INTRODUCTION
The striped dolphin, Stenella coeruleoalba (Meyen, 1833), is a world-wide cetacean species, distributed across the temperate to tropical waters of the world (Perrin et al., Reference Perrin, Wilson, Archer, Ridgway and Harrison1994; Archer, Reference Archer, Perrin, Würsig and Thewissen2000). The species inhabits deep waters beyond the continental shelf, typically over the continental slope out to oceanic waters (Notarbartolo di Sciara et al., Reference Notarbartolo di Sciara, Venturino, Zanardelli, Bearzi, Borsani and Cavalloni1993; Forcada et al., Reference Forcada, Aguilar, Hammond, Pastor and Aguilar1994; Perrin et al., Reference Perrin, Wilson, Archer, Ridgway and Harrison1994; Marini et al., Reference Marini, Consiglio, Angradi, Catalano, Sanna, Valentini, Finoia and Villetti1996; Gannier, Reference Gannier1998, Reference Gannier2005; Aguilar, Reference Aguilar2000; Archer, Reference Archer, Perrin, Würsig and Thewissen2000; Gordon et al., Reference Gordon, Matthews, Panigada, Gannier, Borsani and Notarbartolo di Sciara2000; Cañadas et al., Reference Cañadas, Sagarminaga and Garcia-Tiscar2002; Reeves & Notarbartolo di Sciara, Reference Reeves and Notarbartolo di Sciara2006). Although no separate subspecies are recognized (Archer & Perrin, Reference Archer and Perrin1999), geographical variation has been found in body size throughout the species' range (Perrin et al., Reference Perrin, Wilson, Archer, Ridgway and Harrison1994; Archer, Reference Archer, Perrin, Würsig and Thewissen2000) suggesting distinct populations. A number of studies suggest that there is limited gene flow between Mediterranean and Atlantic populations (Calzada & Aguilar, Reference Calzada and Aguilar1995; Archer, Reference Archer1996; Garcia-Martinez et al., Reference Garcia-Martinez, Moya, Raga and Latorre1999). Moreover, morphological (Calzada & Aguilar, Reference Calzada and Aguilar1995), toxicological (Monaci et al., Reference Monaci, Borrell, Leonzio, Marsili and Calzada1998) and genetic differences (Gaspari, Reference Gaspari2004) suggest limited dispersal range between areas within the Mediterranean.
Based on stomach content analyses, the feeding ecology of striped dolphins has been investigated in the Mediterranean Sea, the Atlantic Ocean (Bay of Biscay) and in Japan (Miyazaki et al., Reference Miyazaki, Kusaka and Nishiwaki1973; Desportes, Reference Desportes1985; Würtz & Marrale, Reference Würtz and Marrale1993; Astruc, 2005 unpublished results; Ringelstein et al., Reference Ringelstein, Pusinieri, Hassani, Meynier, Nicolas and Ridoux2006; Spitz et al., Reference Spitz, Richard, Meynier, Pusinieri and Ridoux2006). The diet of striped dolphin was found to be primarily composed of cephalopods and fish, and secondarily of crustaceans. Prey are typically small-sized (up to 200–300 mm length), pelagic, schooling and vertically migrating organisms. Seasonal or inter-annual variability in environmental conditions and prey resources are suspected to generate shifts in the distribution of striped dolphin (Gannier, Reference Gannier1999; Laran & Drouot-Dulau, Reference Laran and Drouot-Dulau2007; Azzelino et al., Reference Azzelino, Gaspari, Airoldi and Nani2008). However, the species may be less mobile than other cetacean species as a consequence of its foraging plasticity (Azzelino et al., Reference Azzelino, Gaspari, Airoldi and Nani2008). Differences in feeding ecology could then drive population structure of striped dolphins, a hypothesis supported by different stomach contents according to the habitat (Astruc, 2005 unpublished results; Spitz et al., Reference Spitz, Richard, Meynier, Pusinieri and Ridoux2006).
However, it is widely recognized that stomach contents analyses have a number of limitations. In particular, they are strongly influenced by the most recent feeding event. Partially digested prey are not always identifiable, limiting the conclusions that can be made about the overall diet, and the varying time of digestion of different prey species can result in an overestimation of some species (Aguiar dos Santos & Haimovici, Reference Aguiar dos Santos and Haimovici2001; Santos et al., Reference Santos, Clarke and Pierce2001). Stable isotope analysis offers a powerful complementary method to study marine mammal feeding ecology (Smith et al., Reference Smith, Hobson, Koopman and Lavigne1996; Hobson et al., Reference Hobson, Sease, Merrick and Piatt1997; Burns et al., Reference Burns, Trumble, Castellini and Testa1998; Das et al., Reference Das, Lepoint, Loizeau, Debacker, Dauby and Bouquegneau2000). The method is based on the fact that stable isotope ratios of carbon (13C/12C, normally expressed as δ13C) and nitrogen (15N/14N or δ15N) of a consumer are the weighted average of the isotopic composition of the ingested prey animals (DeNiro & Epstein, Reference DeNiro and Epstein1978, Reference DeNiro and Epstein1981), modified by some enrichment associated with metabolic activities within the individual (Mook & de Vries, Reference Mook, de Vries and Mook1989). Lighter isotopes (14N and 12C) are preferentially mobilized in biochemical reactions and are therefore more likely to be excreted (Minawaga & Wada, Reference Minawaga and Wada1984; Peterson & Fry, Reference Peterson and Fry1987). This results in heavier isotopes (15N and 13C) being preferentially retained in the consumer (Das et al., Reference Das, Lepoint, Loizeau, Debacker, Dauby and Bouquegneau2000). Thus consumer tissues exhibit a higher isotopic value than those of their prey.
