Introduction
The re-ensiling process encompasses the steps of unloading, transporting, compacting and sealing silage into a new silo. This is a common practice on farms in several countries due to the need for transporting the silage to other farms; bulk selling to other producers; among other reasons (Chen and Weinberg, Reference Chen and Weinberg2014). In some cases, silages are transported over long distances or remain on the farm for an extended period before being re-ensiled, which may increase dry matter (DM) losses due to exposure to air.
However, the impact of such alterations depends on the ensiled crop and the microbial populations in the silage at the time of re-ensiling. Recent studies have shown that although the corn, wheat and sorghum crops' fermentation profile is not affected, their nutritional value is, with reductions in in vitro DM digestibility (Chen and Weinberg, Reference Chen and Weinberg2014; Michel et al., 2017). Among the crops used for silage making, sugarcane is an important forage source for ruminants in tropical regions because of its low nutrient requirements and elevated DM yield (20–100 t/ha) (Siqueira et al., Reference Siqueira, Reis, Schocken-Iturrino, Pires, Bernardes and Amaral2007).
The main characteristics of sugarcane silage are high ethanol levels produced by yeasts that result from elevated concentrations of soluble carbohydrates in the plant, which culminate in DM losses and decreased nutritional value (Pedroso et al., Reference Woolford2005; Oliveira et al., Reference Oliveira, Garcia, Pires, Oliveira, Almeida, Silva, Nascimento Filho and Abreu Filho2015). The inoculant Lactobacillus buchneri has shown to be effective in controlling yeasts and reducing the ethanol concentration in sugarcane silages and, in some cases, improving aerobic stability (Ávila et al., Reference Ávila, Pinto, Oliveira and Schwan2012; Carvalho et al., Reference Woolford2012; Santos et al., Reference Santos, Nascimento, Magalhães, Silva, Silva, Santana and Soares2015). Nevertheless, the effect of applying this inoculant during the re-ensiling process is unknown.
In this scenario, to maintain the quality and increase the nutritional value of sugarcane silage by mitigating the effects of aerobic exposure, it is necessary to understand how the re-ensiling time affects its fermentation profile and chemical composition and observe the responses to L. buchneri application at the time of re-ensiling. Thus, the present study proposes to examine the effects of re-ensiling time and Lactobacillus buchneri on the fermentation profile, chemical composition and aerobic stability of sugarcane silages.
Materials and methods
Experimental area and ensiling
Sugarcane (Saccharum officinarum) variety RB92579 was grown on Muricy Farm, located in the municipality of São Gonçalo dos Campos - BA, Brazil (12°23′49.5″S and 38°52′43.5″W; 234 m altitude). According to the Köppen classification, the region's climate is an As type (semi-humid tropical with dry summers) with mean temperatures above 18°C and average annual precipitation of 900–1200 mm, classified as semi-arid (Alvares et al., Reference Alvares, Stape, Sentelhas, Goncalves and Sparovek2014).
The sugarcane was harvested at 14 months after planting and ground through a stationary forage chopper (EN93B, NOGUEIRA MÁQUINAS AGRÍCOLAS, São João da Boa Vista, SP, Brazil). Subsequently, the forage was ensiled in four plastic drums of 200-L capacity each, aiming at a density of 700 kg/m3 of fresh matter (FM).
Experimental design and re-ensiling
The experiment was set up as a repeated measure design with a 4 × 2 factorial arrangement consisting of four air-exposure periods (EP) (0, 6, 12 and 24 h) and use of microbial additive (A)(Lactobacillus buchneri; or lack of there), with five replicates.
Drums were opened after 210 days of storage. A sample of approximately 1 kg was collected, and the remaining material was separated into two stacks, one of which was inoculated with L. buchneri (105 cfu/g FM). Next, each replicate's stacks were re-ensiled at the pre-established re-ensiling times in PVC silos (0.5 m height × 0.1 m diameter) with Bunsen valves on the lids to allow gas outlet (average density 700 kg/m3 FM). One kilogram of sand was placed at the bottom of each bucket and covered with a screen to separate the ensiled material to capture fluids and determine effluent losses. Silos were weighed on the day of filling and at the opening.
The experimental silos were opened at 120 days after re-ensiling, totalling 330 days from the moment the material was first ensiled. Upon opening, FM samples of approximately 300 g were collected for laboratory analyses.
