INTRODUCTION
Modern survey techniques and tools have recently shed light on many aspects of gelatinous midwater animals. Taxonomic work, including new species descriptions, benefits greatly from both specimens sampled in pristine condition (e.g. Haddock et al., Reference Haddock, Dunn and Pugh2005; Kitamura et al., Reference Kitamura, Lindsay and Miyake2005; Pugh, Reference Pugh2006) and from the in situ imagery that can be obtained of fragile gelatinous forms (e.g. Harbison et al., Reference Harbison, Matsumoto and Robison2001; Matsumoto et al., Reference Matsumoto, Raskoff and Lindsay2003; Hopcroft & Robison, Reference Hopcroft and Robison2005). Aspects of their ecology such as interspecies interactions are often also only able to be characterized due to in situ observations with modern technologies (e.g. Lindsay et al., Reference Lindsay, Hunt and Hayashi2001; Drazen & Robison, Reference Drazen and Robison2004; Pagès et al., Reference Pagès, Corbera and Lindsay2007). Recognizing this need, submersible platforms such as the PICASSO system (Plankton Investigatory Collaborating Autonomous Survey System Operon) designed specifically for in situ surveys of fragile planktonic organisms are now under development (Yoshida & Lindsay, Reference Yoshida and Lindsay2007).
The midwater zone is perhaps best characterized by the lack of hard substrate, such as rocks, mud or sand, for the attachment of sessile forms. Understandably, the most common representatives of the gelatinous midwater fauna are groups that undergo direct development from a pelagic planula or larval stage to a pelagic adult, such as trachymedusae, siphonophores and ctenophores. Another common gelatinous midwater taxon, the Narcomedusae, also undergo direct development, but some members of this group possess tentacled larvae that parasitize other medusae, polychaetes or fish, thereby garnering a biological substrate upon which to develop (Bouillon et al., Reference Bouillon, Gravili, Pagès, Gili and Boero2006). Some of these parasitic larvae develop stolo-prolifers that can give rise to numerous medusae, somewhat reminiscent of the colony formation of polyps found in the more diverse taxon Hydroidolina (=Leptolina sensu Schuchert, Reference Schuchert2007a). The present study focuses on midwater representatives of the Hydroidolina, the Anthomedusae, a taxon generally understood to include a polyp stage in their life cycles.
Although hard, abiotic substrates for polyp attachment are not available in the midwater zone, the polyps of some anthomedusae have been reported to colonize living substrates. For example, the polyp of Bythotiara dolioeques Raskoff & Robison, Reference Raskoff and Robison2005 (family Bythotiaridae Maas, 1905) is known to colonize the anterior (intake) opening of the doliolid tunicate Doliolula equus Robison, Raskoff & Sherlock, Reference Robison, Raskoff and Sherlock2005 (family Doliopsidae Godeaux, 1996) (Raskoff & Robison, Reference Raskoff and Robison2005; Robison, Raskoff & Sherlock, Reference Robison, Raskoff and Sherlock2005), while the polyp of Pandea conica (Quoy & Gaimard, 1827) (family Pandeidae Haeckel, 1879) has been reported as being an epizoite on the shell of the holoplanktonic pteropod gastropod Clio cuspidata (Bosc, 1802) (Picard, Reference Picard1956).
The eastern seaboard of Japan off the Sanriku coast and above the Japan Trench is an extremely productive oceanic area with Oyashio-derived cold water masses, Kuroshio-derived warm water masses and eddies, and frontal and mixing zones. The variety of water masses ensures that planktonic diversity is high and this in turn suggests that a wide range of possible planktonic substrates for polyp attachment should exist. Information on the gelatinous zooplankton community is still sparse, with some submersible-based surveys concentrated in the far northern reaches where cold water masses predominate (Vinogradov & Shushkina, Reference Vinogradov and Shushkina2002 and references therein; Toyokawa et al., Reference Toyokawa, Toda, Kikuchi, Miyake and Hashimoto2003), a dive with the French bathyscaphe ‘F.N.R.S.III’ off the Boso Peninsula (Pérès, Reference Pérès1959), a dive with the Japanese submersible ‘Shinkai 6500’ off the Sanriku coast at 39°53′N 144°11′E (Lindsay, Reference Lindsay2005), and a series of dives with the ROV ‘HyperDolphin’ (Lindsay et al., Reference Lindsay, Furushima, Miyake, Kitamura and Hunt2004), also off the Sanriku coast. Only two reports contain information on anthomedusae. The first is a record by Pérès (Reference Pérès1959) of a medusa identified as Charybdeidae at 650 m during the dive on 5 July 1958 which we believe is Pandea rubra Bigelow, Reference Bigelow1913, based on his description of its morphology. The second is a report from the east of Hokkaido Island (42°10.5′N 144°10.5′E), of Euphysa spp. (flammea or japonica) occurring in high abundances at 1250 m depth in the benthopelagic layer at 10–20 m altitude above the bottom and at lower densities at 1200 m depth, with a few scattered individuals also present in the midwater at 400 m depth on 14 and 16 August 1999 (Toyokawa et al., Reference Toyokawa, Toda, Kikuchi, Miyake and Hashimoto2003). Much smaller numbers also occurred on 30 August 1998 and 16 September 2000 at the same survey point (Toyokawa et al., Reference Toyokawa, Toda, Kikuchi, Miyake and Hashimoto2003). The present study aims to shed light on the anthomedusan fauna of the waters above the Japan Trench, far removed both bathymetrically and geographically from possible abiotic substrates for polyp attachment and it is hoped that information on the pelagic adult phase of these organisms might stimulate further work on their sessile, but not necessarily benthic, polyp stages.
The tools and survey platforms used for this work include crewed submersibles (‘Shinkai 6500’ and ‘Shinkai 2000’), remotely-operated vehicles (ROVs ‘HyperDolphin’ and ‘Kaiko’), towed camera arrays (6000 m-class Deep-Tow Camera) and visual plankton recorders (VPRs) mounted on towfish. Such modern in situ observational techniques have revealed aspects of the biology, ecology and taxonomy of the major anthomedusan forms above the Japan Trench and these results are introduced in the present paper.
MATERIALS AND METHODS
Surveys of the midwater fauna off the eastern seaboard of Japan, proximal to or directly over the Japan Trench, have been carried out by the Japan Agency for Marine–Earth Science and Technology (JAMSTEC, previously Japan Marine Science and Technology Center) since 1999. A variety of survey tools and strategies have been employed, as outlined below. Data from other submersible surveys in Japanese waters, not near the Japan Trench, have also been included where relevant.
ROV ‘HyperDolphin’ surveys
A transect that included Oyashio-derived cold waters, Kuroshio-derived warm waters, and their respective mixing and frontal zones was run along the eastern seaboard of Japan using the RV ‘Kaiyo’ from 20 April–6 May 2002. Water masses were identified using satellite imagery and XCTD (eXpendable Conductivity Temperature Depth) probes, and dive surveys using the ROV ‘HyperDolphin’ were carried out to determine the vertical distributions of the macroplanktonic gelatinous organisms (Figure 1A). Eight dives from this cruise were analysed for this study (Table 1). All dives were conducted during daylight hours with the exception of the latter halves of dives 98 and 103, where the ROV surfaced at 20:31 and 20:35, respectively. Sunset on these two days was at 18:09 and 18:13, respectively. Anthomedusae have also been observed during other ROV ‘HyperDolphin’ surveys and these dives were added to the analysis (Table 1).

Fig. 1. Maps of water temperatures at the 100 m isobath over the Japan Trench. (A) between 1–31 May 2002 with ROV ‘HyperDolphin’ (HD) dive sites (white circles) superimposed; (B) between 1–30 June 2000 with ‘Shinkai 6500’ (6K) submersible dive sites (white) superimposed; (C) between 1–30 April 1999 with ROV ‘Kaiko’ (10K) dive sites (white) superimposed; (D) between 1–30 September 1999 with 6000 m-class deeptow (DT) towed camera array survey sites (white) superimposed; (E) between 1–31 July 2003; and (F) between 1–31 July 2006 with VPR survey sites superimposed (white circles, stations where Euphysa japonica (Maas, Reference Maas1909) occurred; white triangles, stations where E. japonica was not observed).
Table 1. Submersible surveys analysed in the present study.