Generally, the nitrogen stable isotope ratio of a consumer is enriched on average by 3.4‰ over that of its diet (Minawaga & Wada, Reference Minawaga and Wada1984; Vander Zanden & Rasmussen, Reference Vander Zanden and Rasmussen2001), although considerable variation can be found in the amount of enrichment between one trophic level and the next (Minawaga & Wada, Reference Minawaga and Wada1984; Wada et al., Reference Wada, Terazaki, Kabaya and Nemoto1987; Hobson & Welch, Reference Hobson and Welch1992; Burns et al., Reference Burns, Trumble, Castellini and Testa1998). Nitrogen isotope ratios (δ15N) can thus give insights into consumer trophic level (Hobson & Clark, Reference Hobson and Clark1992; Vander Zanden et al., Reference Vander Zanden, Cabana and Rasmussen1997; Burns et al., Reference Burns, Trumble, Castellini and Testa1998; Das et al., Reference Das, Lepoint, Loizeau, Debacker, Dauby and Bouquegneau2000, Reference Das, Lepoint, Leroy and Bouquegneau2003b; Witteveen et al., Reference Witteveen, Worthy, Wynne, Hirons, Andrews III and Markel2011) (assuming that there is information available on δ15N at the base of the food web to allow calibration)—although the resolution provided in relation to diet composition is coarse—and can potentially allow reconstitution of trophic food webs (Wada et al., Reference Wada, Terazaki, Kabaya and Nemoto1987; Fry, Reference Fry1988; Hobson & Welch, Reference Hobson and Welch1992; Hobson et al., Reference Hobson, Piatt and Pitocchelli1994). In contrast, a small increase (typically 1‰) of carbon isotope ratios (δ13C) between prey and predator is often reported (DeNiro & Epstein, Reference DeNiro and Epstein1978; Fry & Sherr, Reference Fry and Sherr1984; Peterson & Fry, Reference Peterson and Fry1987; Vander Zanden & Rasmussen, Reference Vander Zanden and Rasmussen2001), thus providing information about the origin of the prey, since δ13C values at the base of a trophic web are often characteristic of the ecosystem (Schell et al., Reference Schell, Saupe and Haubenstock1989; Smith et al., Reference Smith, Hobson, Koopman and Lavigne1996; Marcoux et al., Reference Marcoux, Whitehead and Rendell2007). For example, in the marine environment, δ13C can allow discrimination between inshore and offshore (Rau et al., Reference Rau, Mearns, Young, Olson, Scheafer and Kaplan1983; Schell et al., Reference Schell, Saupe and Haubenstock1989; Hobson et al., Reference Hobson, Piatt and Pitocchelli1994; Walker et al., Reference Walker, Potter and Macko1999; Das et al., Reference Das, Beans, Holsbeek, Mauger, Berrow, Rogan and Bouquegneau2003a, Reference Das, Lepoint, Leroy and Bouquegneaub) or pelagic and benthic foraging areas (France, Reference France1995; Burns et al., Reference Burns, Trumble, Castellini and Testa1998; Das et al., Reference Das, Lepoint, Loizeau, Debacker, Dauby and Bouquegneau2000).
As stable isotopes ratios derive from assimilated food, the isotopic composition of an animal's tissue reflects the dietary inputs over the turn-over time of the analysed tissue (DeNiro & Epstein, Reference DeNiro and Epstein1978, Reference DeNiro and Epstein1981). Stable isotope measurements can therefore provide time-integrated dietary information, over a series of time-scales if analyses are performed on tissues with different turnover rates (DeNiro & Epstein, Reference DeNiro and Epstein1978, Reference DeNiro and Epstein1981; Tieszen et al., Reference Tieszen, Boutton, Tesdahl and Slade1983; Das et al., Reference Das, Lepoint, Loizeau, Debacker, Dauby and Bouquegneau2000; Knoff et al., Reference Knoff, Hohn and Macko2008). However, caution is needed due to differences in the biochemistry of different tissues, which could also affect fractionation (DeNiro & Epstein, Reference DeNiro and Epstein1978; Tieszen et al., Reference Tieszen, Boutton, Tesdahl and Slade1983; Hobson & Clark, Reference Hobson and Clark1992; Hobson et al., Reference Hobson, Schell, Renouf and Noseworthy1996; Pinnegar & Polunin, Reference Pinnegar and Polunin1999).