Fermentation parameters
The pH was measured in samples of fresh silage using a portable digital pH meter, following the methodology described by Bolsen et al. (Reference Woolford1992). Fresh samples were also used to determine the buffering capacity (BC), following the technique proposed by Playne and McDonald (Reference Playne and McDonald1966).
Organic acids and lactic acid were determined at the Federal University of Viçosa (UFV) by high-performance liquid chromatography (HPLC), using an Aminex® HPX-87H column (30 cm × 4.5 mm, Bio-Rad Laboratories Ltd.) in a Thermo Scientific™ chromatograph, following the methodology described by Muck and Dickerson (Reference Muck and Dickerson1988). A total of 20 μl of each sample was injected and the acids were detected using water in 0.05 mmol/l sulfuric acid (H2SO4) as a mobile phase with a column flow of 0.8 ml/min, under 87 kgf pressure. The compounds were monitored with a UV detector (model SPD10A VP) regulated at a wavelength (λ) of 210 nm.
The ammoniacal nitrogen concentration (N-NH3) was determined by colorimetry, using the aqueous extract from the silage, as described by Chaney and Marbach (Reference Chaney and Marbach1962).
Dry matter and effluent losses
Prior to being opened at 120 days after re-ensiling, the experimental silos were weighed and then opened and weighed again. Upon opening, the weight of the silos with sand was also recorded to calculate effluent losses. The parameters were estimated according to equations described by Jobim et al. (Reference Woolford2007).
Aerobic stability
Approximately 1 kg of silage was placed on an aluminium tray and the temperature was recorded in triplicate, at 1-h intervals, over 96 h (room temperature: 25°C). Aerobic stability was calculated as the time taken by the silage mass to reach a temperature of 2°C higher than room temperature (Taylor and Kung Jr., Reference Taylor and Kung2002).
Chemical composition
Samples were pre-dried in a forced-air oven at 55 °C for 72 h and ground through a Wiley mill with a 1-mm sieve. Next, the concentrations of DM (Method 934.01), organic matter (OM; Method 924.05), crude protein (CP; Method 920.87) and ether extract (EE; Method 920.39) were determined by following the methodologies described by AOAC (1990). The concentration of neutral detergent fibre (NDF) was determined as proposed by Van Soest et al. (Reference Van Soest, Robertson and Lewis1991), using amylase and without using sodium sulphite. Non-fibrous carbohydrates (NFC) were estimated according to the equation proposed by Mertens (Reference Mertens1997), while soluble carbohydrates (SC) were estimated as suggested by Dubois et al. (Reference Dubois, Gilles, Hamilton, Rebers and Smith1956).
Statistical analyses
All variables were subjected to statistical analysis (PROC MIXED – SAS), adopting a repeated measures design (Kaps and Lamberson, Reference Kaps and Lamberson2017) in which the following statistical model was employed:
where Yijk = observed response to additive i at exposure time j, in replicate k; μ = observed overall mean; Ai = fixed effect of additive i (i = Without, With); ℧ik = random error associated with additive i; EPj = fixed effect of air-exposure period j (j = 0, 6, 12, 24 h); EPj × Ai = interaction effect between additive and exposure period; ɛijk = random error associated with air-exposure period and interaction.
For the effect of the air-exposure period, the variables were evaluated by orthogonal polynomial trend test (POLYANOVA), the coefficient for unequally spaced polynomial Linear (−0.592 −0.254 0.085 0.761) and Quadratic (0.564 −0.322 −0.645 0.403) was estimated using PROC IML (SAS). For variables in which a significant interaction effect was detected, the interaction effect was decomposed (PROC MIXED – SAS), with the additive effect being evaluated within each exposure time (F test) and the effect of the air-exposure period within each additive was described before (PROC MIXED – SAS). For all the evaluated models, a significance level of 0.05 was considered for type-I error.
Results
Fermentation parameters
There was no interaction effect between L. buchneri and air exposure time before re-ensiling (P < 0.05) for pH, N-NH3, acetate, propionate, or butyrate contents (Table 1). However, an interaction effect was detected for ethanol and lactate concentrations, both of which responded quadratically. However, at 6 h of air-exposure, the additive led to a reduction of ethanol levels, but the opposite effect occurred in the other periods (Tables 1 and 2). The increasing air-exposure period to which the silages were subjected before re-ensiling promoted a quadratic response on propionate and N-NH3 contents.
