Introduction
Grasses (Poaceae) are the major component of the host range of numerous phytophagous arthropods (Tscharntke & Greiler, Reference Tscharntke and Greiler1995; Frost & Ridland, Reference Frost, Ridland, Lindquist, Sabelis and Bruin1996). Many grass-associated insects and mites are known as important pests since they attack and feed on crop grasses and, thus, reduce crop yields and increase production costs (Metcalf, Reference Metcalf and Ruberson1999). The losses may increase due to viral diseases which are vectored by phytophagous arthropods (Thresh, Reference Thresh1983). Herbivorous arthropods can colonize new host species and, thus, change or extend their host ranges, by shifting from one to another host (Bush, Reference Bush1994). An important question from an applied point of view is whether grass-feeding arthropods, especially those potentially vectoring grass viruses, can effectively colonize crop grasses, particularly those that are widely used as food crops (such as corn, sugarcane, rice and wheat).
Mites are second only to the insects in their economic importance as pests of Poaceae. One of the most notable among mites causing losses in cultivated grasslands is the cereal rust mite, Abacarus hystrix (Nalepa) (Prostigmata: Eriophyidae). It is able to seriously damage its host plants since its feeding causes leaf discoloration and inhibition of seed production, and at high densities the mite causes withering of plants and retards their growth (Frost & Ridland, Reference Frost, Ridland, Lindquist, Sabelis and Bruin1996). Abacarus hystrix is also known as a vector of two plant viruses. It transmits ryegrass mosaic virus (RgMV), one of the most serious and widespread viruses infecting perennial ryegrass and a wide variety of other pasture grasses, which causes chlorosis and necrosis of leaves. The second virus is agropyron mosaic virus (AgMV), which commonly infects quackgrass and occasionally spreads to wheat, on which it causes severe chlorosis and stunting (Oldfield & Proeseler, Reference Oldfield, Proeseler, Lindquist, Sabelis and Bruin1996). Since passive air dispersal has been reported for the cereal rust mite (Nault & Styer, Reference Nault and Styer1969), it would appear that the mite and the pathogens it transmits could spread very quickly through pastures and crops.
The eriophyid mites are regarded as the most highly adapted for plant feeding among the Acari. The majority of eriophyoid species are specialized feeders intimately associated with their hosts and usually reported from a single or a few closely related host plants (Oldfield, Reference Oldfield, Lindquist, Sabelis and Bruin1996). The cereal rust mite is one of a few exceptional eriophyoid species for which a broad host range has been reported. It has been found infesting wheat, barley, oats, rye, rice, ryegrass, quackgrass, timothy, orchardgrass and many other (ca. 60–70) cultivated and wild grass species (many of them of great economic importance) throughout the world (Amrine & Stasny, Reference Amrine and Stasny1994).
In some regions (mostly in North America), quackgrass Elymus repens (L.) Gould was reported as a major host of the cereal rust mite (Nault & Styer, Reference Nault and Styer1969), whereas in other regions (e.g. in Australia and Great Britain) the mite has been recorded mostly from ryegrass (Lolium spp.) (Gibson, Reference Gibson1974; Frost & Ridland, Reference Frost, Ridland, Lindquist, Sabelis and Bruin1996). In Poland among 40 other grass species, A. hystrix has been found on both quackgrass and ryegrass at relatively high densities: 68.6 (59.5–79.7, 95% confidence interval (CI)) and 33.2 (27.6–40.0, 95% CI) specimens per shoot, respectively, as well as on wheat, but at a much lower density: 3.5 (2.3–5.6, 95% CI) (Skoracka, Reference Skoracka2004).
The differences in the colonization ability of A. hystrix on different host plants have been explained as the likely existence of biotypes adapted to specific members of the Poaceae (Gibson, Reference Gibson1974), and thus host specialization is possible in this species. Recent detailed studies have shown that populations of A. hystrix inhabiting quackgrass and perennial ryegrass are highly adapted to their native hosts and have no success in the colonization of the opposite host (Skoracka & Kuczyński, Reference Skoracka and Kuczyński2006a; Skoracka et al., Reference Skoracka, Kuczyński and Rector2007). It has also been shown that the two populations differ in their phenotypes (i.e. body shape, overall body size and life history traits) (Skoracka et al., Reference Skoracka, Kuczyński and Magowski2002; Skoracka & Kuczyński, Reference Skoracka and Kuczyński2006b). Moreover, reproductive barriers between these two populations have been demonstrated (Skoracka, Reference Skoracka2008). Finally, preliminary observations on host-dependent genetic variation have shown that the two populations can be differentiated on the basis of the mitochondrial marker COI (Skoracka & Dabert, unpublished data). Such host-associated differences, correlated with strong host fidelity, distinct phenotypes and reproductive isolation, were, however, found only between quackgrass and ryegrass populations of A. hystrix. It has not been shown, so far, whether both these mite populations can successfully colonize other grass species, including cereals.