In this study, we use stable isotope analyses to explore a number of aspects of the feeding ecology of striped dolphin in the north-western Mediterranean Sea. Specifically we look at whether there is evidence of geographical and seasonal variation in diet, and how it varies ontogenetically. We analysed ratios of stable nitrogen and carbon isotopes in tissue samples of dolphins from six geographical areas. δ15N was used to assess the trophic levels at which the animals fed, while δ13C was used as an indicator of the source of food resources and thus of habitat differences. Analysis of tissues with slow and fast protein turnover rates can in theory identify animals that are switching to an isotopically novel diet (Tieszen et al., Reference Tieszen, Boutton, Tesdahl and Slade1983). We measured isotope values in skin and muscle. The turnovers of both tissues are unknown for the striped dolphin. But, based on studies on beluga Delphinapterus leucas (St Aubin et al., Reference St Aubin, Smith and Geraci1990) and bottlenose dolphin Tursiops truncatus (Hicks et al., Reference Hicks, St Aubin, Geraci and Brown1985; Knoff et al., Reference Knoff, Hohn and Macko2008), we expected the isotopic composition of striped dolphin skin to reflect the input of recent diet (over the last two months). According to the body size of the species, and relying on previous studies (Tieszen et al., Reference Tieszen, Boutton, Tesdahl and Slade1983; Hobson & Welch, Reference Hobson and Welch1992; Sponheimer et al., Reference Sponheimer, Robinson, Cerling, Tegland, Roeder, Ayliffe, Dearing and Ehlinger2006), we estimated a turnover rate for striped dolphin muscle of approximately several months. Finally, we also analysed the isotope composition of muscle and mantle tissues in some fish and cephalopod species in order to allow us to put dolphin isotope signatures in an ecosystem context (Hobson & Welch, Reference Hobson and Welch1992; Hobson et al., Reference Hobson, Sease, Merrick and Piatt1997; Burns et al., Reference Burns, Trumble, Castellini and Testa1998; Das et al., Reference Das, Lepoint, Leroy and Bouquegneau2003b).
MATERIALS AND METHODS
Sample origin and preparation
Skin and muscle samples derived from stranded and by-caught dolphins collected between 2001 and 2008 along the French Mediterranean coast and between 2001 and 2007 from the French Atlantic coast, by the members of the French Stranding Network. Samples were frozen (–20°C) immediately after collection.
Samples of a variety of potential prey species were also analysed (Table 3). The chosen species were those commonly reported in the diet of striped dolphins and available from markets, fishers or research surveys. These included six fish species (Boops boops, Engraulis encrasicolus, Merluccius merluccius, Micromesistius poutassou, Sardinella aurita and Sardina pilchardus), two squid genera (Illex coindetii and Loligo vulgaris), and two cuttlefish species (Sepia officinalis and S. orbignyana) for the Gulf of Lion and Ligurian Sea. Cephalopod and fish samples were obtained from a local fish market in Sète (Gulf of Lion), directly from fishers in Cagnes-sur-mer (Ligurian Sea) and during trawls surveys off Monaco (Ligurian Sea) (Figure 1). Mantle tissue of cephalopod and muscle tissue from the dorsal region of fish were used for analyses.

Fig. 1. Striped dolphin samplings localities in the Atlantic (area 1, indicated by crosses) and in the north-western Mediterranean Sea: Gulf of Lion (area 2, squares), Provençal Basin (area 3, circles), Ligurian Sea (area 4, triangles), Corsica (area 5, stars) and offshore area (across areas 2 and 3, diamonds). Preys were collected off Sète (area 2), Cagnes-sur-mer, Monaco, and Baie des Anges area (area 4).
All samples were dried at 50°C or freeze-dried for at least 48 hours prior to analysis and ground to a fine powder. Lipids are depleted in 13C compared to the whole body or proteins, such that any material which is rich in lipids will have relatively lower δ13C (DeNiro & Epstein, Reference DeNiro and Epstein1978; Tieszen et al., Reference Tieszen, Boutton, Tesdahl and Slade1983). Therefore, lipids were extracted from all samples using cyclohexane (Cherel et al., Reference Cherel, Fontaine, Richard and Labat2010). About 100 mg of sample were put in a screw-capped tube along with 4 ml of cyclohexane p.a., left under rotary agitation for 1 hour at room temperature, and centrifuged (1000 g, 10 minutes). After centrifugation, the pellet was rinsed with 2 ml of cyclohexane, centrifuged again and dried using a dry bath at 45°C under a hood. For very fatty samples, when the supernatant appeared coloured, the 4 ml extraction was repeated. Carbonates were removed from fish muscles with dilute acid (HCl 0.1 N), since inorganic carbonates tend to be enriched in 13C compared to other body fractions (DeNiro & Epstein, Reference DeNiro and Epstein1978).
Stable isotope analyses
Stables isotope analyses were completed at the University of La Rochelle, France, using an isotope ratio mass spectrometer (Thermo Scientific Delta V Advantage, Bremen, Germany) coupled to an elemental analyser (Thermo Scientific Flash EA1112, Milan, Italy). Stable carbon and nitrogen isotope ratios are expressed in delta (δ) notation, defined as parts per thousand (‰, or per mil) deviations from a universal standard, according to the following formula:
where X is 13C or 15N and R is the corresponding ratio 13C/12C or 15N/14N. The standards are atmospheric nitrogen for δ15N and Pee Dee Belemnite carbonate (PDB) for δ13C and the reference materials used for calibration were IAEA CH-6 and IAEA-N1. Replicate measurements of a laboratory standard (acetanilide, Thermo Scientific) analysed with the samples indicated that analytical precision of the measurements was <0.1‰ for δ13C and <0.15‰ for δ15N. Results on elemental composition (%C and %N) of tissues obtained during isotope analyses were used to calculate the sample C:N ratio, which indicates good lipid removal efficiency when C:N <4.