Table 1. Fermentation profile of sugarcane silages with and without L. buchneri re-ensiled after four air-exposure periods

a % of total nitrogen.
b g kg−1 DM; A = additive effect; EP = effect of air-exposure period; A × EP = interaction effect between additive use and air-exposure period. SEM = standard error of the mean. L = linear effect of air-exposure period; Q = quadratic effect of air-exposure period. *Significance level of 5% for the F test and regression models.
Table 2. Decomposition of the interaction effects on the fermentation profile of sugarcane silages with and without L. buchneri, re-ensiled after four air-exposure periods

a (g/kg−1 DM); *Means in the same column followed by different lowercase letters differ significantly according to the F test at the 5% significance level.
The butyrate levels declined (P < 0.05) in the silages containing L. buchneri as independently of re-ensiling times (Table 2).
The lowest ethanol concentration (148.64 g kg−1) was observed in the silage without L. buchneri at 18.8 h of exposure to air before re-ensiling. However, with the inclusion of the additive, the lowest concentration (161.80 g kg−1) was detected at 11.6 h of air exposure, before re-ensiling (Table 2).
When the interaction effect was decomposed into separate factors, the lactate content in the silage treated with L. buchneri reduced linearly as the air-exposure period increased.
Dry matter and effluent losses
There was an effect (P < 0.05) of microbial additive (L. buchneri) and aerobic exposure time (0, 6, 12 and 24 h) before re-ensiling for effluent losses in the sugarcane silages. The use of L. buchneri also resulted in increased effluent losses (Table 3).
Table 3. Mean values of dry matter losses (DML) and effluent losses (EL) in sugarcane silages with and without L. buchneri, re-ensiled after four air-exposure periods

A = additive effect; A × EP = interaction effect between additive use and air-exposure period. SEM = standard error of the mean. *Means in the same column followed by different lowercase letters differ significantly according to the F test at the 5% significance level. DML in g kg−1 DM and EL in kg t −1 FM. L = linear effect of air-exposure period; Q = quadratic effect of air-exposure period.
An interaction effect between the use of L. buchneri and re-ensiling time was observed (P < 0.05) for DML (Fig. 1).

Fig. 1. Decomposition of the interaction for dry matter losses in sugarcane silages subjected to different air-exposure periods (EP), with or without L. buchneri.
Aerobic stability
The use of L. buchneri did not influence (P < 0.05) the aerobic stability of the sugarcane silages. The silages treated with L. buchneri lost stability at 41.98 h of air exposure. However, those without inoculant had their stability disrupted at 42.26 h of air exposure (Table 4).
Table 4. Aerobic stability (AS), maximum temperature achieved (maxT), time to reach maximum temperature (HT) and difference between maximum and minimum temperatures (D) in sugarcane silages with and without L. buchneri, re-ensiled after four air-exposure periods

N = without additive; Y = with additive.
a Time when aerobic stability was disrupted (silage temperature 2°C above room temperature).
b Temperature in °C.
c Time in hours; A = additive effect; A × EP = interaction effect between additive use and air-exposure period before re-ensiling. SEM = standard error of the mean. *Significance level of 5% for the F test and regression models.
There was a linear decrease in the time for disruption of aerobic stability (P < 0.05) in the silages as the re-ensiling time progressed. The longest time for the disruption of stability was at 0 h of exposure to air. The worst result was seen in the silages exposed to air for 24 h before re-ensiling.
Chemical composition
An interaction effect was observed for all chemical composition variables, except the CP and SC concentrations (Table 5).
Table 5. Chemical composition (g/kg DM or otherwise stated) of re-ensiled sugarcane silages as a function of air-exposure period and use of microbial additive (L. buchneri)

a g/kg (as-is basis); DM = dry matter.
b MM = mineral matter.
c CP = crude protein.
d NFC = non-fibrous carbohydrates.
e NDF = neutral detergent fibre.
f SC = soluble carbohydrates. A = additive effect; A × EP = interaction effect between additive use and air-exposure period before re-ensiling; SEM = standard error of the mean; L = linear effect of air-exposure period; Q = quadratic effect of air-exposure period.*Significance level of 5% for the F test and regression models.