≅ observer Dhugal Lindsay; υobserver James Hunt; :observer Hiroshi Miyake; ?observer Tomonori Hamatsu.
The ROV ‘HyperDolphin’ was equipped with an HDTV (high-definition television) camera integrating an ultra sensitive super HARP (High gain Avalanche Rushing Photo-conductor) tube. Video footage from this highly sensitive camera, recorded at 1080i and 30 frames per second, allowed resolution and identification of forms larger than approximately 1 cm diameter when within the focal field. Further information on camera and lighting specifications during the present cruise is reported elsewhere (Lindsay, Reference Lindsay2003; Lindsay et al., Reference Lindsay, Furushima, Miyake, Kitamura and Hunt2004).
Physico-chemical data were collected using a SeaBird SBE19 CTD (conductivity–temperature–depth meter) and an SBE43 oxygen sensor attached to the vehicle on all dives. Water mass profiles were plotted using linear interpolation. Specimens were collected for positive identification using a suction sampler into either a 12 cm-diameter intake hose and thence into a single clear acrylic canister of 30 cm diameter, or into one of three gate valve samplers. These tools are described elsewhere (Lindsay, Reference Lindsay2003; Lindsay et al., Reference Lindsay, Furushima, Miyake, Kitamura and Hunt2004). Sampling of voucher specimens allowed recognition of species in the HDTV video record based on their external macromorphology and behaviour. Animals collected in the single cannister of the suction sampler were transferred to shipboard aquaria, phototanks or planktonkreisels (Hamner, Reference Hamner1990) for behavioural observation and positive identification, while those sampled with the gate samplers were observed within the original collection device. Still digital photographs of specimens were taken with a Nikon D1H digital camera with a macro lens (AF Micro Nikkor 105 mm 1:2.8 D) and recorded in TIFF-RGB format. Illumination was provided by National Ref Lamp colour-balanced flood bulbs (PRF-500WB) which lit the specimen from the side before a black felt backdrop. High-definition recordings of the live animals were also made in the laboratory using a Sony HDTV camera (HDW-750) under the same lighting regime as above.
‘Shinkai 6500’ crewed submersible surveys
A cruise on the RV ‘Yokosuka’ (YK00-04) using the crewed submersible ‘Shinkai 6500’ was implemented in June 2000. During Leg 2 of this cruise between 6 and 12 June 2000, the location of cold and warm core rings and the frontal zone were determined using satellite imagery and XBT (eXpendable Bathymetry Temperature) probes. Four dives on the ‘Shinkai 6500’ were then carried out to determine the endemic midwater fauna of these water masses and to identify the physical parameters affecting vertical distributions of the midwater organisms (Table 1; Figure 1B). Distribution profiles were made over several thousand metres in order to determine the lower distributional limit for each midwater form. Information on camera and lighting specifications and on sampling equipment used during cruise YK00-04 is reported elsewhere (Lindsay, Reference Lindsay2003, Reference Lindsay2005). No anthomedusae were sampled during this cruise and all information is therefore from in situ observations, with the vast majority being direct observations by eye.
During Cruise YK07-15 on the RV ‘Yokosuka’, held between 13 and 28 October 2007, two midwater surveys were undertaken (Table 1). Video footage from two 3-chip CCD video cameras in pressure housings was recorded onto DV-Cam videotapes with depth, time and other text superimposed via an analogue composite video connection. Observations made with the naked eye were recorded on the audio track of the DV-Cam video tapes through a microphone set next to the scientist's viewport. During this cruise the ‘Shinkai 6500’ was outfitted with seven forward-pointing 400W metal halide (HMI) lights. Physico-chemical data were collected using a SeaBird SBE19 CTD and an SBE43 oxygen sensor attached to the top of the vehicle next to the hatch turret and correlated to the presence of a given animal by matching the timecode on the CTD series to the timecode on video.
ROV ‘Kaiko’ survey
Two midwater surveys using the ROV ‘Kaiko’ were carried out in April 1999 (Table 1) within a warm core eddy and in an outlying area (Figure 1C) as identified by satellite imagery and the SeaBird SBE19 CTD profiles. The ROV ‘Kaiko’ was equipped with a pan-tiltable Victor/TV3100XDB three chip CCD camera and three tiltable Sony/XC-999 cameras mounted in an array to give a 118 degree angle of view. There were six lights: two 500-W MaxSeaLite ML-120/500 halogen lamps, two 500-W Deep-SeaLite ML-120/500 halogen lamps and two 400-W SeaArc2 HMI metal halide lamps. Video footage from all four of the ROV ‘Kaiko’ cameras was recorded in NTSC format on ST-120PRO S-VHS tapes and that from the Victor/TV3100XDB three chip CCD camera was also recorded on BCT-90MLA BetacamSP tapes. Specimens were collected using a hydraulically-powered 6-cannister suction sampler with a hose diameter of 9 cm.
‘Shinkai 2000’ crewed submersible surveys
Dives with the crewed submersible ‘Shinkai 2000’ were implemented east of Hokkaido Island just landwards of the Japan Trench in August 1999 and September 2000, respectively (Table 1). Anthomedusae occurring above the Japan Trench have also been sampled on another ‘Shinkai 2000’ dive and these data were added to the analysis. The ‘Shinkai 2000’ in Sagami Bay was equipped with a Victor GF-S1000 HU three chip CCD camera specially modified for the vehicle. There were eight lights: five 250-W SeaLine SL-120/250 halogen lamps and three 400-W SeaArc HMI/MSR metal halide lamps. One metal halide and one halogen lamp were attached to the pan-tiltable CCD camera unit on the port side and were used to illuminate organisms that drifted within the shadow of the predominantly forward-pointing lights. Video footage was recorded continuously and simultaneously on both ST-120PRO S-VHS and BCT-D124L Digital Betacam tapes. Physico-chemical data were collected using a SeaBird SBE19 CTD and an SBE43 oxygen sensor attached to the forehead of the vehicle just above the forward porthole.
6000 m-class ‘Deep-Tow Camera’ survey
Five midwater deployments of the 6000 m-class ‘Deep-Tow Camera’ were carried out from 29 August to 1 September 1999 (Table 1) within the frontal transition zone between Oyashio- and Kuroshio-derived waters (Figure 1D) as identified by satellite imagery and the SeaBird SBE9plus CTD profiles. The 6000 m-class ‘Deep-Tow Camera’ was equipped with a SuperHarp three chip video camera tilted forward twenty degrees from the vertical plane and a black and white video camera pointing horizontally forward. Four 250-W Deep-SeaLite MC-120/250 halogen lamps provided illumination. Video footage from both cameras was recorded in NTSC format on ST-120PRO S-VHS tapes with CTD depth and temperature superimposed on the image.
Video/visual plankton recorder (VPR) surveys (2003, 2004, 2005 and 2006)
Surveys were carried out using the new video plankton recorder (VPRII; Davis et al., Reference Davis, Hu, Gallager, Tang and Ashjian2004, Reference Davis, Thwaites, Gallager and Hu2005; Ichikawa et al., Reference Ichikawa, Segawa and Terazaki2005) off the Sanriku coast in July 2003 and in June to July 2004–2005, and with the colour autonomous VPR (AVPR) in June 2006 from the RV ‘Shunyo-Maru’. The VPRII was attached to a v-fin, equipped with a CTD (Falmouth Scientific Inc. MCTD), fluorometer (Falmouth Scientific Inc. SCF) and turbidimeter (Seapoint Sensors Inc. Turbidity Meter), and was deployed to a maximum of 500 m depth. The camera of the VPRII was a black and white CCD (Pulnix Inc. TM-1040, 1024 × 1024 pixels), the field of view was 40 × 40 mm (0.039 mm pixel−1), and images were recorded at 25 frames per second. Plankton (including anthomedusae) were detected automatically as regions of interest (ROIs) and were written to disc using a filename that represented the elapsed time in milliseconds since midnight at which each ROI was detected. The field of view of the AVPR was set to 50 × 50 mm (0.049 mm pixel−1) and full frames were recorded every 1/15 second. The AVPR was deployed on a custom-made frame and was equipped with a CTD (Falmouth Scientific Inc. MCTD). Separate CTD (Sea-Bird Electronics, Inc. SBE911-plus with SBE43 DO sensor) observations were carried out before each VPRII or AVPR deployment. CTD data for the AVPR deployments are from this separate CTD cast. After each cruise, images were identified by hand to taxon or species using an image browser (Thumbs Plus 6.0J). Depth, temperature, salinity and any other environmental information was correlated to CTD data files associated with the VPR using a time stamp (milliseconds). Maximum numbers of anthomedusae were recorded during the surveys in July 2003 and June 2006. These survey stations, overlaid on oceanographical data for the survey region, are shown in Figure 1E & F.
Data analysis
Direct visual observation through the portholes of the crewed submersibles ‘Shinkai 2000’ and ‘Shinkai 6500’ allowed accurate identifications of anthomedusae in situ due to the superior resolution and focusing speed of the human eye over video cameras mounted on hydraulic pan-tilt units. The volume of water investigated was also greater in comparison to that surveyed by ROVs per unit time because of the slow response speed of the focusing and zoom functions on the video camera compared to peripheral vision and rapid focusing in the human eye.
Although the resolution of the video taken during the ROV ‘Kaiko’ surveys was only 525 lines (NTSC), we were able to resolve organisms as small as the pteropod Clio, phaeodarian radiolarians, euphausiid krill, and the trachymedusa Crossota rufobrunnea (Kramp, 1913). We feel confident therefore that we would have been able to detect and recognize anthomedusae as small as Euphysa japonica (Maas, Reference Maas1909) if they had occurred in numbers large enough to have been detected with the water volume surveyed. All NTSC video material that was not already in Digital BetaCam format was dubbed to the latter format for storage and analysis with the exception of the ‘Deep-Tow Camera’ footage, which was analysed on the original SVHS tapes. Although phaeodarian radiolarians and the trachymedusa C. rufobrunnea were also observed in the deep-tow NTSC video record we cannot be sure that we might not have missed E. japonica due to the small cross-sectional area of the animals when viewed from above by the downward-pointing colour video camera.
Digital BetaCam and HDCam video was analysed using a Sony HDW-M2100 Deck with an editing jog controller that allowed us to move frame by frame through a recorded observation to resolve morphological details such as tentacle number and estimate bell contraction rate. Images were captured from videotape through the HDSDI or SDI output using an HVD04 capture card and saved in Quicktime TIFF format. To increase the accuracy of morphological measurements the gamma value and exposure of frame grabs or digital stills was sometimes adjusted to make edges more visible.
RESULTS
Anthomedusae generally with an evenly rounded umbrella; without apical canal; without exumbrellar cnidocyst tracks; manubrium stout and cylindrical, usually not extending beyond umbrella margin; mouth simple, circular; with 1–4 marginal tentacles, either unequally developed or of similar length, all of same structure, usually moniliform or modified moniliform, without ocelli; gonads undivided and encircling almost entire length of manubrium.
Remarks: the genus Euphysa contains only two described species with 4 marginal tentacles of equal length when adult, E. japonica (Maas, Reference Maas1909) and E. flammea (Linko, Reference Linko1904). When young, E. flammea has been reported to have only one well-developed tentacle at a bell height of 5 mm (Kramp & Damas, Reference Kramp and Damas1925; Kramp, Reference Kramp1926) while E. japonica has been reported to already have 4 well-developed tentacles at a bell height of only 1 mm (Uchida, Reference Uchida1927). These species have been reported to be indistinguishable in their adult stages (Arai & Brinkmann-Voss, 1980; Brinckmann-Voss & Arai, Reference Brinckmann-Voss and Arai1998) but our data and a review of the literature suggest that several characters serve to distinguish them.
Mayer (Reference Mayer1910) comments that both the manubrium and tentacle bulbs of E. (=Sarsia) flammea are light fiery-red or orange and the bell height to width ratio in the accompanying figure (No. 27, p. 64) is 1.3, the tentacles are constricted proximally and the nematocyst rings arm the tentacle from close to the proximal end. Likewise, Linko (Reference Linko1904) reported in the original description that the bell height to width ratio was between 1.25 and 1.5 and that the bases of the tentacles were orange-red. In contrast, E. (=S.) japonica is reported by Mayer (Reference Mayer1910) with a bell height to width ratio of 2.5–3.0, to have tapering tentacles, and for the tentacular nematocyst rings to start only on the distal two-thirds of the tentacles (p. 720). The bell height was 2.14 times the bell width in the figure provided with the original description of E. (=S.) japonica (Maas, Reference Maas1909) and the colour of the manubrium, gonads and tentacle bulbs was reported as golden-brown, apparently due to fixation. The colour of these structures when alive was not recorded. Euphysa japonica collected by the present authors (H.M.) from close to the type locality have cream to tan tentacle bulbs when live.
In plate II (Figure 5) of E. (=S.) flammea in Bigelow (Reference Bigelow1920) the tentacles are slightly thinner in their proximal portion and the bell height is approximately 1.3 times the bell diameter in the 8 mm tall specimen. Specimens of 19 mm bell height were also collected. Bigelow mentions that a colour sketch, taken from life by Mr Johansen (Station 27r) shows the manubrium and tentacle bulbs to be reddish orange and that the tentacles themselves are pale bluish. This is not the colour combination seen in our specimens of E. japonica from the type locality.
Uchida (Reference Uchida1927) presents an illustration of a 6 mm high female of E. (=S.) japonica with a bell height to width ratio of 1.2 (Figure 24) and notes that the manubrium and tentacles were coloured bluish brown in preserved specimens. These characters suggest that the illustrated specimen was actually E. flammea, although at least one of the animals he collected in waters near Hokkaido Island either off Oshoro, Rebun or Takashima, would seem to have been E. japonica based on his report of a 1 mm high specimen that already had developed 4 tentacles.
Notes on Euphysa were made by Kramp (Reference Kramp1928) on specimens caught in the Strait of Georgia, Vancouver in July. The bell height to width ratio was 1.24 in the specimen illustrated in longitudinal section in Figures 2 and 6, while it was reported as 1.5 in the text describing the largest captured specimen (7.5 mm in height). A proximal constriction is seen in the tentacles below the tentacle bulb (Figure 6) and the nematocyst rings start directly below this constriction. Although there are no notes as to the colour of the tentacles and bulbs, the above characters suggest that the figured medusae were in fact E. flammea. Some small individuals of 2.5 mm in bell height were also captured and these had four well-developed tentacles, suggesting that both E. japonica and E. flammea may have been present in the examined material.