Data analysis
For the statistical analyses, dolphin samples were classified according to the location of the stranding or the by-catch. Samples derived from strandings from Brittany to the Bay of Biscay along the French Atlantic coast (area 1), were all grouped in the same (Atlantic) pool. Samples derived from strandings in the Mediterranean were separated into four areas, i.e. the Gulf of Lion (area 2), Provençal Basin (area 3), Ligurian Sea (area 3), and Corsica (area 5). By-catches collected in the Mediterranean Sea were grouped as ‘offshore’ samples (Figure 1).
Previous studies based on stomach content analysis showed evidence of ontogenetic, seasonal, and geographical patterns in the diet of striped dolphins (Astruc, 2005 unpublished results). Thus, we focused our analyses to determine relationships (Anderson et al., Reference Anderson, Burnham and Thompson2000) between isotopic values in dolphin tissues and the explanatory variables body length, sex, year, month, and area. Data exploration has been undertaken following Zuur et al. (Reference Zuur, Ieno and Elphick2010). Specifically, pair plots of the explanatory variables have been examined to avoid non-linear or bivariate collinearity and variance inflation factors (VIF) have been calculated to check for multivariate collinearity. Generalized additive models (GAMs) (Hastie & Tibshirani, Reference Hastie and Tibshirani1990) were fitted to C and N isotope ratio data. Body length was fitted as a continuous variable while year, month, sex and area were fitted as nominal variables. In order to take account of the effect of trophic level on carbon values, we added nitrogen as a continuous explanatory variable in the carbon model. A Gaussian distribution with identity link was applied since isotope ratios appeared to be approximately normally distributed. GAMs were fitted using a backward selection procedure and the optimal model was identified by Akaike's information criterion (AIC). Generally, the best model is that with the lowest value for the AIC, in which all remaining explanatory variables have significant effects. We started with a full model which included all variables, and at each step the least significant variable was excluded from the model, the process continuing until no further fall in the AIC values was obtained. Stepwise procedures have recently received considerable criticism (Whittingham et al., Reference Whittingham, Stephens, Bradbury and Freckleton2006; Mundry & Nunn, Reference Mundry and Nunn2009) even if compared to other methods of variable selection they generate similar models (Murtaugh, Reference Murtaugh2009). To avoid the inclusion of non-significant variables in models, which could be indicative of a Type I error (Mundry & Nunn, Reference Mundry and Nunn2009), extra model selection steps (F-tests) were applied to test differences between nested models with and without the non-significant variable. A significant difference implies that the model is improved by the additional terms, which can therefore be retained, otherwise it is dropped (Zuur et al., Reference Zuur, Ieno and Smith2007). Once final models were obtained, they were checked to ensure that there were no obvious patterns in the residuals and residuals appeared to be approximately normally distributed. All analyses were performed using the statistical software R (R Development Core Team, 2008). For both additive models (for N and C respectively), we present both the smoothing function and a plot of the predicted values of the ratios against the relevant explanatory variable. Predicted values were calculated using the final models for nitrogen and carbon. Assumption has been made on length values: we used the range from 80 to 220 cm, with an interval of 5 cm.
Following the literature, an average increase of 3.4‰ in δ15N (Minawaga & Wada, Reference Minawaga and Wada1984; Vander Zanden et al., Reference Vander Zanden, Cabana and Rasmussen1997; Vander Zanden & Rasmussen, Reference Vander Zanden and Rasmussen2001) and 1‰ in δ13C is typically observed between a consumer and its diet (DeNiro & Epstein, Reference DeNiro and Epstein1978; Fry & Sherr, Reference Fry and Sherr1984; Minawaga & Wada, Reference Minawaga and Wada1984; Peterson & Fry, Reference Peterson and Fry1987; Vander Zanden et al., Reference Vander Zanden, Cabana and Rasmussen1997; Vander Zanden & Rasmussen, Reference Vander Zanden and Rasmussen2001). We estimated the isotopic enrichment (IE) between newly weaned dolphins (155 cm length) and mature dolphins (200 cm length). We calculated this enrichment using nitrogen and carbon ratios from fitted GAM, according to the following equation:
where δX is δ15N or δ13C of mature and newly weaned dolphins, E is the enrichment of 3.4‰ for nitrogen and 1‰ for carbon assumed for a difference of one trophic level.
Analysis of tissues that have slower and faster turnover rates can identify whether an individual changed its diet or its feeding area over time (Tieszen et al., Reference Tieszen, Boutton, Tesdahl and Slade1983; Das et al., Reference Das, Lepoint, Loizeau, Debacker, Dauby and Bouquegneau2000). Temporal variation was investigated by comparing skin and muscle isotopic compositions in animals for which both tissues were available. If the isotope ratio of skin was consistently higher or lower than muscle across all individuals within a season, size-class or area, this would suggest a change in the diet or feeding area over time. Chi-squared tests were used to assess whether there were any such patterns between the stable isotope ratios of skin and muscle. For this analysis, comparisons were conducted between seasons, areas and body size-classes. The reported body size of striped dolphins in the western Mediterranean is 90–120 cm for calves, 120–160 cm for juveniles, and 160–190 cm for sub-adults (Calzada et al., Reference Calzada, Aguilar, Lockyer and Grau1997). Accordingly, and examining the smoothers resulting from the GAMs, size-classes were chosen as 80–130 cm for calves, 130–155 cm for juveniles, 155–200 cm for subadults and above 200 cm for adults. For each grouping, if a significant difference was found between both ratios, a second test was performed to investigate if the pattern was related to season, area and length.