The silage DM content responded quadratically in the silages without microbial inoculant and linearly in those with the additive, with the increasing re-ensiling periods (Fig. 2).

Fig. 2. Dry matter (DM) content of re-ensiled sugarcane silages as a function of air-exposure periods (EP) with or without microbial additive (L. buchneri).*Differ significantly within air-exposure periods according to the F test (P < 0.05).
For the NFC concentration, a linear effect was observed in the silages inoculated with L. buchneri. However, no regression equation was fitted for the non-inoculated silages regarding the NFC (Fig. 3).
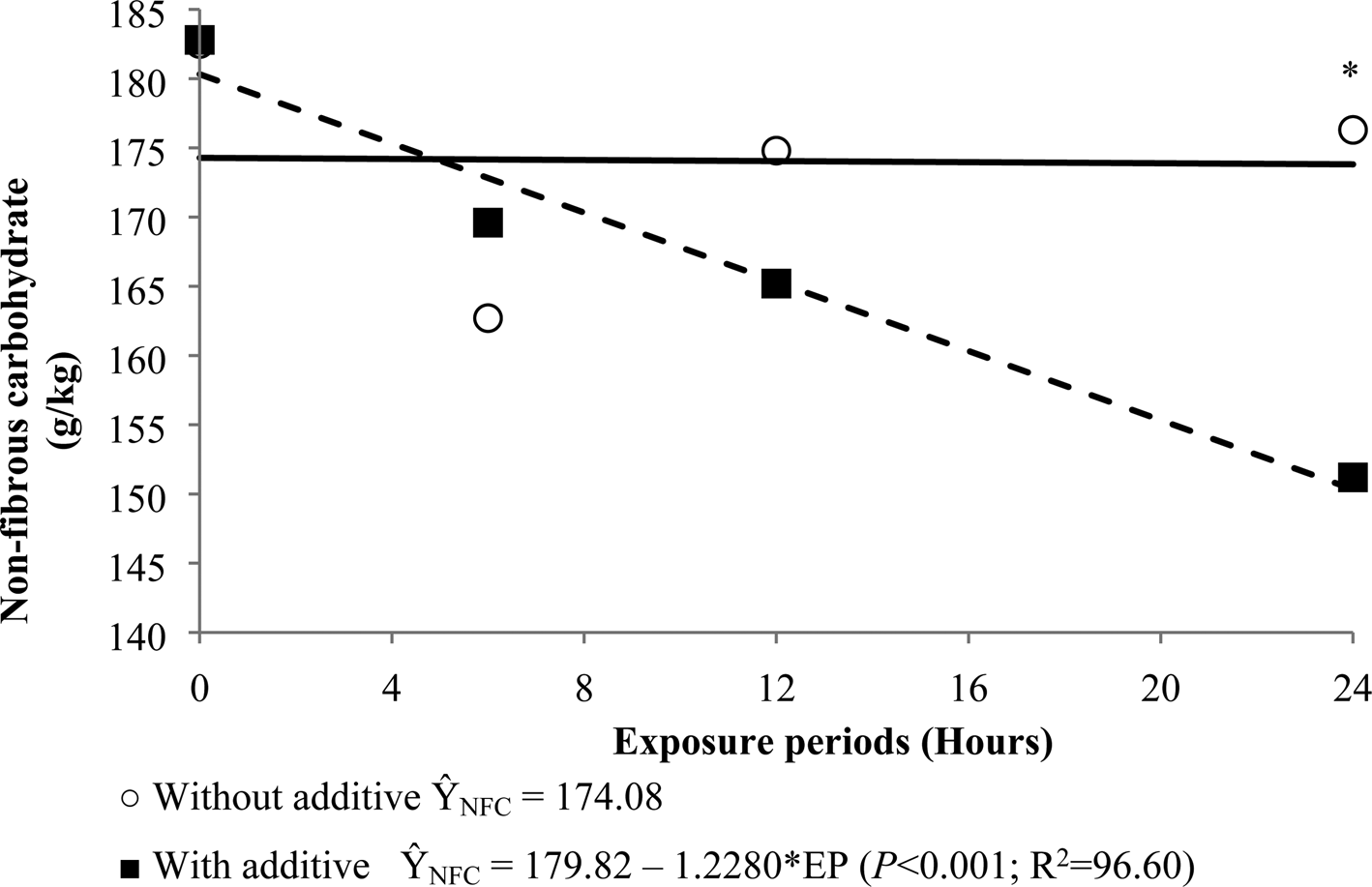
Fig. 3. Non-fibrous carbohydrates (NFC) content of re-ensiled sugarcane silages as a function of air-exposure periods (EP) with or without microbial additive (L. buchneri). *Differ significantly within air-exposure periods according to the F test (P < 0.05).