In the present study, wheat was tested as a cereal capable of being colonized by quackgrass- and ryegrass-adapted mites. Regarding habitat requirements and phylogenetic relationships, quackgrass is more closely related to wheat than to perennial ryegrass. Apart from on roadsides, balks and abandoned land, quackgrass commonly grows in wheat fields and acts as a serious weed of this cereal. Ryegrass frequently grows in grasslands, grass plots, sport fields and near airfields (Chapman, Reference Chapman2002). Morphological, serological, anatomical, chromosomal and molecular analyses showed that Lolium L. (ryegrass) is a different lineage than Elymus L. (quackgrass) and Triticum L. (wheat) (Kellogg, Reference Kellogg1998; Mathews et al., Reference Mathews, Tsai and Kellogg2000). Thus, Lolium belongs to the tribe Poeae, whereas Elymus and Triticum belong to the tribe Triticeae; both tribes belong to the subfamily Pooidae (Grass Phylogeny Working Group, 2001; Clayton et al., Reference Clayton, Harman and Williamson2006). The host range of phytophagous arthropods is often limited to a group of closely related host plant species (Ehrlich & Raven, Reference Ehrlich and Raven1964; Jaenike, Reference Jaenike1990); thus, the relatedness of wheat to quackgrass and ryegrass may influence the host range of the studied mite species. Considering the above observations, the potential for wheat colonization by the quackgrass-associated population of A. hystrix appears more likely than by the ryegrass-associated mites. Here, the above hypothesis was tested by comparing the colonization performance (assessed by female survival and fecundity) of quackgrass- and ryegrass-associated populations of the cereal rust mite on their familiar hosts and on wheat.
Material and methods
Plants
Three grass species – quackgrass, Elymus repens; perennial ryegrass, Lolium perenne L.; and common wheat, Triticum aestivum L. – were used as experimental plants. Ryegrass and quackgrass rhizomes were collected in September 2006 from two separate field sites in Poznan, Poland (ryegrass: E 16° 56.0′, N 52° 28.0′; quackgrass: E 16° 55.6′, N 52° 27.9′). In the laboratory, rhizomes were put in boxes with loamy and sandy soil, respectively. Plants were kept at room temperature and exposed to artificial light for 19 h per day. The boxes were covered with nylon taffeta fastened to the wooden frame in order to protect plants from infestation by arthropods. When sufficiently grown, plants were used for the experiment. For this purpose, shoots (8–10 cm in height) were transplanted from boxes to plant pots (7.5 cm in height; 6 cm in diameter). Each grass shoot was transplated to one pot. Ryegrass shoots were transplated to pots with loamy soil, and quackgrass shoots were transplated to pots with sandy soil. Wheat was cultivated directly from seeds (originating from the wheat of one field near Poznan, E 17° 15.1′, N 52° 10.0′) by putting them in plant pots (the same diameter as above) with loamy soil. Ryegrass and wheat were watered twice a week, and quackgrass was watered once a week.
Experimental set-up
Mites for the experiments were collected from field-grown ryegrass and quackgrass. They were obtained from separate field sites in Poznań, Poland, from three sites with quackgrass: (i) E 16° 55.6′, N 52° 27.9′; (ii) E 16° 55.7′, N 52° 22.9′ and (iii) E 16° 53.0′, N 52° 24.5′ and three sites with ryegrass: (i) E 16° 55.4′, N 52° 22.2′; (ii) E 16° 56.0′, N 52° 28.0′; (iii) E 16° 56.3′, N 52° 24.1′. In the laboratory, plants were checked under the stereomicroscope and females from field-collected plants were transferred to uninfested experimental plants grown in pots. Females were transferred either to the same grass species as they originated from (familiar host) or to wheat. Mites were transferred using an eyelash glued to a preparatory needle. Females of A. hystrix can be distinguished from other species or conspecific males and immatures by the presence of a dorsal ridge or by the body shape and dimensions, respectively. A few specimens from each sample were mounted on slides and deposited in the Department of Animal Taxonomy and Ecology, Adam Mickiewicz University, Poznań, Poland.