Comparisons between prey and dolphin isotopic compositions were made within the same area (Gulf of Lion or Ligurian Sea). We used isotopic ratios measured in mature dolphins (>200 cm length). We calculated their isotopic composition and compared them to prey isotopic composition.
RESULTS
In the Mediterranean, adult and sub-adult striped dolphins (i.e. those larger than 155 cm length) exhibited an average δ13C of –17.7 ± 0.6 in skin and –17.4 ± 0.4 in muscle, and an average δ15N of 10.0 ± 0.9 in skin and 9.6 ± 0.7 in muscle (Table 1). In the Atlantic, the average δ13C in skin and muscle were –17.5± 0.7 and –17.5 ± 0.3 respectively, and an average δ15N of 12.1 ± 0.8 in skin and 10.9 ± 0.6 in muscle (Table 1).
Table 1. Skin and muscle mean isotopic values (± SD) of adult and sub-adult striped dolphins (>155 cm length) from the Atlantic and the Mediterranean (with the five regions grouped and separated).

Isotopic signatures of striped dolphins: statistical modelling results
VARIATION IN δ15N VALUES
Of the five variables tested, only dolphin length and geographical area showed significant relationships with the nitrogen signature (length: t = 8.86, P < 0.0001; area: t = 9.93, P < 0.0001). The final model explained 59.2% of deviance in δ15N values. In terms of the relationship with length, nitrogen isotope ratios decreased with increasing length for dolphins between 80 and 155 cm (Figure 2a). The curve inflects at 155 cm and δ15N shows an increasing pattern for larger individuals, although this increase may reach an asymptote for dolphins larger than 200 cm. In terms of actual nitrogen isotope ratios, they decreased on average by 3.50‰ between dolphins of 80 and 155 cm, while they increase by 0.57‰ between 155 and 200 cm (Figure 2b).

Fig. 2. Plots for nitrogen signature. (a) Effect of dolphin length on nitrogen signature. The solid line is the estimated smoother, the dashed line is the 95% confidence interval; (b) predicted nitrogen signature for muscle according to length and area, using a fitted generalized additive model. From top to bottom, the lines indicate samples from the Atlantic, Corsica, Ligurian Sea, Gulf of Lion, Offshore and Provençal Basin, respectively.
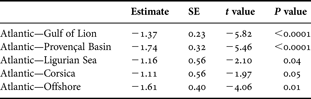
In terms of areas of origin, Atlantic dolphin samples had a higher δ15N (a general enrichment of more than 1‰) than dolphin samples from the Mediterranean Sea (Table 2; Figure 2b). There were no significant differences found amongst the five Mediterranean areas.
Table 2. Comparison between nitrogen isotope ratios for dolphins from the Atlantic and Mediterranean: estimated parameters, standard error (SE), values of Student's t and probability (P)
VARIATION IN δ13C VALUES
Of the six variables included in the original model, only length had a significant effect on carbon isotope composition (t = 8.48, P < 0.0001). The final model explained 31.1% of deviance in δ13C. In terms of the relationship with body length, carbon isotope ratios increased for dolphins of lengths between 130 and 200 cm (Figure 3a), and the actual enrichment was 0.56‰ (Figure 3b).

Fig. 3. Plots for carbon signature. (a) Effect of dolphin length on carbon signature. The solid line is the estimated smoother, the dashed line is the 95% confidence interval; (b) predicted carbon signature for muscle according to length using a fitted generalized additive model.
Isotopic enrichment according to length
Based on the model, we estimated that dolphins of 155 cm typically have a δ15N of 10.55‰ and a δ13C of –17.83‰. Dolphins of 200 cm would exhibit a δ15N of 11.12‰ and a δ13C of –17.41‰. Mature dolphins would exhibit, therefore, an isotopic enrichment of 0.17 when inferred from nitrogen isotope ratios and an inferred enrichment of 0.41 for carbon, compared to smaller dolphins. The difference of enrichment between newly-weaned and mature dolphins is therefore twice as high for carbon as it is for nitrogen.
Variation in isotopic composition over time
VARIATION IN NITROGEN COMPOSITION
Nitrogen isotope ratios were higher in skin than in muscle. This did not vary with season (χ23 = 0.87, P = 0.83) (Table 3). However, it did vary with body length and, while the nitrogen isotope ratios were lower in skin than in muscle in dolphins between 80 and 130 cm, they were higher in skin than in muscle in dolphins over 130 cm (χ23 = 11.73, P = 0.01) (Table 3). In relation to area, nitrogen isotopes in skin were higher than in muscle in all areas except Corsica (χ25 = 14.66, P = 0.01) (Table 3).
Table 3. Percentages of striped dolphins for which skin had higher or lower isotope value than muscle.

VARIATION IN CARBON COMPOSITION
Carbon isotope ratios tended to be lower in skin than in muscle in all seasons except winter when the ratio was higher in skin. This pattern was however not significant (χ23 = 7.18, P = 0.07) (Table 3). Carbon isotope ratios between skin and muscle did not vary significantly according to length-class (χ23 = 1.37, P = 0.71) (Table 3) nor according to area (χ25 = 9.43, P = 0.09) (Table 3).