Decomposing the interaction effect observed for the silages' NDF content into separate factors revealed a quadratic response of this variable to the re-ensiling times in the silages with L. buchneri. The highest concentration of NDF (755.35 g kg−1) was observed at 14.3 h of air exposure when the inoculant was not used (Fig. 4). Besides, a linear effect was observed in the NDF content of silages inoculated with L. buchneri.

Fig. 4. Neutral detergent fibre (NDF) content of re-ensiled sugarcane silages as a function of air-exposure periods (EP) with or without microbial additive (L. buchneri). *Differ significantly within air-exposure periods according to the F test (P < 0.05).
The silage MM content responded quadratically in the silages without microbial additive, with the increasing re-ensiling periods. However, no regression equation was fitted for the inoculated silages regarding the MM content (Fig. 5).

Fig. 5. Mineral matter (MM) content of re-ensiled sugarcane silages as a function of air-exposure periods (EP) with or without microbial additive (L. buchneri). *Differ significantly within air-exposure periods according to the F test (P < 0.05).
Discussion
At 24 h of air exposure, additive use had reduced the DM content in the silage, although the opposite effect was found at 8 h of exposure. This can be explained by the increasing effluent losses observed, which followed the same trend.
The increase observed in the NDF content of the silages with L. buchneri as the air-exposure time progressed may be related to NFC's reduction. Freitas et al. (Reference Freitas, Pereira, Rocha, Costa, Leonel and Ribeiro2006), Pedroso et al. (Reference Pedroso, Nussio, Loures, Paziani, Ribeiro, Mari, Zopollatto, Schmidt, Mattos and Horii2008), Mendes et al. (Reference Mendes, Susin, Nussio, Pires, Rodrigues and Urano2008) and Valeriano et al. (Reference Valeriano, Pinto, Ávila, Evangelista, Tavares and Schwan2009) also reported an increase in the NDF content of sugarcane silages inoculated with the same additive.
Erickson et al. (Reference Erickson, Whitehouse and Spangler2012) also described a lack of statistical differences in pH and acetate and propionate levels in the silage regardless of the air-exposure time before re-ensiling. Those authors evaluated corn silages treated with a commercial microbial additive containing lactic acid bacteria (L. plantarum, Pediococcus pentosaceus and Enterococcus faecium) that were exposed to air for seven different periods (0, 24, 48, 72, 120, 240 and 336 h) and observed no differences between the silages with and without inoculant. They also reported that the pH values remained close to 3.8 for up to 72 h of exposure.
The silages treated with L. buchneri showed no reduction (P > 0.05) of N-NH3 levels. In both silages with or without the inoculant, the N-NH3 levels were below the threshold of 10% of the total nitrogen proposed by Woolford (1984), McDonald et al. (Reference McDonald, Henderson and Heron1991) Van Soest (Reference Van Soest1994). This result should not be evaluated separately as an indicator of silage quality because sugarcane has a low protein content. This fact was observed in the current trial, in which the CP content of fresh sugarcane was 21.2 g/kg DM.
The decreasing butyrate levels observed in the silages with L. buchneri might have been a consequence of the improved fermentation environment. However, no such finding was reported by Silva et al. (Reference Silva, Pereira, Silva, Leandro, Paula, Santos, Ribeiro and Valadares Filho2018), who evaluated the effect of additives containing five strains of this heterofermentative lactic bacteria isolated from corn silages, in a tropical environment, in the making of sugarcane silages.
When the interaction effect between the bacterial additive and air-exposure time before re-ensiling was decomposed into factors, lower value to ethanol and lactate were observed in the silages enriched with L. buchneri in the periods of 12 h and 24 h of air exposure, respectively. The average ethanol content in the silages with inoculant was 175.1 g/kg, whereas the non-enriched silages averaged 164.6 g/kg for this variable. The present values are lower than those described by Freitas et al. (Reference Freitas, Pereira, Rocha, Costa, Leonel and Ribeiro2006), who reported the same trend, in which the silages with and without the microbial additive exhibited ethanol contents of 193 and 178 g/kg, respectively. Pedroso et al. (Reference Pedroso, Rodrigues, Barioni Júnior and Souza2011), on the other hand, added an L. buchneri-based additive (5 × 104 cfu/g) in the ensiling of sugarcane and obtained an 18% reduction in ethanol concentration.
