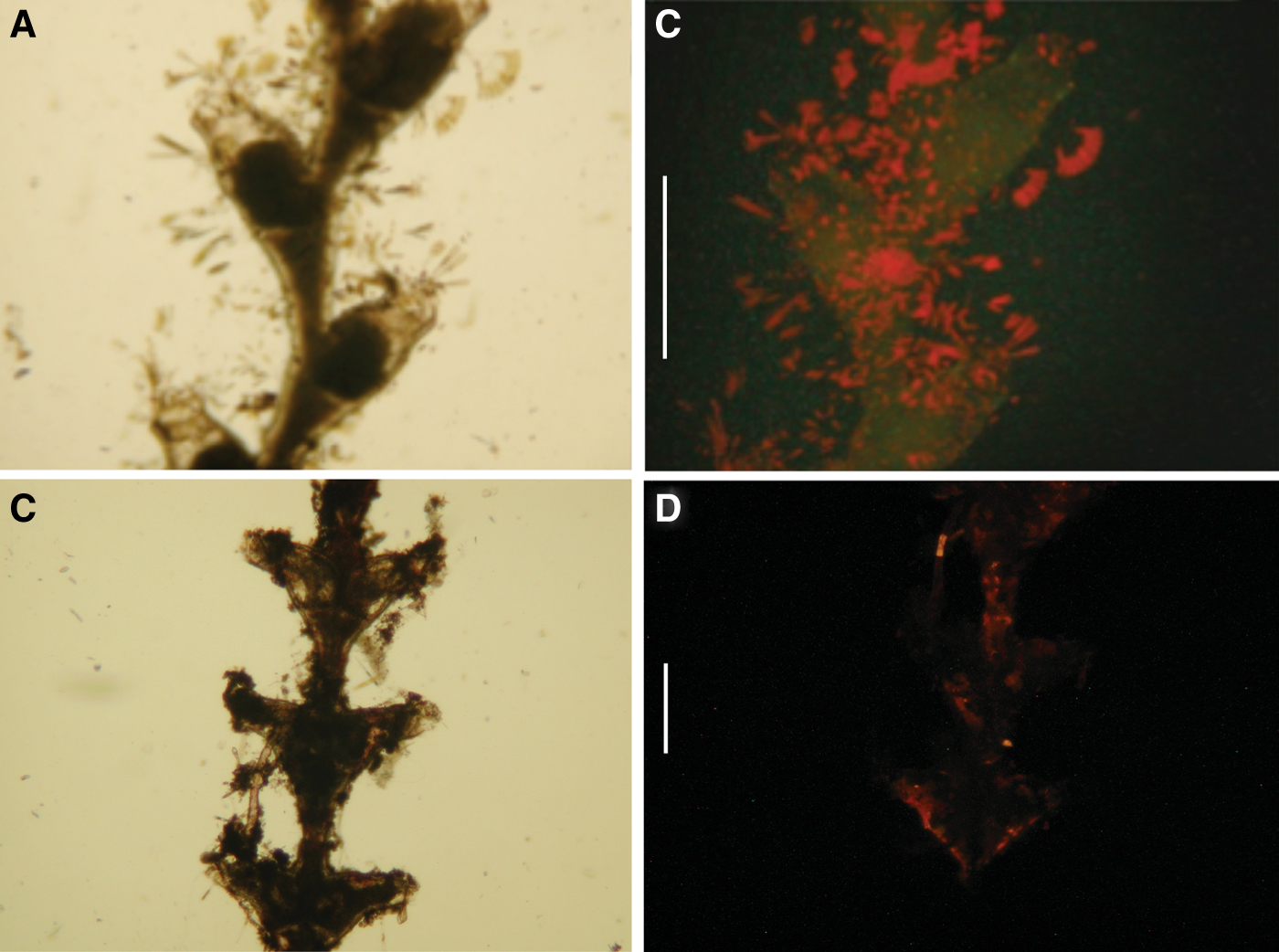
Fig. 6. Sertularella ellisii photomicrographs, living material: (A) hydrothecae at transmitted light; (B) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Sertularia perpusilla photomicrographs, living material: (C) hydrothecae at transmitted light; (D) hydrothecae at epifluorescence (green, luminous bacteria; red, microalgae). Scale bars: (A & B) 1 mm; (C & D) 500 µm.