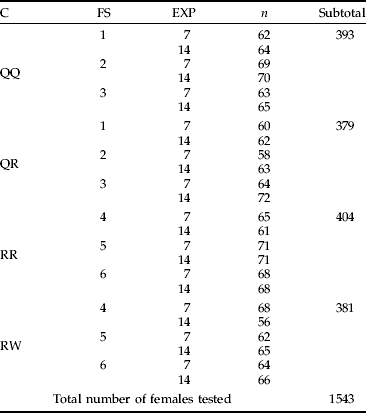
Four combinations were tested: (i) QQ – females from quackgrass transferred to quackgrass (control); (ii) QW – females from quackgrass transferred to wheat; (iii) RR – females from ryegrass transferred to ryegrass (control); and (iv) RW – females from ryegrass transferred to wheat. Each combination was repeated 15 times using mites from three separate field sites. Within each combination and each field site five repetitions were carried out. One repetition was defined as a single plant with 15 females transferred to it. After ten minutes, plants were checked to count the number of females that had successfully settled on the leaf. This number was assumed to be the number of females engaged in the experiment. Females that looked dead or injured were removed from the plants. Pots with experimental plants were put into cages consisting of nylon taffeta fastened to a metal frame, one pot per cage. They were maintained under room conditions (21°C, 60%±5 RH) for seven or 14 days. Afterwards, the plants were checked and the numbers of mites (experimental females and their progeny) were counted using a stereomicroscope. Thus, two types of experiments differing in the longevity of their duration were carried out; and, for each type of experiment, 15 replications were carried out. From the seven-day long (EXP7) experiment the numbers of eggs and larvae were obtained; whereas from the 14-day experiment (EXP14) the numbers of all immature stages (eggs, larvae and nymphs), as well as young F1 adults, were obtained. Data received from these two experiments were analyzed separately. A summary of experimental procedures is given in table 1.
Table 1. Numbers of females used for experiments (n).
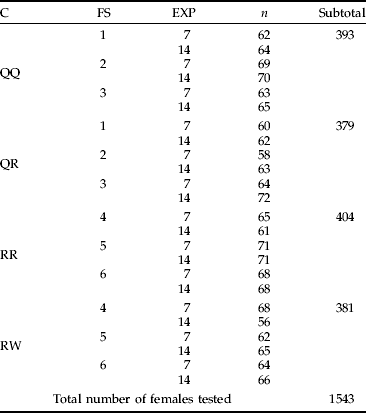
C, combination; FS, the field site from which mites were collected (described in the text); EXP, the type of experiment (seven or 14 days long).
Data analysis
Three components of colonization ability were measured: female fecundity and survival, and the mean number of progeny of respective stage (i.e. egg, larva, nymph or adult F1) obtained within each trial. Mean fecundity of females was calculated as the total number of progeny (all stages) divided by the number of female-days (total number of observation days of all females). Weekly or bi-weekly (depending on the length of the experiment, seven or 14 days) rates of female survival were calculated from the formula proposed by Trent & Rongstad (Reference Trent and Rongstad1974), S=D m, where S is the estimate finite survival rate per m days, m is the number of days (duration of the experiment) and D is the estimated daily survival calculated from the formula D=(x−y)/x, where x is the total number of female-days observed over the period and y is the total number of deaths observed over the period.
One-way ANOVA was used to test the differences in all parameters (fecundity, survival and mean number of progeny) between field sites within each combination. No significant differences were found (P>0.1 in all cases); thus, data obtained from three field sites within each combination were pooled.
To test the differences between means, one-way ANOVA followed by Tukey test and the criterion of confidence intervals (CI) overlapping (i.e. means were regarded as ‘significantly different’ when their CI did not overlap) were applied. For all computations, the S-PLUS software was used (S-PLUS 7.0: Insightful Corpation, 2005).
Results
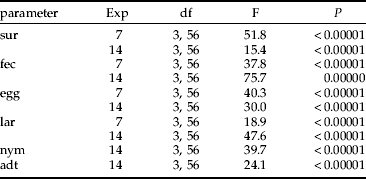
Highly significant differences in female fecundity, survival and mean number of progeny of all stages were found between all combinations tested (table 2).
Table 2. Results of ANOVA comparing colonization ability, expressed as female survival (sur), fecundity (fec), and mean number of progeny: eggs (egg); larva (lar); nymphs (nym); adults (adt) between combinations.