Fig. 2. Images of Euphysa japonica. (A) Individual photographed in a shipboard aquarium collected at 508 m depth on ‘Shinkai 2000’ Dive 1218, scale bar 1 mm; (B) illustration of type specimen from Maas Reference Maas1909; (C) individual photographed in a shipboard aquarium collected at 1156 m depth on ‘Shinkai 2000’ Dive 1129, mesoglea shrunken due to overnight maintenance in aquarium; (D) individual videotaped during ROV ‘HyperDolphin’ Dive 59 at indeterminate depth.
Uchida (Reference Uchida1933) reports a Euphysa species from off Kamchatka (51°33′N 156°20′E) with orange tentacle bulbs and a bell height to width ratio of 1.3 to 1.5 (1.47 in text, 1.36 and 1.3 measured from photographs in Figure 3) as E. japonica. These characters are the same as those reported by Linko (Reference Linko1904), Mayer (Reference Mayer1910) and Bigelow (Reference Bigelow1920) for E. flammea.

Fig. 3. In situ images of Euphysa japonica (scale bars 2 mm). (A) 467 m depth on 27 June 2005; (B) 487 m depth on 11 July 2006; (C) 442 m depth on 11 July 2006; (D) 406 m depth on 25 July 2003; (E) 516 m depth on 28 June 2004; (F) 477 m depth on 29 June 2004; (G) 451 m depth on 11 July 2006.
The figure of Corymorpha (=Euphysa) flammea in Naumov (Reference Naumov1960) shows a constriction in the tentacles in the proximal portion that is not present in our images of E. japonica, the nematocyst rings on the tentacles start very close to the proximal end, and the bell height is approximately 1.2 times the bell diameter in both the black and white figure on page 212 and in the colour plate XXIX (Figure 4). The text however states that the medusa is 17 mm high and 8 mm wide (ratio 2.13) so it is possible that both species were present in Naumov's material.

Fig. 4. Horizontal and vertical distributions of the euphysid anthomedusa Euphysa japonica (purple bars) and the pandeid anthomedusa Pandea rubra (red circles) over the Japan Trench between 6–12 June 2000, and correlations with water temperature. Black triangles signify latitudes at which XBT probes were deployed and red triangles where ‘Shinkai 6500’ (6K) dives were conducted.
The figure of E. japonica in Arai & Brinckmann-Voss (Reference Arai and Brinckmann-Voss1980) suggests that the proximal portion of the tentacles is thinner than the distal face of the marginal nematocyst bulbs and the bell height is figured at 1.75 times the bell diameter in the 8 mm tall specimen. Nematocyst rings on the tentacles start very close to the proximal end. The specific identification of the figured animal is uncertain. The accompanying description mentions scarlet tentacle bulbs with a moderately thick umbrella suggesting identification as E. flammea and the sketch on page 3 also resembles this species in its bell height to width ratio.
Zhang & Lin (Reference Zhang and Lin2001) present a figure of E. flammea based on a specimen from the Chukchi Sea. Bell height is approximately 1.3 times the bell diameter. The note on the fiery-red colour of the manubrium and tentacle bulbs in this species suggests that this was also the case in their specimens.
In Miyake et al. (Reference Miyake, Lindsay and Kubota2004) a photograph of E. japonica is shown in their figure 4. Bell height is 1.64 times the bell diameter in this moribund specimen. Tentacle bulbs taper into the tentacles, which are not constricted proximally and are cream to tan rather than red as in E. flammea.
Nouvian (Reference Nouvian2007) includes a photograph of E. flammea taken by Dr Per Flood on page 75. This individual was hand collected in January 1996 from the surface waters drifting past the docks of Friday Harbor Laboratories, San Juan Island, Washington State, USA (Per Flood, personal communication). The bell height is 1.14 times the bell width, a constriction in the tentacles in the proximal portion is visible, and the nematocyst rings on the tentacles start very close to the proximal ends. Three of the tentacles are short and one longer, as has been reported for smaller individuals of E. flammea (Kramp & Damas, Reference Kramp and Damas1925), whereas in E. japonica all tentacles are of similar length even at sizes as small as 1 mm (Uchida, Reference Uchida1927; Brinkmann-V0ss & Arai, Reference Brinckmann-Voss and Arai1998). The individual in Nouvian (Reference Nouvian2007) was approximately 8–10 mm in height (Per Flood, personal communication). In contrast to the cream-tan colour of bulbs and tentacles evident in E. japonica (Miyake et al., Reference Miyake, Lindsay and Kubota2004), the tentacle bulbs of E. flammea in Nouvian (Reference Nouvian2007) were bright yellow with the tentacles being green and becoming pale blue distally, as also recorded for this species in Bigelow (Reference Bigelow1920). This particular specimen is somewhat malformed as the longest tentacle is bifurcated. However, in photographs of similar specimens photographed during the same period, the tentacles were more or less of equal length and in all specimens the bell height to width ratio was well below 1.4 (Per Flood, personal communication).
Euphysa japonica: Maas, Reference Maas1909; Foerster, Reference Foerster1923 (in part, as E. (=S.) japonica); Uchida, Reference Uchida1927 (in part); Kramp, Reference Kramp1928 (in part); Arai & Brinckmann-Voss, Reference Arai and Brinckmann-Voss1980 (in part); Brinckmann-Voss & Arai, Reference Brinckmann-Voss and Arai1998 (in part); Miyake et al., Reference Miyake, Lindsay and Kubota2004, figure 4.
MATERIAL EXAMINED
Images: digital still macrophotographs of 2 individuals in shipboard phototanks collected at 1156 m depth during the ‘Shinkai 2000’ Dive 1129 and at 508 m depth during the ‘Shinkai 2000’ Dive 1218 and recorded by H. Miyake (JAMSTEC). In situ images of 20 individuals were photographed using a visual/video plankton recorder VPR (Table 2). In situ HDTV images were recorded of an individual videotaped at indeterminate depth during the ROV ‘HyperDolphin’ Dive 59.
Table 2. Records of Euphysa japonica occurrence based on digital photographs recorded by the video plankton recorder (VPR) and colour autonomous visual plankton recorder (AVPR). Question marks indicate individuals where bell dimensions could not be ascertained because the entire animal was not visible in a single frame.