Comparison with isotopic compositions of potential prey species
The average carbon isotopic ratio was −18.99 ± 0.57‰ for fish and –17.96 ± 0.45‰ for cephalopods (Table 4). The nitrogen isotopic ratios were 7.55 ± 0.71‰ and 8.87 ± 1.13‰ respectively. The nitrogen isotopic ratio seemed higher for larger prey, and this pattern was more obvious for cephalopods (Figure 4). For example, specimens of Illex coindetii measuring 27.5cm length were enriched by 1.6‰ in δ15N compared to specimens half their length (14.9 cm). The average carbon and nitrogen signatures of mature dolphins were –17.22 ± 0.47‰ and 9.94 ± 0.94‰ respectively (N = 8). Nitrogen fractionation was 2.39‰ from fish to dolphins and 1.07‰ from cephalopods to dolphins. On average, carbon isotope ratios in dolphins were 1.77‰ greater than fish, and 0.74‰ greater than cephalopods (Figure 5).

Fig. 4. Nitrogen isotopic ratios according to the length (cm) of fish (open symbols: Boops boops (Bboops), Engraulis encrasicolus (Eencr), Merluccius merluccius (Mmerl), Sardinella aurita (Saur), Sardina pilchardus (Spilch)) and cephalopods (filled symbols: S. orbignyana (Sorb), Sepia officinalis (Soff), Loligo vulgaris (Lvulg), Illex coindetii (Icoin)) from Gulf of Lion and Ligurian Sea. Mean ± standard deviation.

Fig. 5. Carbon and nitrogen isotopic ratios for fish, cephalopods, and mature striped dolphins from the Gulf of Lion and Ligurian Sea. Mean ± standard deviation.
Table 4. Prey samples: species, area of origin (LS, Ligurian Sea; BA, Baie des Anges; M, Monaco; GL, Gulf of Lion; S, Sète; na, unknown location), number of items (N), carbon and nitrogen isotopic ratios (mean ± SD). The length from the tip of the snout to the tip of the longer lobe of the caudal fin was measured for fish and the dorsal mantle length was measured for squids.

†, mean and standard deviation on 6 items.
DISCUSSION
Isotopic signatures of striped dolphins
VARIATION IN δ15N VALUES: EFFECT OF BODY LENGTH
Variation in δ15N suggests ontogenetic changes in striped dolphin diet according to their size. The decrease in nitrogen isotope signatures up to a body length of 155 cm is likely to be associated with animals changing from primarily feeding on milk to primarily feeding on live prey. Feeding on milk is known to increase N ratios above those typically found in mature animals of the same species (Steele & Daniel, Reference Steele and Daniel1978; Hobson et al., Reference Hobson, Sease, Merrick and Piatt1997; Das et al., Reference Das, Lepoint, Leroy and Bouquegneau2003b; Knoff et al., Reference Knoff, Hohn and Macko2008; Fernandez et al., Reference Fernandez, Garcia-Tiscar, Santos, Lopez, Martinez-Cedeira, Newton and Pierce2011) and thus explains the higher nitrogen ratios found in small individuals. Our results suggest that weaning occurs at around 155 cm, as indicated by the point of inflection in the smoother for effect of length on nitrogen isotope ratio. In animals with body lengths >155 cm, nitrogen isotope ratios increased with body length. This pattern could be explained by larger individuals feeding on larger prey and/or changing the composition of their diet. Indeed, comparisons between the diets of striped dolphins of different size-groups in the Atlantic indicated that larger dolphins fed on larger prey (Ringelstein et al., Reference Ringelstein, Pusinieri, Hassani, Meynier, Nicolas and Ridoux2006). Moreover, higher nitrogen values are typically found in larger prey, and this pattern has been shown for fish and cephalopods (Hooker et al., Reference Hooker, Iverson, Ostrom and Smith2001; Jennings et al., Reference Jennings, Pinnegar, Polunin and Warr2002; Ruiz-Cooley et al., Reference Ruiz-Cooley, Gendron, Aguiniga, Mesnick and Carriquiry2004, Reference Ruiz-Cooley, Villa and Gould2010; Fernandez et al., Reference Fernandez, Garcia-Tiscar, Santos, Lopez, Martinez-Cedeira, Newton and Pierce2011). In the north-western Mediterranean, the composition of the diet was found to change according to the maturity of striped dolphins, with a higher proportion of fish in the diet of immatures (Astruc, 2005 unpublished results). As cephalopods were found to have consistently higher isotopic values than fish species (Figures 4 & 5; Table 4), higher nitrogen values are expected to be found in adult compared to younger individuals. Both suggestions are consistent with previous stomach contents analysis (Astruc, 2005 unpublished results; Ringelstein et al., Reference Ringelstein, Pusinieri, Hassani, Meynier, Nicolas and Ridoux2006) and are not necessarily mutually exclusive.