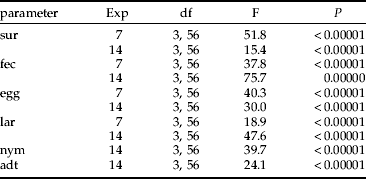
Exp, the type of experiment.
The mean number of progeny produced by ryegrass-associated females (R-females) transferred to wheat (combination RW) was significantly lower compared to the mean number of progeny obtained in all other combinations (RR, QQ, QW). There were no significant differences between the mean numbers of progeny of all stages produced by quackgrass-associated females (Q-females) reared on quackgrass (QQ, familiar host) and on wheat (QW). The mean number of progeny produced by Q-females was slightly higher on wheat than on quackgrass, except the mean number of adults, which was slightly higher on quackgrass. On the other hand, the mean number of progeny of all stages produced by R-females was significantly higher on ryegrass (RR, familiar host) compared to wheat (RW). The mean number of larvae, nymphs and adults produced by Q-females on their familiar host was significantly higher compared to R-females, whereas the differences in the mean number of eggs produced by Q-females and R-females on their familiar host were significant only in the seven-day experiment (fig. 1).

Fig. 1. Mean number of progeny produced by Abacarus hystrix females when reared on their familiar hosts (combinations QQ and RR) and on wheat (QW and RW) during seven- and 14-day experiments. Bars represent 95% CI around means. Different letters represent significant differences (P<0.05) between combinations based on Tukey test performed after Anova. White bars represent control combinations.
The survival and fecundity of R-females on wheat (RW) was significantly lower when compared to all other combinations (QQ, QW, RR). The survival of Q-females was similar on both familiar host (QQ) and on wheat (QW), whereas the survival of R-females was significantly higher on familiar host (RR) compared to wheat (RW). The results were consistent for both types of experiments (seven and 14 days) performed. The survival of Q-females on their familiar host and wheat (QQ and QW) was comparable to the survival of R-females on their familiar host (RR) in the seven-day experiment; whereas, in the 14-day experiment, the survival of R-females on ryegrass (RR) was significantly lower than the survival of Q-females on both types of hosts (QQ and QW) (fig. 2).

Fig. 2. Survival (sur) and fecundity (fec) of females of Abacarus hystrix reared on their familiar hosts (combinations QQ and RR) and on wheat (QW and RW) during seven- and 14-day experiments. Bars represent 95% CI around means. Different letters represent significant differences (P<0.05) between combinations based on Tukey test performed after Anova. White bars represent control combinations.
The fecundity of Q-females was significantly lower on their familiar host (QQ) compared to wheat (QW) in the seven-day experiment, whereas in the 14-day experiment the fecundity of Q-females was similar on both types of hosts. The fecundity of R-females was significantly lower on wheat (RW) compared to the familiar host (RR), consistently in both types of experiments. The fecundity of R-females on their familiar host (RR) was significantly lower compared to fecundity of Q-females on both their hosts (QQ and QW) in the 14-day experiment; whereas, in the seven-day experiment, the fecundity of R-females on the familiar host (RR) was not significantly different compared to fecundity of Q-females on their familiar host and wheat (QQ and QW) (fig. 2).
Discussion
Faunistic and ecological observations carried out in many regions of the world have shown that ryegrass and quackgrass are of major importance as host plants for the cereal rust mite, Abacarus hystrix. The mite, however, can also feed on cereals, mainly wheat (Amrine & Stasny, Reference Amrine and Stasny1994; Frost & Ridland, Reference Frost, Ridland, Lindquist, Sabelis and Bruin1996). Several experiments have demonstrated that populations of this mite inhabiting perennial ryegrass and quackgrass are different biotypes (at least host races, and probably separate species) since they are highly adapted to their familiar hosts, differ in some phenotypic features and are reproductively isolated (Skoracka & Kuczyński, Reference Skoracka and Kuczyński2006a,Reference Skoracka and Kuczyńskib; Skoracka et al., Reference Skoracka, Kuczyński and Rector2007; Skoracka, Reference Skoracka2008). The present study, in addition, shows that the two host-adapted populations of the cereal rust mite have different potential for wheat colonization, and thus different host ranges.
The ryegrass population of the cereal rust mite had no success in wheat colonization, which was expressed by extremely low fecundity and female survival, as well as the low number of progeny of all stages. In contrast, the quackgrass population survived and developed on wheat quite well. Female survival and fecundity on wheat were as good as on the familiar host; moreover, in the seven-day experiment, survival on wheat was even notably higher than on quackgrass. Also, the numbers of immature stages of A. hystrix were slightly higher on wheat. Thus, the hypothesis that the ability to colonize wheat is more likely for a quackgrass-adapted than a ryegrass-adapted population was confirmed. Furthermore, the results suggest that only the quackgrass-adapted cereal rust mite is able to effectively infest wheat.