JST, Japan Standard Time; Temp., temperature (°C); Oxy., dissolved oxygen concentration (ml/L); SigmaT, Sigma T (kg/m3); HxW, bell height and width; n.a., data not available due to a faulty oxygen sensor.
SPECIFIC CHARACTERS
Bell height was greater than 2 times bell width in healthy specimens. Proximal end of tentacles developed along the entire surface of the marginal bulb thereafter tapering into tentacles, both bulbs and tentacles cream to tan. Nematocyst ring armament on tentacles some distance from tentacle bulbs. Four tentacles of equal length from immediately after liberation.
DESCRIPTION
Evenly rounded umbrella, up to 26 mm in height, greater than 2 times bell width in healthy specimens (2.14 in original description and 2.17 (SD 0.14) in present material, N = 14); without apical canal; without exumbrellar cnidocyst tracks; orange-red manubrium cylindrical, usually not extending beyond umbrella margin; mouth simple, circular; with 4 marginal cream-tan tentacles of similar length, all of same moniliform structure, without ocelli, proximal end not constricted, tapering from cream-tan marginal bulb. Nematocyst ring armament on tentacles some distance from tentacle bulbs; gonads undivided and encircling almost entire length of manubrium. Small subunit ribosomal DNA sequence (18S) submitted under GenBank Accession Number EU301605.
Remarks
Although E. japonica has been reported from the surface layer (0–80 m, 27.7°C) in the Banda and Aru Seas (van der Spoel & Bleeker, Reference Van der Spoel and Bleeker1988) we consider this identification dubious given the obvious difficulties in distinguishing between net-caught, preserved Euphysa species (e.g. Arai & Brinckmann-Voss, Reference Arai and Brinckmann-Voss1980; Brinckmann-Voss & Arai, Reference Brinckmann-Voss and Arai1998). A FASTA search on 10 April 2008 identified Corymorpha intermedia Schuchert, 1996 as the most closely related sequence to E. japonica but no other members of either the Euphysidae or the Corymorphidae Allman, 1872 were evident in the DBGET integrated database retrieval system on this date. It is hoped that molecular markers will both serve to aid identification of such morphologically similar species as E. japonica and E. flammea, as well as to shed light on the evolution of hydrozoans within this still largely unstudied clade.
DISTRIBUTION
All unequivocal records of these species have been from sub-boreal Pacific Ocean waters. It has been recorded twice in recent years in the Japan Sea, on 19 July 2001 at 421 m (water temperature 0.7°C, salinity 34.08 psu, dissolved O2 5.0 ml/l, sigmaT 27.33 kg/m3) and at 620 m (water temperature 0.5°C, salinity 34.08 psu, dissolved O2 4.7 ml/l, SigmaT 27.33 kg/m3) near Shiribeshi seamount (43°34′N 139°33′E) in the northern Japan Sea (Miyake et al., Reference Miyake, Lindsay and Kubota2004). Previous records in the Japan Sea were off Rebun Island by Uchida (Reference Uchida1927). Although it appears able to survive such low temperatures as occur in the deep Japan Sea it is not common and indeed was not observed at all during a dive in Toyama Bay (‘Shinkai 2000’ Dive 1119; 37°16.5′N 137°33.5′E, observer J.H.) on 28 July 1999 (Lindsay & Hunt, Reference Lindsay and Hunt2005), even though it was very common during a dive on 18 August 1999 off the east coast of Hokkaido Island, near the type locality (Table 1). Abundances of over 35 individuals per video frame have also been recorded at 315 m depth, altitude above sea floor of 10 m, during the ‘Shinkai 2000’ Dive 1217, also off the east coast of Hokkaido Island (Table 1, identified from video record by D.L.) (2.5°C, 33.60 psu, 3.4 ml/l oxygen, SigmaT 26.81 kg/m3). The shallowest occurrence recorded during this dive was at 231 m depth (2.1°C, 33.47 psu, 4.1 ml/l oxygen, SigmaT 26.74 kg/m3).
A single large individual was observed at 582 m during the ‘Shinkai 6500’ Dive 1037, quite far off the east coast of Honshu Island (Table 1) (4.6°C, 34.07 psu, 1.9 ml/l dissolved O2, SigmaT 26.98 kg/m3). During the ‘Shinkai 6500’ Dive 1039, closer to the east coast of Honshu Island (Table 1), three individuals were observed, one at 385 m (5.9°C, 33.75 psu, 4.4 ml/l dissolved O2, SigmaT 26.57 kg/m3), one at 474 m (3.8°C, 33.71 psu, 3.6 ml/l dissolved O2, SigmaT 26.77 kg/m3) and one at 510 m (3.5°C, 33.73 psu, 3.4 ml/l dissolved O2, SigmaT 26.83 kg/m3). No E. japonica were observed in late April 1999 during the ROV ‘Kaiko’ survey or between 22 April and 2 May 2002 during the ROV ‘HyperDolphin’ survey (Table 1) suggesting that this is too early in the year for its occurrence.
The distribution of E. japonica in relation to water masses off the east coast of Honshu Island was investigated between 7 and11 June 2000 during the ‘Shinkai 6500’ survey YK00-04 and is shown in Figure 4. In this area and seasonal period it appears to be a mesopelagic species and was less common in the water column directly below an overlying warm water mass towards the south. It could be supposed that it is released from its polyp at the lower edge of the contintental shelf in northern Japan from around May and is advected southwards, gradually becoming displaced to deeper depths as the North Pacific Intermediate Water is subducted below waters of warm Kuroshio Current origin and becoming less common further south. The southernmost record in our dataset is at 37°8.9′N 141°58.2′E. There is no evidence, however, for its transport via water of Oyashio Current origin as surveys using a visual/video plankton recorder (VPR) in 2003 and 2006 failed to correlate its presence with the temperature minima indicative of Oyashio-derived water (Figures 5 & 6). Information is desired on current flow and direction below the cold water tongues of Oyashio water penetrating into the mesopelagic zone as this seems to be the water mass in which E. japonica is entrained. No evidence of diel vertical migration was observed in our data set (Table 2). Foerster (Reference Foerster1923) reports (as E. flammea) individuals of a Euphysa species off the coast of British Columbia with a bell height to width ratio of between 1.4 and 1.8 and with white tentacles when live. It is possible that these could be damaged individuals of E. japonica with partially contracted bells but the description is not precise enough to be certain. Brinckmann-Voss & Arai (Reference Brinckmann-Voss and Arai1998) report E. japonica, identified by the presence of four equal tentacles immediately after liberation from its hydroid, to be present in Canadian Pacific waters.
Euphysa flammea: Linko, Reference Linko1904; Mayer, Reference Mayer1910, figure 27; Bigelow, Reference Bigelow1920, plate II figure 5; Foerster, Reference Foerster1923, plate 1 figure 1; Kramp, Reference Kramp1926, plate I, figures 12–14; Kramp, Reference Kramp1928 (in part, as E. japonica), figure 2, 5 & 6; Uchida, Reference Uchida1927 (in part, as E. japonica), figure 24; Uchida, Reference Uchida1933 (as E. japonica), figure 3; Naumov, Reference Naumov1960, figure 98, plate XXIX, figure 4; Arai & Brinckmann-Voss, Reference Arai and Brinckmann-Voss1980 (in part), figure 1; Brinckmann-Voss & Arai, Reference Brinckmann-Voss and Arai1998, figure 11b (as E. japonica); Nouvian, Reference Nouvian2007, page 75 middle.

