Introduction
Protective host immune responses to helminthic parasites are generally thought to be TH2 dependent, requiring the presence of CD4+ Helper T cells (TH) and the production of TH2-associated effector cytokines such as IL-4, 5, 9 and 13 in order to mediate expulsion (Sher and Coffman, Reference Sher and Coffman1992). The migration and expulsion of the murine hookworm Nippostrongylus brasiliensis during primary infection has been described in detail in several previous studies (Yokogawa, Reference Yokogawa1920, Reference Yokogawa1922; Croll, Reference Croll1977; Tindall and Wilson, Reference Tindall and Wilson1990a, Reference Tindall and Wilsonb). Infective L3 larvae penetrate through the skin and migrate via a cryptic pathway reaching the lungs at day 1–2 post-infection (p.i.), during which time they further mature to the L4 stage. In the lungs the larvae leave the bloodstream and invade the alveoli, then migrate along the trachea, eventually reaching the buccal cavity where they are swallowed. The larvae pass through the stomach, to the small intestines, where they reside mainly concentrated in the jejunum. Nippostrongylus brasiliensis reach sexual maturity at day 5–6 p.i. and adult (L5) females begin to lay eggs at day 6–7 p.i. Immunocompetent mice spontaneously expel adult worms in feces at days 9–12 p.i., resulting in a sterile resolution of infection. Expulsion of N. brasiliensis has been demonstrated to require CD4+ T cells and is dependent on IL-13, IL-4Rα and STAT6 signalling (Barner et al. Reference Barner, Mohrs, Brombacher and Kopf1998; Urban et al. Reference Urban, Noben-Trauth, Donaldson, Madden, Morris, Collins and Finkelman1998). It has further been demonstrated that expulsion is dependent on the expression of IL-13 by cells of the innate immune system (Voehringer et al. Reference Voehringer, Reese, Huang, Shinkai and Locksley2006) and IL-4R expression by non-bone marrow derived cells (Urban et al. Reference Urban, Noben-Trauth, Schopf, Madden and Finkelman2001b). This mechanism of expulsion is thought to be based on increasing the contractility of intestinal smooth muscle (Madden et al. Reference Madden, Whitman, Sullivan, Gause, Urban, Katona, Finkelman and Shea-Donohue2002; Zhao et al. Reference Zhao, McDermott, Urban, Gause, Madden, Yeung, Morris, Finkelman and Shea-Donohue2003) and altering intestinal epithelial cell function (Madden et al. Reference Madden, Yeung, Zhao, Gause, Finkelman, Katona, Urban and Shea-Donohue2004), in conjunction with goblet cell production of Relm-β (Herbert et al. Reference Herbert, Yang, Hogan, Groschwitz, Khodoun, Munitz, Orekov, Perkins, Wang, Brombacher, Urban, Rothenberg and Finkelman2009). Interestingly, whilst STAT6 is required for expulsion of N. brasiliensis it is not an absolute requirement for the induction of a robust TH2 response, with significant levels of TH2 cytokines (IL-4, 5 and 13) being produced by CD4+ T cells following infection with the parasite. However, in the absence of STAT6 signalling, type II associated effector responses are significantly diminished (Finkelman et al. Reference Finkelman, Morris, Orekhova, Mori, Donaldson, Reiner, Reilly, Schopf and Urban2000; van Panhuys et al. Reference van Panhuys, Tang, Prout, Camberis, Scarlett, Roberts, Hu-Li, Paul and Le Gros2008).
Conversely expulsion of N. brasiliensis from the rat intestine is thought to be mediated by a bimodal mechanism, whereby adult larvae are initially subject to irreparable damage by the immune system (Ogilvie and Hockley, Reference Ogilvie and Hockley1968) via a serum-based factor (Love et al. Reference Love, Ogilvie and McLaren1975), before being expelled by a cellular action (Dineen et al. Reference Dineen, Ogilvie and Kelly1973). Immune-mediated damage is not thought to be essential for the clearance of N. brasiliensis infection in mice. However, it can be demonstrated that immune damage occurs in the mouse model because following the surgical transfer of normal and damaged N. brasiliensis into the small intestine damaged N. brasiliensis are expelled faster than normal N. brasiliensis (Ishiwata et al. Reference Ishiwata, Nakao, Nakamura-Uchiyama and Nawa2002). Interestingly, although STAT6 signalling-mediated events are required for the clearance of N. brasiliensis in the mouse model (Urban et al. Reference Urban, Noben-Trauth, Donaldson, Madden, Morris, Collins and Finkelman1998) it was found that the mechanism responsible for mediating N. brasiliensis damage was induced independently of a requirement for STAT6 signalling (Ishiwata et al. Reference Ishiwata, Nakao, Nakamura-Uchiyama and Nawa2002). Therefore STAT6-independent damage to gut-dwelling parasites may represent an important alternative pathway for the interruption of the parasite life cycle, and the elucidation of its mechanism may lead to new possibilities for therapeutic intervention against clinically important enteric parasites.