Four Hydrozoa species, namely the thecates Clytia hummelincki, Clytia linearis and Hydranthea margarica, and the athecate Coryne muscoides, showed a slight fluorescence. In C. muscoides fluorescence was localized on the hydrorhiza as well as in the perisarc enveloping hydrocladia and hydrocaulus.
Microalgae were always recovered together with the luminous bacteria in the strongly, medium and slightly fluorescent hydroids.
Five species, namely the thecates Campanularia hincksi, Clytia hemisphaerica, Monotheca obliqua and Obelia dichotoma, and the athecate Eudendrium capillare, did not show fluorescence and luminous bacteria were absent on their surface.
The hypothesis that fluorescence on chitinous structures was due to luminous bacteria was tested by cultural analysis using Marine Agar 2216 (Figure 7). Tests demonstrated that luminous bacteria represented a conspicuous component (about 20%) of the total culturable surface bacteria in all the fluorescent hydroid species. Furthermore, concentrations of total and luminous bacteria differed significantly (P < 0.05) among the strongly, medium and slight fluorescent hydroids (Table 2). In particular, in the strongly fluorescent species the mean value of total surface bacteria was 3.0 × 107 CFU/g whilst the mean density of luminous bacteria was 6.0 × 106 CFU/g. In the medium florescent hydroids the mean abundance of total surface bacteria was 1.1 × 107 CFU/g whilst the mean density of luminous bacteria accounted for 2.3 × 106 CFU/g. Finally, in the slight fluorescent species the mean densities of total surface and luminous bacteria were 5.0 × 106 CFU/g and 8.0 × 104 CFU/g, respectively.

Fig. 7. Epibiotic bacteria from one of the studied species. (a) Total culturable bacteria grown after incubation in Petri dish containing Marine Agar 2216; (b) luminous bacteria grown after incubation in Petri dish and detected in a dark room by emission of visible light.
Table 2. Viable count of total culturable and luminous bacteria isolated from the studied species.

CFU, colony forming units.
DISCUSSION
Despite their potentially important role in marine ecology, reports on associations between epibiotic bacteria and marine macroorganisms are scarce and often circumstantial. Available information on the interactions of luminous bacteria with the surfaces of marine invertebrates is limited (Ramesh & Venugopalan, Reference Ramesh and Venugopalan1984); moreover, little is known about their physiological characteristics. By contrast, a wide literature exists on the symbiotic colonization of Vibrio fischeri and the Hawaiian bobtail squid, Euprymna scolopes (Boettcher & Ruby, Reference Boettcher and Ruby1990; Ruby & Lee, Reference Ruby and Lee1998; DeLoney et al., Reference DeLoney, Bartley and Visick2002; McCann et al., Reference McCann, Stabb, Millikan and Ruby2003; Whistler & Ruby, Reference Whistler and Ruby2003): the squid houses the bacteria in a specialized light-emitting organ within the mantle cavity, and uses it during its nocturnal activities, probably to escape from predators (Visick & McFall-Ngai, Reference Visick and McFall-Ngai2000; McCann et al., Reference McCann, Stabb, Millikan and Ruby2003; Haddock et al., Reference Haddock, Moline and Case2010). The specificity of the association suggests that the specialized colonization mechanisms in the bacterial symbiont have coevolved with recognition mechanisms in the squid host (Visick & McFall-Ngai, Reference Visick and McFall-Ngai2000). By contrast, the molecular mechanism involved in the interaction between luminous bacteria and the examined hydrozoans is unknown. Luminous bacteria probably feed on the chitinous structures of the hydroids. Some luminous bacteria elaborate an extracellular chitinase but, to date, only few studies have investigated their association with chitin-producing organisms. A previous research reported that surface chitin-containing structures of the hydroid Aglaophenia octodonta (Stabili et al., Reference Stabili, Gravili, Piraino, Boero and Alifano2006) are heavily colonized by luminous bacteria belonging to the genus Vibrio. Moreover, a recent study by Gorelova et al. (Reference Gorelova, Baulina, Lobakova and Kosevich2010) demonstrated a feeding activity of bacteria on the surface of the hydrozoans Dynamena pumila and Gonothyrea loveni, showing micro-perforations within the perisarc containing the microrganisms, presumably due to chitin lysis. In the present study, the occurrence of luminous bacteria in the investigated hydrozoan species could be related to differences in the chitin localization as well as presence or absence of the perisarc. Hydroids with thick chitinous exoskeleton belonging to the Aglaopheniidae, Plumulariidae and Halopteriidae, showed strong fluorescence; by contrast, in Dynamena disticha, Sertularella ellisii, Sertularia perpusilla and Antennella siliquosa, with medium fluorescence, the perisarc is moderately thick and less developed than in the species exhibiting strong fluorescence. In the case of the athecate A. dinema a medium fluorescence is mainly observed in the chitinous hydrorhiza, whereas the hydrocauli are covered only by a thin perisarc. Finally, the species belonging to the families Campanulariidae, Coryniidae and Lovenellidae possess a thin chitinous envelope, and showed slight fluorescence. Campanularia hincksi, Clytia hemisphaerica, Eudendrium capillare, Monotheca obliqua and Obelia dichotoma, possess chitinous structures but, under epifluorescence microscopy, they exhibited neither green fluorescence due to luminous bacteria nor red fluorescence due to microalgae.