According to Ranjit and Kung Jr. (Reference Ranjit and Kung2000), inoculants containing L. buchneri induced a fermentation profile that produces acetic acid over lactic acid, inhibiting yeasts' growth, which is responsible for alcoholic fermentation. The lack of differences (P > 0.05) in the concentration of acetic acid between the silages with and without microbial additive explains the unchanged ethanol contents in the silages with additive, which might have been due to the characteristics of the material used in ensiling.
At the air-exposure time of 0 h, no differences were detected in ethanol content between the silages with and without L. buchneri. The additive effectively reduced the ethanol concentration in the silages re-ensiled after 6 h of aerobic exposure, which had the lowest concentration of the alcohol (143.7 g/kg). However, these values can still be considered high when compared with those reported by Ávila et al. (Reference Ávila, Pinto, Oliveira and Schwan2012). They inoculated sugarcane with a strain identified in sugarcane silage (UFLA SL 72) before ensiling and obtained a significant reduction in ethanol content, which decreased from 61.4 g/kg, in the control treatment to 21.1 in the silages with the additive.
Another possible explanation for the elevated ethanol levels observed in the present experiment is the fact that the sugarcane used contained a large amount of SC (up to 70.0 g/kg DM). Additionally, its BC (2.59 meq100/g DM) was much lower than the 7.0–10.8 meq.100/g described in the literature for sugarcane (Siqueira et al., Reference Siqueira, Reis, Schocken-Iturrino, Pires, Bernardes and Amaral2007; Silva et al., Reference Silva, Borgatti, Meyer, Marino and Rodrigues2008; Valeriano et al., Reference Valeriano, Pinto, Ávila, Evangelista, Tavares and Schwan2009). This elevated sugar content associated with low BC contributes to a rapid pH decline, favouring the growth of ethanol-producing yeasts (Driehuis and Wikselaar, Reference Driehuis and Wikselaar2000).
The lowest effluent losses were recorded at the air-exposure time of 0 h before re-ensiling. This was an expected finding since exposure to air causes water to be lost to the environment, which was verified by the increase in this parameter up to 6 h of air exposure. However, the reduction in effluent losses observed between 12 and 24 h of aerobic exposure was because the effluent amount had already decreased as the aerobic exposure time progressed.
The slight reduction in DM losses in the silages with L. buchneri from 0 to 6 h of air exposure and the occurrence of the opposite effect from 6 to 12 h of exposure is explained by the effluent losses, whose results are inversely proportional.
The lack of effects of inoculation in the sugarcane silages before re-ensiling on aerobic stability conflicts with literature findings in that, by inducing an increase in acetic acid, L. buchneri should inhibit mold and yeast growth and improve silage preservation. Positive results from the use of L. buchneri in the ensiling of sugarcane were reported by Siqueira et al. (Reference Siqueira, Reis, Schocken-Iturrino, Pires, Bernardes and Amaral2007), who observed an increase of 32–60 h in the time before stability was disrupted.
However, L. buchneri did not increase acetic acid production in the present study, which may explain the lack of significant effects on the stability of the produced silages. Similar results were found by Michel et al. (2017), who used other heterofermentative microorganisms (L. plantarum and Propionibacterium acidipropionici) in the inoculation of sorghum silages and did not observe influences on their aerobic stability.
The reduction of 10.5 h in the time before stability was disrupted and was expected, since, as stated by Wilkinson and Davies (Reference Wilkinson and Davies2012), the presence of oxygen in the ensiled material favours the growth of aerobic microorganisms (yeasts and molds), which use soluble carbohydrates and lactic acid, increasing the temperature and pH.
Conclusions
Lactobacillus buchneri is not effective in improving aerobic stability in re-ensiled sugarcane silages. However, less DM is lost in silages treated with L. buchneri and exposed to air for 24 h.
Re-ensiling sugar cane in up to 24 h of exposure to air does not change final product quality.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Not applicable.