Fig. 7. In situ VPR photograph (A, scale bar 2 mm) and photograph in aquarium (B) of Euphysa flammea. Photograph in (B) of individual 8–10 mm in bell height kindly provided by Dr Per R. Flood, copyright Bathybiologica.
MATERIAL EXAMINED
Images: in situ image of 1 individual photographed using the visual/video plankton recorder VPR on 29 June 2004, 00:43:09; 477 m depth; temperature 3.2°C, salinity 34.09 psu, dissolved oxygen 3.3 ml/l, SigmaT 27.13 kg/m3; 40°00′N 146°18′E; file name roi1.0259275000.tif (Figure 7A). Colour image of 1 individual collected from near Friday Harbor Laboratory, Washington State by Per Flood (Figure 7B). Monochrome image of 2 individuals collected near Kamchatka and photographed by Tohru Uchida (Reference Uchida1933; figure 3).
SPECIFIC CHARACTERS
Bell height less than 1.5 times bell width. Proximal part of tentacles thin. Nematocyst rings arm tentacles from just distal to the proximal constriction. Manubrium and tentacle bulbs light fiery-red, orange or bright yellow, distal ends of tentacles may become pale blue. Tentacles of equal length only in larger individuals.
DESCRIPTION
Evenly rounded umbrella, up to 19 mm in height, less than 1.5 times bell width in healthy specimens (1.36 in original description and 1.29 in the present in situ material, 1.28 (SD 0.09) with all material combined, N = 9); without apical canal; without exumbrellar cnidocyst tracks; fiery-red to orange manubrium, cylindrical, usually not extending beyond umbrella margin; mouth simple, circular; with 4 marginal light fiery-red or orange tentacles of similar length in adults, one long and three shorter in juveniles, all of same moniliform structure, without ocelli, proximal part of tentacles thin, sometimes green; abaxially protruding marginal tentacle bulb; nematocyst rings arm tentacles from just distal to the proximal constriction, distal ends of tentacles sometimes pale blue; gonads undivided and encircling almost entire length of manubrium.
Remarks
A Euphysa species with four equal tentacles and a bell height 1.3 times that of the diameter appears on page 31 of Wrobel & Mills (Reference Wrobel and Mills1998). The cream-tan coloration of the marginal bulbs and tentacles suggests that it is not E. flammea while the aspect of the marginal tentacle bulbs and tentacles with the proximal part of the tentacle being thin and developed from only a part of the marginal bulb, leaving an abaxially protruding nematocyst pad, and the ratio of bell height to width suggests that it is not E. japonica. Diverticulae are prominent on the radial canals and a Euphysa species that resembles E. japonica but with prominent diverticulae and a bell height to width ratio of 1.23 as measured from the figure, has been reported from the north-east Pacific Ocean previously (Arai & Mason, Reference Arai and Mason1982). The possibility needs to be entertained that additional species of Euphysa with four equal tentacles remain to be described. It is hoped that a combination of molecular and morphological data will help clarify the taxonomy of Euphysa spp.
DISTRIBUTION
Only one individual has been reported unequivocally off the eastern seaboard of Japan, photographed using the visual/video plankton recorder (VPR) on 29 June 2004 (09:43:09; 477 m depth; temperature 3.2°C, salinity 34.09 psu, dissolved oxygen 3.3 ml/l, SigmaT 27.13 kg/m3; 40°00′N 146°18′E). Most records are from boreal, Arctic waters. In the North Pacific Ocean it has been reported from the Okhotsk and Bering Seas (Naumov, Reference Naumov1960) as well as off the north-west coast of America.
Bythotiaridae with primarily four unbranched radial canals and with four or more centripetal canals arising from the ring canal, blind or joining the cruciform base of the stomach. Gonads transversely folded, frequently forming eight adradial rows of deep transverse furrows; basal portion of tentacles adnate to umbrella margin; all tentacles hollow, nematocysts only in the terminal knob; no ocelli.
Remarks
The most recent published diagnosis for the genus Calycopsis (Bouillon et al., Reference Bouillon, Gravili, Pagès, Gili and Boero2006) includes ‘marginal tentacles of similar structure, with cnidocysts only on the terminal knob and with adnate base.’ The above diagnosis differs in part to the diagnosis of Bouillon et al. (Reference Bouillon, Gravili, Pagès, Gili and Boero2006) because although it is true that all tentacles are of similar structure with respect to their hollowness, the presence of a terminal nematocyst-laden bulb, and with their bases being adnate with the margin of the umbrella, there are several species that have both long and short tentacles that alternate in an ordered fashion. In C. nematophora Bigelow, Reference Bigelow1913 the tentacles differ in their aspect as well as their size, with the terminal knobs of the longer tentacles being elongated while those of the shorter tentacles are spherical.

Fig. 8. Images of Calycopsis nematophora. In situ frame grab of captured individual HD100GS1a (A, scale bar 5 mm), still photographs taken in shipboard aquarium of animal when relaxed (B, scale bar 5 mm), partially contracted while attempting to ingest a copepod (C, scale bar 5 mm), and close-up of manubrium (D, scale bar 1 mm).
Calycopsis nematophora: Bigelow, Reference Bigelow1909, figure 2 (as Sibogita simulans Bigelow, Reference Bigelow1909, in part); Bigelow, Reference Bigelow1913, plate II figure 8, plate III figures 1–3; Naumov, Reference Naumov1960, figure 79, plate XXIX figure 2; Renshaw, Reference Renshaw1965, figure 2; Arai & Brinckmann-Voss, Reference Arai and Brinckmann-Voss1980, figure 39; (not Calycopsis nematophora van der Spoel & Bleeker, Reference Van der Spoel and Bleeker1988, figure 14); Brinckmann-Voss & Arai, Reference Brinckmann-Voss and Arai1998, figure 3.
MATERIAL EXAMINED
Specimens: 1 specimen captured at 400 m depth at 14:36 during the ROV ‘HyperDolphin’ Dive 100 (JAMSTEC sample code HD100GS1a, temperature 5.3°C, salinity 34.13 psu, dissolved oxygen 2.0 ml/l, SigmaT 26.96 kg/m3). Observed live and after preservation in 70% ethanol.
Images: in situ HDTV images of captured specimen (time code on original tape stored at JAMSTEC Yokohama Institute, 01:33:03:04–01:40:57:01). HDTV video footage of above animal in shipboard aquarium recorded by J. Tanada (JAMSTEC). Digital still macrophotographs of above animal in shipboard phototank recorded by D. Lindsay (JAMSTEC). In situ images of 3 other individuals. First: ROV ‘HyperDolphin’ Dive 98; 19:20; 388 m depth; temperature 2.9°C, salinity 33.82 psu, dissolved oxygen 4.5 ml/l, SigmaT 26.95 kg/m3; 06:17:32:26–06:17:38:18. Second: ROV ‘HyperDolphin’ Dive 99; 14:21; 234 m depth; temperature 2.3°C, salinity 33.54 psu, dissolved oxygen 3.5 ml/l, SigmaT 26.78 kg/m3; 01:08:40:11–01:08:46:11. Third: ROV ‘HyperDolphin’ Dive 101; 10:47; 588 m depth; temperature 2.5°C, salinity 33.56 psu, dissolved oxygen 3.5 ml/l, SigmaT 26.78 kg/m3; 02:11:37:18 –02:11:47:18.
SPECIFIC CHARACTERS
Mouth with yellow-green labial nematocyst clusters.
DESCRIPTION
Sampled individual (live specimen): exumbrella 28 mm wide, 33 mm high. Subumbrella 19 mm wide, 29 mm high. Length of brown-pigmented manubrium 2/5 of subumbrella height. Manubrium with slit-like pits adradially and a line of oval pits interradially. Gonads in adradial slit-like pits. Lips intensively folded, armed with yellow oblong nematocyst clusters on stalks. Four thick primary radial canals joined to manubrium by well-developed mesenteries, forming a cross when viewed aborally. Primary radial canals trifurcate on subumbrella at distalmost extent of mesenteries with a further interradial canal branching off from midway along each mesentery. Sixteen canals therefore meet the ring canal at the bell rim. Each canal bordered by a band of longitudinal muscles. Six long, hollow, thick tentacles (and two thick stubs) with long sausage-shaped terminal nematocyst bulb, pink in colour with proximal end of bulb tinged orange. Thirty-six short, thin tentacles with spherical to oblong terminal nematocyst bulbs, pink in colour. All tentacles adnate with umbrella at base to varying degrees.
Remarks
We are somewhat ill-at-ease with placement of this species within the genus Calycopsis. It seems equivocal that the apparent branching of the four main radial canals is actually due to their merging with centripetal canals issuing from the ring canal, given the large number of specimens collected to date (see Brinckmann-Voss & Arai, Reference Brinckmann-Voss and Arai1998) and the extremely low number with centripetal canals of any form. It is hoped that further material will become available that may shed light on the development of canals in this species. Perhaps more disturbing is the presence of complexly-folding oral lips with characteristic stalked labial nematocyst knobs, yellow in live specimens, that are also confined to this species, with all other species of the genus having relatively simple lips with smooth margins. Nevertheless, we follow the lead of previous authors in relegating this species to the genus Calycopsis until a thorough revision of the Bythotiaridae can be made.
When encountered by the ROV, C. nematophora was always oriented with the aboral surface of the bell pointing down and with at least one of its longer tentacles deployed horizontally at a right angle to the bell (Figure 8A). When fed a copepod while being kept in a shipboard aquarium, it caught it with a long tentacle and drew it quickly into the bell. The lips opened and the manubrium moved as if searching for food. The rim of the bell crumpled inwards and the nematocyst bulbs of the shorter tentacles thereby formed a minefield in three-dimensional space depending on how adnate their bases were with the rim of the exumbrella, effectively blocking the copepod from escape through the bell opening (Figure 8C). The three-dimensional deployment of nematocyst bulbs within the bell is the origin of this medusa's common Japanese name kiraikurage (minefield medusa). This specimen was kept for approximately one month at 4°C with the aboral surface of the bell resting on the bottom of the aquarium. It was fed wild-caught copepods and over the course of the month the pigment in the manubrium gradually disappeared until it was bleached white. The tentacle bulbs remained pink and the labial nematocyst clusters yellow. It is not known whether this signifies that the manubrial pigment is unable to be synthesized by the medusa and comes from its diet. Some brown pigments common in midwater medusae, such as protoporphyrin, are known to react with light. However, protoporphyrin becomes toxic upon light-mediated breakdown and since the medusa remained healthy we doubt that this was the origin of the manubrial pigment.
DISTRIBUTION
Only four definite observations of this medusae have been made, and all of these during the ROV ‘HyperDolphin’ survey in April 2002. Although it is premature to conjecture on the seasonal occurrence of this species, its distribution in relation to water masses (Figure 9) suggests that it is a cold water, boreal species that can be subducted to deeper mesopelagic depths as the cold Oyashio Current waters in which it resides are displaced deeper by warm, overlying waters of Kuroshio Current origin. Based on the illustration (Figure 14) in van der Spoel & Bleeker (Reference Van der Spoel and Bleeker1988) where the illustrated medusa has simple lips with no labial nematocyst clusters, we consider the Banda/Aru Sea report of its occurrence in the Philippine Archipelago to be dubious. All other records to date have been in cold waters, centred around the boreal North Pacific Ocean (Renshaw, Reference Renshaw1965; Brinckmann-Voss & Arai, Reference Brinckmann-Voss and Arai1998).
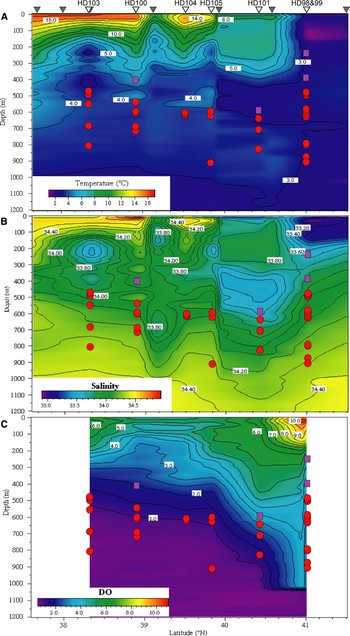
Fig. 9. Horizontal and vertical distributions of the calycopsid anthomedusa Calycopsis nematophora (purple squares, 1 per individual) and the pandeid anthomedusa Pandea rubra (red circles, 1 per individual) over the Japan Trench during April/May 2002, and correlations with (A) water temperature, (B) salinity, and (C) dissolved oxygen (DO) concentrations. Upturned grey triangles signify latitudes at which XCTD probes were deployed and upturned white triangles where ROV ‘HyperDolphin’ (HD) dives were conducted.
Medusa with or without apical projection, with or without longitudinal ridges and nematocyst tracks on exumbrella; radial canals ribbon-like; gonads at first in the adradii and eventually covering the manubrium, forming a complex reticulated network of ridges with pits in between; lips large and folded; more than eight hollow marginal tentacles, without rudimentary marginal tentacles or marginal warts; with or without ocelli.
Remarks: the genus Pandea currently contains two described species of definite status, with P. minima von Lendenfeld, 1885 being of doubtful status (Bouillon et al., Reference Bouillon, Gravili, Pagès, Gili and Boero2006) and P. cybeles Alvariño, 1988 suspected to be invalid (Pagès et al., Reference Pagès, Corbera and Lindsay2007). The polyp stage of the type species, P. conica, is thought to be Campaniclava cleodorae (Gegenbaur, 1854), which is an epizoic associate of the euthecosome pteropod Clio cuspidata (Bosc, 1802) (Figure 10). This conspecificity has been widely accepted in relevant studies of hydrozoan systematics (Kramp, Reference Kramp1961; Bouillon et al., Reference Bouillon, Medel, Pagès, Gili, Boero and Gravili2004, Reference Bouillon, Gravili, Pagès, Gili and Boero2006; Schuchert, Reference Schuchert2007b) although the published evidence for this linkage seems inconclusive (Picard, Reference Picard1956). Polyps of Campaniclava clionis Vanhöffen 1910, the only other species in the genus and an epizooite on the pteropod Clio recurva (Childern, 1823) (Figure 11, also see Lalli & Gilmer, Reference Lalli and Gilmer1989 p. 127), were linked to the anthomedusa Pandea rubra by Rees (Reference Rees1967) in a review of symbiotic associations between cnidarians and molluscs. Nevertheless, no evidence supporting this linkage was provided, as noted in subsequent systematic studies (Bouillon & Boero, Reference Bouillon and Boero2000).