Microalgae were always recovered together with luminous bacteria in the strongly, medium and slightly fluorescent hydroids. Luminous bacteria, therefore, co-occurred with microalgae. Bacteria glow continuously, emitting light, when they reach sufficiently high concentrations to initiate quorum sensing (Waters & Bassler, Reference Waters and Bassler2005; Nealson & Hastings, Reference Nealson and Hastings2006). These specific properties make bacteria uniquely suitable as photogenic symbionts. Hence, their continuous luminescence might support microalgal photosynthesis. Further studies will need to be accomplished to test this hypothesis. Several studies undertaken on other marine invertebrates reported the coexistence of microalgae and bacteria. In particular, Bavestrello et al. (Reference Bavestrello, Cerrano, Cattaneo Vietti, Sarà, Piraino, Boero, Bouillon, Cornelius and Gili1996, Reference Bavestrello, Cerrano, Di Camillo, Puce, Romagnoli, Tazioli and Totti2008), Siqueiros-Beltrones et al. (Reference Siqueiros-Beltrones, Serviere-Zaragoza and Argumedo-Hernandez2001), Di Camillo et al. (Reference Di Camillo, Puce, Romagnoli, Tazioli, Totti and Bavestrello2005, Reference Di Camillo, Bo, Lavorato, Morigi, Reinach, Puce and Bavestrello2008) and Romagnoli et al. (Reference Romagnoli, Bavestrello, Cucchiari, De Stefano, Di Camillo, Pennesi, Puce and Totti2007) described epibiotic bacteria, diatoms, and foraminiferans from both Mediterranean and tropical hydroids. Moreover, Gorelova et al. (Reference Gorelova, Baulina, Lobakova and Kosevich2010) observed by electron microscopy that the epibiotic community of the hydroid perisarc of Dynamena pumila and Gonothyraea loveni consisted of different microalgae and bacteria. These findings suggest many interactions between the hydroids and epibiotic microorganisms.
Future researches will be conducted to characterize phenotypically and genotypically all the luminous bacteria isolated from the surface of the examined hydrozoans as well as to better understand whether the interaction observed is only related to chitin utilization. Furthermore, since some luminous bacteria are considered opportunistic pathogens (Maldonado et al., Reference Maldonado, Sánchez-Tocino and Navarro2010; Vezzulli et al., Reference Vezzulli, Previati, Pruzzo, Marchese, Bourne and Cerrano2010), as suggested by the name of the disease commonly referred to as luminous vibriosis, the observed associations between these bacteria and hydrozoans might have epidemiological implications. These hydrozoan species, indeed, are widely distributed in the Mediterranean Sea (Bouillon et al., Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004; Gravili et al., Reference Gravili, Boero, Licandro and Relini2008) and might constitute natural reservoirs of the pathogens. The pathogenic effects of luminous Vibrio species are critical also in aquaculture settings, where organisms are reared at high densities under artificial and unstable conditions. The studied hydrozoans might behave as a reservoir of antibiotic multiresistant bacteria if present in aquaculture farms taking into account the results obtained by Stabili et al. (Reference Stabili, Gravili, Boero, Tredici and Alifano2010), on the resistance to antibiotics of a luminous Vibrio growing in association with its hydroid host A. octodonta.
ACKNOWLEDGEMENTS
We thank Alessandro Colombelli for his contribution to the paper, and Christian Vaglio for his help in the field. Financial support was provided by the Ministero dell'Università e della Ricerca Scientifica e Tecnologica (PRIN2008 protocol 2008YBEANX) and the European Community (MARBEF, SESAME and VECTORS).