VARIATION IN δ13C VALUES: EFFECT OF BODY LENGTH
Carbon isotope ratio increases (+0.56‰) with dolphin body length (between 130 and 200 cm length) and this enrichment could be explained by dolphins feeding at higher trophic levels or on larger prey, as suggested for the increase in nitrogen isotope ratios. However, the observed increase in the carbon isotope ratio before weaning (155 cm) is in apparent contradiction with the decreasing pattern for nitrogen. We suggest that a change in mother's rearing behaviour occurs during this period. Marine mammal calves are known not to follow the adults in their foraging dives, because of lower physiological capacity constraining their dive depth and duration (Slip, Reference Slip1995; Noren et al., Reference Noren, Lacave, Wells and Williams2002; Noren & Edwards, Reference Noren and Edwards2007). According to the species, the degree of dependence of the calves can also be relatively strong in the first months but decreases with time (Würsig & Clark, Reference Würsig, Clark, Burns, Montague and Cowles1993; Noren & Edwards, Reference Noren and Edwards2007). If striped dolphins exhibit a similar pattern, the observed increase in carbon composition may be explained by a change in mothers' feeding behaviour: once calves reach around 130 cm length they can be left at the surface allowing adults to undertake longer and deeper dives to feed on prey in food webs with higher basal carbon isotope ratios but that do not differ in nitrogen isotope ratios.
VARIATION IN δ15N VALUES: EFFECT OF AREA
Nitrogen isotopic composition revealed some geographical differences in diet according to the study areas. Atlantic dolphins had nitrogen isotope ratios that were on average 1‰ higher than recorded in Mediterranean dolphins. Intrinsic dietary differences (Astruc, 2005 unpublished results; Ringelstein et al., Reference Ringelstein, Pusinieri, Hassani, Meynier, Nicolas and Ridoux2006) could explain the differences observed in cetacean tissues between the two regions. Geographical variation in the δ15N values has been shown for various marine consumers (Walker et al., Reference Walker, Potter and Macko1999; Takai et al., Reference Takai, Onaka, Ikeda, Yatsu, Kidokoro and Sakamoto2000; Ruiz-Cooley et al., Reference Ruiz-Cooley, Gendron, Aguiniga, Mesnick and Carriquiry2004, Reference Ruiz-Cooley, Villa and Gould2010; Marcoux et al., Reference Marcoux, Whitehead and Rendell2007; Bentaleb et al., unpublished results). Difference in the baseline δ15N signatures of the Mediterranean and Atlantic food webs could therefore account for the difference found in dolphin tissues. Isotope data from dolphins revealed no population structuring within the north-western Mediterranean, suggesting that striped dolphin diet might be isotopically homogeneous across this area. This may reflect a wide foraging range: if individual dolphins move between several study areas this would tend to mask any geographical differences in isotopic composition. Absence of differences could also be observed if dolphins fed in distinct feeding grounds but on prey with similar isotopic values, therefore exhibiting a similar overall isotopic composition. Regional differences in diets are reported from stomach content analysis (Astruc, 2005 unpublished results) and would therefore support the second hypothesis. Differences in isotopic signature could exist between stranded and by-caught dolphins, as suggested for other cetaceans (Gannes et al., Reference Gannes, Martinez del Rio and Koch1998; Ruiz-Cooley et al., Reference Ruiz-Cooley, Gendron, Aguiniga, Mesnick and Carriquiry2004). Starvation or disease can affect dolphin metabolism before the stranding (Markussen, Reference Markussen, Blix, Walloe and Ulltang1995; Boily & Lavigne, Reference Boily and Lavigne1997). But again, the absence of differences within the five Mediterranean areas, suggest that by-caught dolphins analysed in the present study exhibit a similar isotopic composition to stranded dolphins.
Isotopic enrichment according to the length
Mature dolphins present higher isotopic values compared to newly-weaned dolphins. However, carbon ratios increased more rapidly than nitrogen ratios, suggesting that the increase in carbon isotope ratios is not driven by solely trophic level enrichment. Polunin et al. (Reference Polunin, Morales-Nin, Pawsey, Cartes, Pinnegar and Moranta2001) found that δ13C of some fish species increases from depths of 200 to 1800 m. Striped dolphins are believed to dive to depths of up to 700 m (Archer, Reference Archer, Perrin, Würsig and Thewissen2000). Although this range reflects physiological diving capacity rather than the usual foraging depth, striped dolphins are known to feed on deep water prey (especially cephalopods) (Astruc, 2005 unpublished results). The increase in δ13C with body length could then be explained by increased feeding on cephalopods in older animals as suggested by stomach content analyses (Astruc, 2005 unpublished results).
Variation in isotopic composition over time
VARIATION IN NITROGEN COMPOSITION
Enrichment in the composition of skin relative to muscle could indicate that the most recent diets are always more enriched than the less recent ones. However, this pattern is observed for weaned dolphins in all seasons and most areas, which makes the explanation highly implausible. The difference in nitrogen isotope ratios between tissues may therefore be caused by a higher fractionation in skin compared to the muscle, as such differences are reported for other tissues (DeNiro & Epstein, Reference DeNiro and Epstein1978; Tieszen et al., Reference Tieszen, Boutton, Tesdahl and Slade1983; Hobson et al., Reference Hobson, Schell, Renouf and Noseworthy1996; Pinnegar & Polunin, Reference Pinnegar and Polunin1999). Therefore, to answer ecological questions, comparisons of isotopic composition should be undertaken on the same tissue. Unfortunately this means that analyses of isotope ratios in different tissues of the same animal cannot provide a reliable indication of diet over different time scales, unless biases due to differences in fractionation between tissues are known.