In this study we aimed to clarify the role of Th2 effector responses mediated by STAT6 signalling in providing protective immunity by analysing the migration of N. brasiliensis during both primary and secondary infections. Nippostrongylus brasiliensis numbers in the lungs and gut were assessed to determine the relative contribution of STAT6 in the establishment of protective immunity at each of these sites. Further, the effects of STAT6-independent damage to N. brasiliensis during a chronic primary infection were assessed in order to determine whether the mechanism responsible for the immune damage was sufficient to clear the parasite. Here we assessed N. brasiliensis numbers in the gut directly which revealed that in the absence of STAT6 N. brasiliensis become trapped in the mucosa resulting in their death and degradation via a mechanism mediated by the innate immune system.
Materials and Methods
Ethics statement
All experimental procedures involving animals were approved by the Victoria University Animal Ethics Committee and carried out in accordance with the guidelines set forth by the MIMR, Victoria University and the Australian and New Zealand Laboratory Animal Association and AAALAC International in conformation with the NZ Animal Welfare Act.
Mice
BALB/c wild type (WT) and BALB/c STAT6−/− and C57B/6 MHCII−/− mice were obtained from the National Institute of Allergy and Infectious Diseases contract facility at Taconic Farms (Germantown, NY). Mice were maintained in a specific pathogen-free animal facility at the Malaghan Institute, according to institutional guidelines. Mice aged 6–12 weeks were used for experimental work and mice were age matched for each individual experiment.
Nippostrongylus brasiliensis infection
Nippostrongylus brasiliensis was maintained by serial passage through 6–12-week-old Lewis rats at the Malaghan Institute as previously described (Camberis et al. Reference Camberis, Le Gros and Urban2003). Balb/c, STAT6−/− and MHCII−/− mice were inoculated with 600 N. brasiliensis L3 via intradermal (i.d.) injection in 25 μl sterile PBS. After 30 days, secondary i.d. infections were administered. At the time points indicated after infection, lung tissues were isolated and processed after incubation overnight in PBS at 37 °C and the number of N. brasiliensis/tissue was determined under a dissecting microscope. For the N. brasiliensis motility assay, the total worm numbers present in the gut were determined by longitudinally opening the gut and counting under a dissecting microscope, followed by overnight incubation in PBS at 37 °C. Motility = number of migrating N. brasiliensis/total number N. brasiliensis in gut. Nippostrongylus brasiliensis egg counts were determined by removal of the caecum and determination of total egg numbers in the egg count chamber via flotation on sucrose. Fecundity was determined as: fecundity = (eggs/caecum)/(N. brasiliensis/gut).
Flow cytometry
Total cell numbers were determined by haemocytometer counting and Trypan blue exclusion. CD4+ cells and B cells in lymphoid tissue were determined by FACS (Min et al. Reference Min, Prout, Hu-Li, Zhu, Jankovic, Morgan, Urban, Dvorak, Finkelman, LeGros and Paul2004) following staining for CD4, B220 and CD45. Flow cytometry reagents were purchased from BD Biosciences. Samples were acquired on a FACSCalibur (Becton Dickinson) flow cytometer. Data were analysed using Flow Jo (Tree Star) software.
Extraction of total RNA, cDNA synthesis, RT-PCR and relative quantification
Total RNA was extracted using TRIZOL Reagent (Life Technologies, Rockville, MD). Two-microgram aliquots of RNA were reverse transcribed in 20 μL of reverse transcription buffer containing 5 mm MgCl2, 1 mm dNTP mixture, 1 U μL−1 RNase inhibitor, 0·25 U μL−1 AMV reverse transcriptase, and 0·125 μ m oligo dT-adaptor primer (Takara RNA LA PCR kit, Takara Biomedicals, Osaka, Japan) at 42 °C for 50 min. One-microlitre aliquots of the synthesized cDNA were mixed with SyBr Green PCR master mix (Applied Biosystems, Foster City, CA, USA) with appropriate primers and were amplified using a real-time PCR system 7300 (Applied Biosystems).