Fig. 10. High definition television video (HDTV) framegrabs of the euthecosome pteropod Clio cuspidata with a colony of the polyp Campaniclava cleodorae (adult probably Pandea conica) attached to its shell (A), collected on 20 March 2006 by an Isaac–Kidd midwater trawl (IKMT) net hauled from 0–1000 m at nighttime in Sagami Bay (Cruise ‘MULTISPLASH’, P.I. Dr Dhugal Lindsay). Close-up of the hydranth (feeding polyp) indicated by the white arrow in A (B), stolonal colonies arose from a ramified hydrorhiza (C), medusae buds born on short pedicels covered by perisarc arose directly from the hydrorhiza with several medusa buds of variable size usually placed between two consecutive hydranths (D).
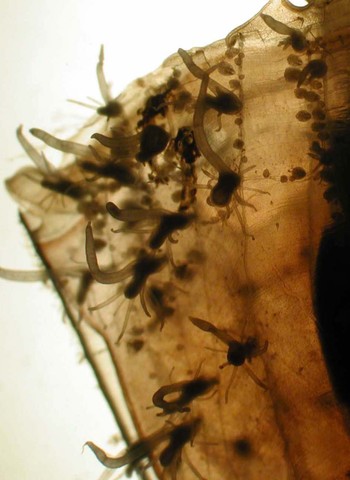
Fig. 11. Close-up of the shell of a specimen of Clio recurva collected by MOCNESS on 16 September 2002 between 215 and 138 m depth in Atlantis Canyon (Cruise ‘The Maine Event: Fall 2002’, P.I. Dr Marsh Youngbluth) with a colony of Campaniclava clionis (adult possibly Pandea rubra) attached to the shell surface. Polyps and gonozooids are visible attached to the mostly linear hydrorhiza and the hydranths with 7–10 filiform tentacles placed in two whorls of 3–5 tentacles each, with a distinctive single, long, hypertrophied tentacle of up to 3.1 mm in length arising from between these filiform tentacle whorls.

Fig. 12. Pandea rubra, specimen HD98GS2c, photographed in a shipboard aquarium. Lateral view in relaxed state (A, scale bar 5 mm), apico-lateral view (B) and oral-lateral view (C) in contracted state.

Fig. 13. Pandea rubra, sketch of specimen HD98GS2c with a portion of the exumbrella removed.

Fig. 14. Pandea rubra, in situ frame grabs of the escape response of specimen 2K1218SS4, of exumbrella width 103 mm, taken at 3 second intervals from the NTSC video record. Sequence from top left downwards, then from top right downwards.