VARIATION IN CARBON COMPOSITION
Enrichment and depletion in carbon isotopic composition of skin relative to muscle has been observed according to the season. Skin showed lower carbon isotope ratios than muscle from spring to autumn, while it showed higher ratios in winter. Although a larger sample size is needed to confirm this pattern, this observation suggests some possible seasonal movements outside the study areas. Migration of the population between the central Spanish Mediterranean Sea (end of autumn–winter) to the Tyrrhenian Sea (summer) has been suggested by Laran & Drouot-Dulau (Reference Laran and Drouot-Dulau2007) and may explain the changes in the composition of the diet observed in our results.
Comparison with isotopic composition of potential prey species
Without a sufficient amount of prey species, covering each study area and a wide spectrum of size-classes, we could not examine the variation of isotopic signatures for prey as we did for dolphins. However, comparison of isotopic composition in dolphins and some prey species enable us to give some insights into prey–predator enrichment. We found a general low enrichment rate: nitrogen fractionation was 2.39‰ from fish to dolphins and 1.07‰ from cephalopods to dolphins, and carbon fractionation was 1.77‰ from fish to dolphins and 0.74‰ from cephalopods to dolphins. The reported enrichment values of 3.4‰ for nitrogen (DeNiro & Epstein, Reference DeNiro and Epstein1981; Minawaga & Wada, Reference Minawaga and Wada1984; Pinnegar et al., Reference Pinnegar, Polunin and Badalamenti2003) and 1‰ for carbon (DeNiro & Epstein, Reference DeNiro and Epstein1978) from one trophic level to the next are averages and values from different predator–prey pairings can vary very widely (Minawaga & Wada, Reference Minawaga and Wada1984; Wada et al., Reference Wada, Terazaki, Kabaya and Nemoto1987; Hobson & Welch, Reference Hobson and Welch1992; Burns et al., Reference Burns, Trumble, Castellini and Testa1998; Ruiz-Cooley et al., Reference Ruiz-Cooley, Gendron, Aguiniga, Mesnick and Carriquiry2004). Lower isotopic fractionations have often been found (Ostrom et al., Reference Ostrom, Lien and Macko1993; Abend & Smith, Reference Abend and Smith1997; Vander Zanden & Rasmussen, Reference Vander Zanden and Rasmussen2001). The enrichment found between putative prey species and dolphins in our study falls within the range of values reported in the literature. However, it could also suggest that dolphins feed on prey species other than those sampled in this study (Bode et al., Reference Bode, Alvarez-Ossorio and Varela2006; Bentaleb et al., unpublished results) as our sampling of potential prey species was not exhaustive. With an apparently opportunistic species like striped dolphins, we can expect a large range of isotopic signatures to occur.
CONCLUSION
This study is the first to explore feeding ecology of striped dolphins in the north-western Mediterranean Sea through stable isotope analyses. Our results give insights that are complementary to previous work based on stomach contents analysis.
Variations in nitrogen isotope ratios allowed confirmation that weaning of calves occur around 155 cm length. Enrichment in nitrogen ratio occurs post-weaning, probably because larger dolphins feed on larger and on higher trophic level prey. The carbon isotope ratio increases with length as well, but this seems not to be driven solely by trophic level enrichment. Dolphins are suspected to forage on deep water prey and in offshore waters.
Comparisons of the nitrogen isotope ratios of skin and muscle revealed that skin presents a higher fractionation, and highlights the need to carefully interpret results of comparisons between samples from different tissues. It cannot be assumed that differences reflect only the timescale of turnover, so inferences about recent feeding versus feeding over a long timescale may not be possible.
A geographical difference in isotopic signatures of striped dolphins between the Atlantic and the Mediterranean was observed, probably because of fundamentally different diets and different isotopic compositions at the base of the food chain. However, no regional population structure based on feeding ecology emerged within the Mediterranean. The small sample size may explain the absence of difference. Another possible hypothesis is that dolphins move between the study areas and therefore exhibit a homogeneous isotopic composition. However, absence of differences in isotopic composition could also be explained if dolphins forage on similar trophic level prey in distinct feeding grounds. Regional differences in diets are reported from stomach content analysis (Astruc, 2005 unpublished results). Stable isotope analyses have their own limitations, and studies on feeding ecology should ideally integrate both stomach content and stable isotope analyses.
Seasonal differences in carbon isotope signatures could suggest seasonal movements, and this hypothesis would be consistent with previous results from analysis of distribution. However, more samples are required to confirm our results. Finally, comparisons between potential prey and striped dolphins suggest a low enrichment rate.
ACKNOWLEDGEMENTS
We would like to thank GECEM (Groupe d'Étude sur les Cétacés en Méditerranée) and CRMM (Centre de Recherche sur les Mammifères Marins) for providing cetacean samples, and acknowledge all the volunteers from the French Stranding Network. We thank Yann Leroy from the Université Internationale de la mer of Cagnes-sur-mer for having provided some preys samples. Particular thanks are extended to Willy Dabin of CRMM for access and assistance in sample collection and processing. We are grateful to Gaël Guillou of the University of La Rochelle for running the stable isotope analyses. Final thanks extend to the University of La Rochelle and Marineland of Antibes for having supported this study. Graham J. Pierce was supported by the ANIMATE project (MEXC-CT-2006-042337). The manuscript was much improved by critical comments from two anonymous referees.