The specificity of each amplified product was confirmed by dissociation analyses that gave a single sharp dissociation peak, the absence of the amplified product without reverse transcription, and the appearance of a band of the expected size on electrophoresis of the amplified product. For the amplification of β-actin and GAPDH, Actb primers and Gapdh primers (Applied Biosystems), and TaqMan PCR master mix (Applied Biosystems) were used. In this case, we assayed SyBr Green and TaqMan in different tubes in the same PCR run. For relative quantification, standard curves of the threshold cycle (Ct) of amplification of each target against log ng total RNA were created using the cDNA samples that had the lowest Ct value in preliminary runs, and relative quantification was performed for each sample. All quantified values were normalized to those of β-actin and GAPDH (quantified value for the target/quantified value for β-actin and GAPDH).
Determination of the number of goblet cells
A segment of the jejunum distal to the pyloric ring was removed, opened longitudinally, fixed in 4% buffered formalin overnight, and embedded in paraffin in such a position that histological sections could be cut perpendicular to the luminal surface. Five-micron tissue sections were stained with periodic acid-Schiff (PAS) reaction and haematoxylin. Ten villi, which were cut as perpendicularly as possible, were selected per animal, and the numbers of goblet cells and epithelial nuclei in each villus were counted under a microscope, and the ratio of goblet cell number/100 epithelial cells was calculated. The mean number of goblet cells/100 epithelial cells in 30 villi per group of animals (n = 3) and s.d. were calculated.
The high iron diamine–alcian blue (pH 2.5; HID-AB2.5) method
Slides were immersed in high-iron diamine solution for 18–24 h (Spicer, Reference Spicer1965). After washing in 3% acetic acid, slides were then immersed in alcian blue 8 GX (10 mg mL−1) solution (pH 2·5) for 30 min. Sulfomucin stained with HID was coloured dark brown, while acidic mucin other than sulfomucin was stained blue by alcian blue.
Fluorescent bead analysis
The fluorescent bead assay (FBA) was adapted for the measurement of IgG1 and IgG2a from van Panhuys et al. (Reference van Panhuys, Tang, Prout, Camberis, Scarlett, Roberts, Hu-Li, Paul and Le Gros2008). Briefly, a polylink coupling kit (Bangslab, IN, USA) was used to bind IgG1 or IgG2a to carboxylated polystyrene beads (Bangslab, IN, USA) as per product protocol. Samples to be tested were diluted in FBA buffer and added to wells. Purified IgG1 and IgG2a were serially diluted in FBA buffer and added to the corresponding wells. Biotinylated detector reagents were added to corresponding wells and incubated at room temperature. Wells were then washed with FBA buffer. Streptavidin-Fitc (BD Pharmingen, NJ, USA) was diluted 1 : 1000 in FBA buffer and added to the wells, followed by incubation at room temperature. Beads were then washed as above and re-suspended in FBA buffer for analysis. Sample acquisition was conducted in a FACS Array (Becton Dickenson, CA, USA) and data analysed using FlowJo (TreeStar, OR, USA); at least 50 beads per sample were analysed. Standard curves and regression analysis was calculated with Prism (GraphPad, CA, USA).