These findings are of great importance from an applied point of view. Wheat is not a plant available for mites constantly (it is not available between harvest and sowing), and A. hystrix is not able to survive in the absence of the green parts of its host (e.g. in the soil or on grains). Considering that quackgrass is a common weed in wheat cultures and that quackgrass-associated mites survive and develop on wheat similarly or even better than on quackgrass, it is likely that quackgrass is, indeed, a source of wheat infestation by the cereal rust mite in the field. Under natural conditions in Poland, the cereal rust mite has been found infesting wheat moderately as compared to quackgrass. The prevalence of mites on wheat was not higher than 31%, whereas on quackgrass it was consistently higher than 60%. The intensity and density of mites were also several times higher on quackgrass compared to wheat (Skoracka, Reference Skoracka2004). Such parameters of infestation might suggest that the cereal rust mite does not have great reproductive potential on wheat, whereas the present study has shown that it does. In addition, it is possible that some as yet unexamined environmental factors may be responsible for inhibiting the growth of the cereal rust mite population on wheat in the field. This issue needs further observation. However, it should be noted that quackgrass-adapted A. hystrix is a potential pest of wheat.
It is known that phytophagous arthropod species may arise without geographic barriers in the process of shifting and adapting to new plants. Thus, a single species of phytophagous insects or mites may consist of populations that have different host ranges. Specialization on physiologically different hosts may result in genetic differentiation, and this incipient stage of such speciation is known as a ‘host race’ (Berlocher & Feder, Reference Berlocher and Feder2002; Drés & Mallet, Reference Drés and Mallet2002). Recognition of host-associated biotypes has a great impact on biological control programmes. It is important if multiple strains of the same biological control agent differ in their host range or are genetically differentiated (Clarke & Walter, Reference Clarke and Walter1995). Because of their high host specificity, several eriophyoid species have been investigated for possible use in the biological control of weeds, for example, Phyllocoptes fructiphilus Keifer against Rosa multiflora Thunb. (Jesse et al., Reference Jesse, Moloney and Obrycki2006), Leipothrix dipsacivagus Petanovic et Rector against Dipsacus spp. (Petanovic & Rector, Reference Petanovic and Rector2007), Floracarus perrepae Knihinicki et Boczek against Lygodium microphyllum (Cav.) R. Br. (Ozman & Goolsby, Reference Ozman and Goolsby2005) and Aceria anthocoptes (Nalepa) against Cirsium arvense (L.) Scop. (Rancić et al., Reference Rancić, Stevanović, Petanović, Magud, Toševski and Gassmann2006). A few of the species have also been used in programmes targeting weeds, e.g. Aceria malherbae Nuzzaci against Convolvulus arvensis L. (Michels et al., Reference Michels, Fritts, Bible and Spencer2000) and Aculus hyperici (Liro) against Hypericum perforatum L. (Briese & Cullen, Reference Briese, Cullen, Halliday, Walter, Proctor, Norton and Colloff2001). Rigorous host-specificity testing is necessary to ensure that a biological weed control agent is sufficiently specialized on the target weed and does not impact on non-target species (Messenger et al., Reference Messenger, Wilson, Whitten, Huffaker and Messenger1976). The present results suggest that the quackgrass-adapted race of A. hystrix should not be considered as a biological control agent on quackgrass, particularly in the presence of wheat culture. On the other hand, its performance on wheat has not been experimentally studied in the field. Thus, this question needs further observation.
The present findings propose an interesting explanation for the results of previous studies regarding the host ranges of two viruses transmitted by A. hystrix, agropyron mosaic virus (AgMV) and ryegrass mosaic virus (RgMV). AgMV is known to infest quackgrass and many varieties of wheat and was found to be experimentally transmissible to wheat (Oldfield & Proeseler, Reference Oldfield, Proeseler, Lindquist, Sabelis and Bruin1996). RgMV infects Lolium spp., Dactylis glomerata L., Festuca spp., Poa annua L., Avena sativa L., Bromus spp., Cynosurus cristatus L. and Holcus lanatus L. but is not transmissible to wheat (Salm et al., Reference Salm, Rey and Wolfson1994; Webster et al., Reference Webster, Beck, Rabenstein, Forster and Guy2005). The present findings show that the ryegrass-adapted cereal rust mite (the only vector of RgMV) is not capable of effectively colonizing wheat, so it seems reasonable that the virus has no potential to be transferred to wheat.