Fig. 15. In situ images of an association between Pandea rubra and a hyperiid amphipod of the supra-family Platysceloidea, belonging to either of the families Platyscelidae or Parascelidae, made at 785 m depth during crewed submersible ‘Shinkai 2000’ Dive 1218. Amphipod in dorsal view (A), attachment position lateral and near a radial canal on the exumbrella (B), ventral view of amphipod with oral view of contracted medusa (C), frontal view (D) and lateral view (E) of amphipod.
Pandea rubra Bigelow, Reference Bigelow1913, plate 2 figures 1–7; Kramp, Reference Kramp1926, plate 2 figure 15; Russell, Reference Russell1953, figures 111–112; Naumov, 1969, figure 75; Kramp, Reference Kramp1968, figure 135; Arai & Brinckmann-Voss, Reference Arai and Brinckmann-Voss1980, figure 34; Bleeker & van der Spoel, Reference Bleeker and Van der Spoel1988, figure 14; Brinckmann-Voss & Arai, Reference Brinckmann-Voss and Arai1998, figure 5; Hunt & Lindsay, Reference Hunt and Lindsay1999, figure 6G (as Hydroidomedusae sp. A); Schuchert, Reference Schuchert2007b, figure 45.
MATERIAL EXAMINED
Specimens: 7 specimens, all preserved in 4–5% buffered formalin–seawater. First: JAMSTEC sample code HD98GS2c; captured at 868 m depth at 15:18 during ROV ‘HyperDolphin’ Dive 98 (temperature 2.9°C, salinity 34.29 psu, dissolved oxygen 3.5 ml/l, Sigma-T 27.33 kg/m3). Observed live and after preservation in 5% buffered formalin–seawater. Second: JAMSTEC sample code 10K116SS2; captured at 810 m depth at 11:13 during ROV ‘Kaiko’ Dive 116 (temperature 4.3°C, salinity 33.86 psu). Third: JAMSTEC sample code 2K1201SS1a; captured at 914 m depth at 11:23 during crewed submersible ‘Shinkai 2000’ Dive 1201 (temperature 3.7°C, salinity 34.36 psu, dissolved oxygen 1.4 ml/l, Sigma-T 27.31 kg/m3). Fourth: JAMSTEC sample code 2K1218SS4; captured at 624 m depth at 11:16 during crewed submersible ‘Shinkai 2000’ Dive 1218 (temperature 3.4°C, salinity 34.10 psu, dissolved oxygen 3.2 ml/L, Sigma-T 27.12 kg/m3). Fifth: specimen collected by a Multinet in the Weddell Sea between 1000–500 m on 15 January 1993 during the ‘Polarstern’ cruise Antarktis X/7 (temperature −0.4 to 0.4 °C, salinity 34.60 to 34.64 psu; 68°38.7′S 55°27.6′W). Sixth: specimen collected by an RMT (rectangular midwater trawl) net in waters north of Terre Adélie and George V Land, eastern Antarctica, between 500–1000 m on 29 January 2008 during a CEAMARC (Collaborative East Antarctic Marine Census) cruise on the TS ‘Umitaka Maru’ (temperature 1.4 to 1.7 °C, salinity 35.84 to 36.18 psu; 62°29.7′S 140°0.3 ′E). Seventh: specimen collected by an IYGPT (international young gadoid pelagic trawl) net in waters north of Terre Adélie and George V Land, eastern Antarctica, between 0–1000 m on 1 February 2008 during a CEAMARC cruise on the TS ‘Umitaka Maru’ (64°0.6 ′S 140°0.8′E)
Images: in situ HDTV images of specimen HD98GS2C (time code on original tape stored at JAMSTEC Yokohama Institute, 02:08:57:26–02:19:42:15). HDTV video footage of above animal in shipboard aquarium recorded by J. Tanada (JAMSTEC). Digital still macrophotographs of above animal in shipboard phototank recorded by D. Lindsay (JAMSTEC). In situ NTSC images of 3 other sampled individuals. Individual 10K116SS2, S-VHS 01;45;59;10–01;46;58;08 (CTL), Digital BetaCam dub 02:45:58:14–02:46:57:19 (TC). Individual 2K1201SS1a, 23:58:22:17–00:01:25:15. Individual 2K1218SS4, 22:17:25:28–22:19:21:22.
SPECIFIC CHARACTERS
Without exumbrellar nematocyst tracks; subumbrella deep-red in mature specimens; gonads forming fine-meshed network of pits; manubrium deep-red; no ocelli.
DESCRIPTION
Umbrella bell-shaped, slightly taller than wide in relaxed, live specimens, just over half as tall as wide in fully contracted/crumpled live specimens; with rounded summit without marked apical process; without exumbrellar nematocyst tracks; jelly very soft and fragile. Maximum recorded size of exumbrella 179 mm wide in seventh specimen (30 tentacles; subumbrella 153 mm wide, 155 mm high, mesoglea 13 mm thick). Subumbrella indented apically, ridged quadratically along margins, deep-red in live mature specimens, deep brownish red to chocolate brown when dead or preserved (e.g. sample 10K116SS2: mature; very damaged but fresh, exumbrella 30–35 mm wide, 41 mm high. specimen 6: 16 tentacles; partially contracted, subumbrella 35 mm wide, 34 mm high), transparent in younger specimens (e.g. specimen 5: 14 tentacles; exumbrella 14 mm wide, 15 mm high). Four ribbon-like radial canals, doubly bifurcating at apex in larger specimens, with wavy or jagged outlines; ring canal thinner than radial canals, with smooth outlines. Fourteen to thirty marginal tentacles in present specimens, much extensible, hollow, smooth, each with large conical basal bulb, relatively laterally compressed in present material, with distinct abaxial spur clasping margin of exumbrella. Abaxial spurs of bulbs pigmented cream-pink in present material. No rudimentary tentacles. No ocelli. Stomach large, with broad base, about one-quarter to one-third height of subumbrellar cavity in present material. Mouth with four lips with intensely folded and crenulated margins. Gonads situated interradially on stomach, forming very close-meshed irregular network of ridges with pits between. Colour stomach, mouth, gonads deep-red when alive, deep brownish red to chocolate brown when dead or preserved. Colour of radial and circular canals, and marginal tentacles cream-pink in sample HD98GS2c (16 tentacles; partially contracted, exumbrella 38 mm wide, 30 mm high, subumbrella 35 mm wide, 26 mm high), marginal tentacles darker proximally in sample 2K1218SS4 (30 tentacles (one developing); relaxed and alive, exumbrella 103 mm wide, 110 mm high).
Remarks
In situ the tentacles of P. rubra are often extended to lengths of greater than 6 times the bell height. The escape response when exposed to light is to slowly retract the tentacles and contract the umbrella as if to swim. However, P. rubra is an extremely slow swimmer and a single contraction of the bell can take up to 33 seconds (Figure 14). When subjected to physical stimulation it crumples the umbrella, much like the folding up of a paper lantern (Figure 12B, C). This is the origin of its common Japanese name akachōchinkurage (red paper lantern medusa).
Pandea rubra was recently reported as a host for pycnogonids and narcomedusae in the midwater (Pagès et al., Reference Pagès, Corbera and Lindsay2007). During the present study, an association between this medusa and a hyperiid amphipod of the supra-family Platysceloidea, belonging to either of the families Platyscelidae or Parascelidae, was also observed though the animal was unable to be sampled (Figure 15). The observation was made at 785 m depth at 12:24 during crewed submersible ‘Shinkai 2000’ Dive 1218. During this same dive, the large individual (2K1218SS4) that was captured at 624 m depth at 11:16 and the escape response of which is recorded in Figure 14, was found to be host to a narcomedusan parasite. Stolo-prolifers were attached at multiple sites on the manubrium (Figure 16A), although many of the larval narcomedusae were ripped free (Figure 16B) in the act of sampling with the slurp gun. They were in varying stages of development (Figure 16B, C & E; Figure 17A–D) and were attached with the exumbrellar surface facing towards the manubrium of their host (Figure 16D). The most developed individuals (e.g. Figures 16C & 17D) had hemispherical exumbrellas, 10–14 tentacles, a peripheral canal system, and three short, globular otoporpae with 3 otocysts per lappet. The younger individuals attached to the manubrium of P. rubra by way of two anchoring tentacles, as was also reported by Bouillon (Reference Bouillon1987) in Figure 9 for Cunina peregrina Bigelow, Reference Bigelow1909. Bouillon (Reference Bouillon1987) also includes an illustration of C. becki Bouillon, 1985 drawn eight hours after liberation from its host and, although adults of Cunina species have perradial manubrial pouches, in this figure (1B) the pouches are hardly developed. Our original tentative identification of the parasitic larval narcomedusae inhabiting the subumbrella of P. rubra was as a species of Pegantha, based on a lack of visible stomach pouches and the presence of otoporpae and a peripheral canal system. Pegantha godeffroyi (Haeckel, 1879), although considered an unrecognizable species by Kramp (Reference Kramp1961), is hemispherical, has 14 tentacles, 14 lappets, and has 2–3 statocysts (and otoporpae?) per lappet. The lappets in this species, however, were semi-circular, in contrast with the rectangular outline of the lappets in the present material. Given the similarity of the stolo-prolifers and developing young with those of C. peregrina, and that the manubrial pouches of Cunina species seem to become more prominent with age and are virtually indistinguishable in recently released medusae (Bouillon, Reference Bouillon1987), another possible identification for the present material would be as Cunina globosa Eschscholtz, 1829. As redescribed by Bigelow (Reference Bigelow1909), C. globosa is hemispherical, has 3 short, globular otoporpae, 3 otocysts per lappet, 13–14 tentacles in mature individuals, and a well-developed peripheral canal system. The shape of the lappets and canal system is also quite rectangular, as in the present material.

Fig. 16. Pandea rubra captured at 624 m depth during crewed submersible ‘Shinkai 2000’ Dive 1218 and its narcomedusan parasites. Apical view of Pandea rubra (sample 2K1218SS4) with larval narcomedusae visible through the translucent subumbrella (A, scale bar 5 mm). Stolo-prolifers and narcomedusae in various stages of development with fragments of an intensely folded and crenulated lip margin and a tentacle also torn free during sampling (B). Lateral view of an individual that was spontaneously released while the host was kept in a shipboard aquarium (C). Larval narcomedusae were attached with the exumbrellar surface facing towards the manubrium of their host (D). Apical and apico-lateral views of some of the larval narcomedusae (E).