The differences in the wheat colonization capability of ryegrass and quackgrass strains of the cereal rust mite are likely attributable to host specialization that may be an effect of phylogenetic relationships among the three grass hosts used in this study. It is recognized (on the basis of phylogenetic analysis and experimental observation) that wheat is more closely related to quackgrass than to ryegrass and that wheat can form hybrids with quackgrass but not with ryegrass. Wheat and quackgrass are morphologically, physiologically and chemically more similar to each other than to ryegrass (Kellog, Reference Kellogg1998; Mathews et al., Reference Mathews, Tsai and Kellogg2000). Such similarity between wheat and quackgrass may explain the ability of the quackgrass strain of the cereal rust mite to successfully perform on wheat, since on wheat the mite meets similar plant compounds to cope with and a similar habitat to inhabit. Related host plants tend to possess the same or similar defences to be overcome by herbivores, and chemical similarities between plants have often been suggested as possible factors in determining the host ranges of phytophagous insects (e.g. Ehrlich & Raven, Reference Ehrlich and Raven1964; Futuyma & McCafferty, Reference Futuyma and McCafferty1990; Becerra, Reference Becerra1997; Renwick, Reference Renwick2001; Murphy & Feeny, Reference Murphy and Feeny2006). It has been shown that in some butterflies and leaf beetles host shifts are most likely to occur among chemically similar plants (reviewed in Jaenike, Reference Jaenike1990). Moreover, it has been demonstrated that many host shifts by phytophagous insects are phylogenetic host shifts between plant species that are closely related. Even species with seemingly wide host ranges are often made up of more specialized populations associated with the same set of plants (Ehrlich & Raven, Reference Ehrlich and Raven1964; Janz et al., Reference Janz, Nyblom and Nylin2001; Agosta, Reference Agosta2006).
The availability of particular host plants (or host plant arrays) as a factor influencing the pattern of host use also has been often mentioned. It has frequently been shown that the biological performance of insects is higher on hosts available on a local or regional scale than on other potential hosts occurring elsewhere in their range but not available (Horton et al., Reference Horton, Capinera and Chapman1988; Jaenike, Reference Jaenike1990; Hébert et al., Reference Hébert, Berthiaume, Bauce and Brodeur2006). Host availability depends on the dispersal ability of phytophagous arthropods and their success rate in finding and settling on a new host (Ward et al., Reference Ward, Leather, Pickup and Harrington1998). Three modes of dispersal between host plants have been proposed for eriophyoid mites: (i) dispersal by wind currents (Nault & Styer, Reference Nault and Styer1969); (ii) phoretic transport by insect carriers (Waite & McAlpine, Reference Waite and McAlpine1992); and (iii) walking between the nearest host plants (Manson & Oldfield, Reference Manson, Oldfield, Lindquist, Sabelis and Bruin1996). For A. hystrix, evidence that wind plays the principal role in mite dispersal has been shown (Nault & Styer, Reference Nault and Styer1969). Thus, when being blown for long distances, both ryegrass- and quackgrass-adapted cereal rust mite might have an opportunity to encounter wheat, and wheat might seem to be an available host for both mite populations. The inability of the ryegrass strain to perform on wheat suggests that the ryegrass mite is adapted to a different phylogenetic tribe of grasses and that colonization of wheat by the cereal rust mite is largely influenced by host phylogeny rather than by the host availability.
Altogether, factors that might play a decisive role in the adaptation of eriophyoid mites to their hosts and influence their host ranges have scarcely been examined. Due to their obligatory and intimate relations with host plants, Eriophyoidea seems to be an ideal subject for studying host specialization. Moreover, because of the important role of eriophyoids as pests, the results of such studies may be of great agricultural significance. The results presented in this study show that (i) quackgrass- and ryegrass-adapted strains of the cereal rust mite have different physiological host ranges and (ii) phylogenetic relationships between host plant species appear to be a driver for host specialization in this mite species.
Acknowledgements
I thank Dr Lechosław Kuczyński (Adam Mickiewicz University), Prof. Greg Spicer (San Francisco State University), Dr David Gillespie (editor of the Bulletin of Entomological Research) and two anonymous reviewers for helpful and valuable remarks on the manuscript. The study was supported by the Polish Ministry of Science and Higher Education (grant no. 3P04C03825).