Fig. 17. Sketches of larval narcomedusae that were attached to the manubrium of Pandea rubra (sample 2K1218SS4) in various stages of development. Lateral views of stolo-prolifers with the two anchoring tentacles visible (A, B), and oral views of more developed individuals with the anchoring tentacles still evident (C), and almost fully resorbed (D).
DISTRIBUTION
Pandea rubra has been recorded in the North Pacific, North and South Atlantic, off India, and in the Southern Ocean (Pagès et al., Reference Pagès, Corbera and Lindsay2007; Schuchert, Reference Schuchert2007b). No records yet exist for the Arctic Ocean or Mediterranean Sea, even though in situ surveys of the gelatinous macrofauna have been made to lower mesopelagic depths in these locations with submersible platforms (Mills et al., Reference Mills, Pugh, Harbison and Haddock1996; Raskoff et al., Reference Raskoff, Purcell and Hopcroft2005). It has been considered a rare, though distinct and easily recognizable species (Schuchert, Reference Schuchert2007b). However, since the establishment of a submersible-based midwater biology programme in Japan in 1996 (Hunt et al., Reference Hunt, Hashimoto, Fujiwara, Lindsay, Fujikura, Tsuchida and Yamamoto1997), we have observed P. rubra often in the waters off Japan, generally between 475–925 m with the deepest record at 1207 m and shallowest at 473 m (Table 3). Although no individuals were observed deeper than 800 m after 18:00:00, maximum survey depth and depth-range of maximum effort were shallower during the night. No evidence of diel vertical migration into depths shallower than the daytime distributional range was observed (Table 3). The distribution of P. rubra in relation to water masses (Figures 4, 9 & 18) suggests that it is neither a boreal nor warm water-associated species but rather that it avoids both warmer waters and the cold, low salinity Oyashio-derived waters when they are subducted into its regular distributional zone. Pandea rubra has been observed from March through to October in Japanese waters.

Fig. 18. Observation records of Pandea rubra (black and white diagonally bisected squares) during the ROV ‘Kaiko’ survey in April 1999, overlaid on the vertical profiles of water temperature versus depth. Dive 115 passed through a warm core-ring and extensions of the subducted cold Oyashio Currents are evident in the profile of Dive 116.
Table 3. Records of Pandea rubra occurrence based on in situ submersible surveys in Japanese waters from 1997–2007.

Temp., temperature (°C); Oxy., dissolved oxygen concentration (ml/L); SigmaT, Sigma T (kg/m3); n.a., data not available due to a faulty oxygen sensor; ≅clear subumbrella with red manubrium; υwith two pycnogonids attached; :in Hunt & Lindsay, 1997; ?Sample code 10K116SS2; τSample code 2K1218SS4; λSample code 2K1201SS1a; νSample code HD98GS2.
DISCUSSION
The anthomedusan species investigated in this study had contrasting distribution patterns. Euphysa japonica was most common near the ocean floor at ‘upper mesopelagic’ depths in far northern Japan. Its distribution maximum was usually just below the main body of the generally southward-flowing subducted Oyashio water mass. It can be conjectured that the adult medusae may be advected away from the site of budding by benthic polyps and cause the decreasing gradient in population density from north to south. This species can also occur in large numbers in the benthopelagic layer at greater depths, such as 1250 m depth off the east coast of Hokkaido Island (Toyokawa et al., Reference Toyokawa, Toda, Kikuchi, Miyake and Hashimoto2003) and it is therefore difficult to surmise the habitat and depth-range of its polyp stage.The ctenophore Beroe abyssicola Mortensen, 1927 has been observed to prey on E. japonica in situ (D.L., personal observation) and it is thought that other mesopelagic carnivores would also take advantage of such abundant, if patchy, prey. An indeterminate species of Euphysa, assigned once to E. japonica (Mackie & Mills, Reference Mackie and Mills1983) but the species level identification subsequently rescinded (Mackie, Reference Mackie1985), occurred at daytime depths between 115 m and 240 m in February and March 1981 (Mackie & Mills, Reference Mackie and Mills1983) and between 150 m and 216 m in November 1982 (Mackie, Reference Mackie1985) in the coastal waters of British Columbia. Nighttime distribution was bimodal with part of the population residing at 13–29 m depth and the remainder between 111–216 m depth in November 1982 (Mackie, Reference Mackie1985). The shallowest daytime observation of E. japonica in the present study was at 231 m depth and no evidence of diel vertical migration was found (Table 2), suggesting a different species identity for the aforementioned Euphysa sp. from British Columbia.
In comparison to trachymedusae and narcomedusae, both the diversity and population density of anthomedusae was low in mesopelagic and bathypelagic waters off the eastern seaboard of northern Japan. Two small corymorphid medusae with pointed exumbrellas were observed in the benthopelagic layer at 5430 m depth during ‘Shinkai 6500’ Dive 1037 but these were the only anthomedusae observed below 1500 m depth during any of the surveys. Submersible surveys are perhaps the only way such benthopelagic animals could be sampled due to the difficulties of towing plankton nets just above the bottom.
The identity and habitats of the polyp stages of all of the anthomedusae dealt with in this study remain ill-defined. In particular, when polyp colonies are attached to pelagic, biotic substrates rather than benthic, abiotic substrates, the linking of these polyp stages to their respective adult medusae can be an arduous task. It entails both the long term maintenance of the fragile planktonic organisms that act as substrates, maintenance of the polyp colonies themselves, and the rearing of newly liberated medusae to the point where unambiguous species identifications can be made based upon their morphologies. It is hoped that molecular data gained from such initiatives as the Cnidarian Tree of Life project (http://cnidarian.info) and the Census of Marine Zooplankton (http://www.cmarz.org) will facilitate the linking of polyp and medusoid generations, and to this end a molecular identifier in the form of the 18S ribosomal sequence was provided for E. japonica. We hope to sequence the medusae of C. nematophora and P. rubra in the near future.
Although the specific identification of the polyps of P. rubra is not perfectly elucidated it seems highly probable that they are epibionts on the shells of euthecosome pteropods such as Clio recurva. A worrying scenario can be envisaged based on the results of the present study. To date, P. rubra has been characterized as a quite rare mesopelagic medusa (Schuchert, Reference Schuchert2007b). However, as the present study shows, it is in fact a stable component of the mesopelagic fauna within the study area and can be relatively abundant in patches, as the observation of three individuals within a three minute period during ROV ‘HyperDolphin’ Dive 106 indicates (Table 3). This medusa is also relatively widespread worldwide, with records in the north and south Atlantic, off India, and in the Southern Ocean (Pagès et al., Reference Pagès, Corbera and Lindsay2007; Schuchert, Reference Schuchert2007b). Pandea rubra has recently been reported to be a host to pycnogonids (Pagès et al., Reference Pagès, Corbera and Lindsay2007; present study). The present study has also shown that it can be host to hyperiid amphipod parasitoids as well as to nurture larval narcomedusae. Recently much attention has been focused on the acidification of the oceans due to global warming and the effect this will have on calcifying organisms (Orr et al., Reference Orr, Fabry, Aumont, Bopp, Doney, Feely, Gnanadesikan, Gruber, Ishida, Joos, Key, Lindsay, Maier-Reimer, Matear, Monfray, Mouchet, Najjar, Plattner, Rodgers, Sabine, Sarmiento, Schlitzer, Slater, Totterdell, Weirig, Yamanaka and Yool2005; Royal Society, 2005). Detrimental effects of oceanic acidification on pteropods such as Clio will not only directly affect matter transport into the deep sea and affect the food webs in which these pteropods play a major role, but will also deprive epibiotic polyps of their hosts. The number of associations of this type presently known comprises 5 hydroid and 5 pteropod species with a high interspecificity, as summarized by Lalli & Gilmer (Reference Lalli and Gilmer1989). A detrimental effect on Clio recurva would impact the population of P. rubra and this would in turn impact mesopelagic hyperiid amphipods, pycnogonids and narcomedusae. Very little is known about the importance and character of possible run-on effects of oceanic acidification such as these due to the limited amount of data on interspecies interactions in mesopelagic waters. Modern technologies and methodologies such as those employed in the present study provide one way to gather much needed data on such inter-species relationships within the most unexplored biome on our planet—the ocean's midwater zone.
ACKNOWLEDGEMENTS
The authors would like to express their sincere thanks to Hirotaka Nakamura and Jun Tanada for technical help with the video analysis, to Per Flood for his photograph and other information on E. flammea, to Wolfgang Zeidler for checking the amphipod identification, to Mamoru Sano for access to some of the CTD data, and to Kaoru Hidaka and other JAMSTEC library staff and to Minoru Kitamura for literature support. We also acknowledge the Tohoku National Fisheries Research Institute for allowing us to use the Off Tohoku Temperature Field maps. We would like to express our appreciation to the captains and crews of the RV ‘Yokosuka’, RV ‘Kaiyo’, RV ‘Kaiko’, RV ‘Natsushima’, RV ‘Shunyo-Maru’ and TV ‘Umitaka Maru’, as well as to the operations teams and commanders of the manned submersibles ‘Shinkai 6500’ and ‘Shinkai 2000’, the ROVs ‘HyperDolphin’ and ‘Kaiko’, and the 6000 m-class deeptow camera operations team. Recognition is also due to Takashi Ishimaru and the other staff and students of the Faculty of Marine Science, Tokyo University of Marine Science and Technology, to Verónica Fuentes, Russell Hopcroft, Graham Hosie, and to the cadets and other members of the CEAMARC TV ‘Umitaka Maru’ cruise for their tireless efforts in procuring plankton samples from frigid Antarctic waters. VPR data were funded from the Agriculture, Forests and Fisheries Research Council (FY2002-2006). Supported in part by a grant to M. Youngbluth from the National Science Foundation (NSF-002493). We appreciate the opportunity given D.L. for a 5-month sabbatical at CSIC, Barcelona, by the Japan Society for the Promotion of Science. This study is a contribution of the Census of Marine Zooplankton (CMarZ), and of the Census of Antarctic Marine Life (CAML), both Census of Marine Life ocean realm field projects. This manuscript is CEAMARC contribution Number 2.

