Results
Characterization of N. brasiliensis infection in the absence of STAT6
To analyse the role of STAT6 in the establishment of a protective immune response to N. brasiliensis, WT and STAT6−/− animals were infected i.d. with 600 L3 N. brasiliensis. As animals deficient in STAT6 are unable to expel the L3 from the gut (Urban et al. Reference Urban, Noben-Trauth, Donaldson, Madden, Morris, Collins and Finkelman1998), STAT6−/− animals were treated on days 9 and 10 p.i. with the anthelmintic drug pyrantel pamoate to eliminate the larvae from the gut, and ensure that there was a similar antigenic stimulation during the primary response in both WT and STAT6−/− mice (Urban et al. Reference Urban, Katona, Paul and Finkelman1991). At day 30 post-primary infection, animals were re-infected at the same i.d. site with 600 L3 N. brasiliensis. The N. brasiliensis migrate from the dermis to the lung via a cryptic pathway and are first detected at 18 h p.i. (Weinstein, Reference Weinstein2006). Similar numbers of N. brasiliensis were detected in the lung at 48 h p.i. following primary infection in WT and STAT6−/− animals and following secondary infection in STAT6−/− animals (Fig. 1A). Secondary infection in WT animals induced a substantial protective effect with very few N. brasiliensis detected at 48 h p.i. in comparison to naïve and STAT6-deficient animals, indicating that STAT6 is essential for inducing a strong protective response against parasites during the lung phase of a secondary response. Interestingly, during secondary infection with N. brasiliensis an effective protective response is not detected at the site of infection (Harvie et al. Reference Harvie, Camberis, Tang, Delahunt, Paul and Le Gros2010), further N. brasiliensis could not be detected in the blood, kidneys, liver or heart at 24 h p.i. (data not shown). Analysis of N. brasiliensis migration to the gut reveals a further requirement for STAT6 in protection (Fig. 1B), since during the secondary response N. brasiliensis were not detected in the gut of WT mice on day 7 p.i. whereas significant numbers were observed during primary infection in both WT and STAT6−/− mice. Interestingly in the absence of STAT6 a degree of partial protection was observed during the secondary response, as a significant decrease in the number of N. brasiliensis was observed in the gut on day 7 p.i. (Fig. 1B). Whilst previous studies have indicated that STAT6 deficient animals are unable to efficiently clear N. brasiliensis (Barner et al. Reference Barner, Mohrs, Brombacher and Kopf1998; Urban et al. Reference Urban, Noben-Trauth, Donaldson, Madden, Morris, Collins and Finkelman1998), because a partially protective effect was observed here we sought to determine whether N. brasiliensis are also cleared during secondary infection. On day 14 p.i. gut N. brasiliensis numbers were analysed (Fig. 1C), WT mice cleared N. brasiliensis as previously described, and following secondary infection a significant decrease in the number of N. brasiliensis present in the gut was detected in comparison to primary infection. Whilst there was no significant decrease (P = 0·27) in the number of N. brasiliensis present during primary infection from day 7 to day 14, following secondary infection a significant decrease (P = 0·003) in the number of N. brasiliensis was detected at day 14 p.i., indicating the presence of a protective response which occurs independent of STAT6 signalling. Nippostrongylus brasiliensis present in WT and STAT6−/− mice at day 7 following primary infection were observed to be free swimming and exhibited a high degree of motility (Fig. 1D, left panel). In contrast, when N. brasiliensis present in STAT6−/− mice at day 14 p.i. were examined in situ they appeared largely immobilized and were engulfed in a dense layer of mucus (Fig. 1D, right panel). Additionally, significant numbers of degraded N. brasiliensis were present in the mucosa where only the GI tract of the N. brasiliensis was evident (Fig. 1E), which indicated that a late-phase protective response was occurring through the trapping and killing of N. brasiliensis in the mucosa. Further, 95% of N. brasiliensis removed from the gut at day 10 and cultured in vitro for 20 days remained viable (data not shown), indicating that the decrease in N. brasiliensis numbers was not because of death and passive degradation due to a limited lifespan. Thus, the immobilization and degradation of N. brasiliensis was due to an active gut associated process which may involve either the direct killing of N. brasiliensis or alteration of their ability to feed leading to starvation following trapping. As such, these observations indicate the presence of a novel secondary clearance mechanism distinct from the expulsion-mediated mechanism observed in STAT6-sufficient animals.

Fig. 1. Deletion of STAT6 induces an alternative programme of protection. Balb/c and STAT6−/− mice were inoculated with 600 Nippostrongylus brasiliensis larvae via i.d. injection (primary infection). After 30 days, secondary i.d. infections (600 L3) were administered. At the time points indicated after infection (A) lungs were isolated and processed, and after incubation overnight in PBS at 37 °C the number of migratory N. brasiliensis/lung was determined. (B and C) Gut tissue was dissected longitudinally and numbers of N. brasiliensis present in each gut were counted under a dissecting microscope. Data points shown indicate mean±s.e. from 3 individual animals. (D) STAT6−/− mice were inoculated with N. brasiliensis (600 larvae) via i.d. injection (primary). On day 7 (left) and day 14 (right) guts were excised and micrographs taken of N. brasiliensis present in the gut. (E) Representative micrographs of degraded worms (indicated by arrows) on day 14 post primary infection. Statistical significance determined by ANOVA and Tukey multiple comparison post-test; * P < 0·05, ** P < 0·01 and *** P < 0·001. Data shown are representative of 3 independent experiments.
Characterization of mucosal trapping in the absence of STAT6
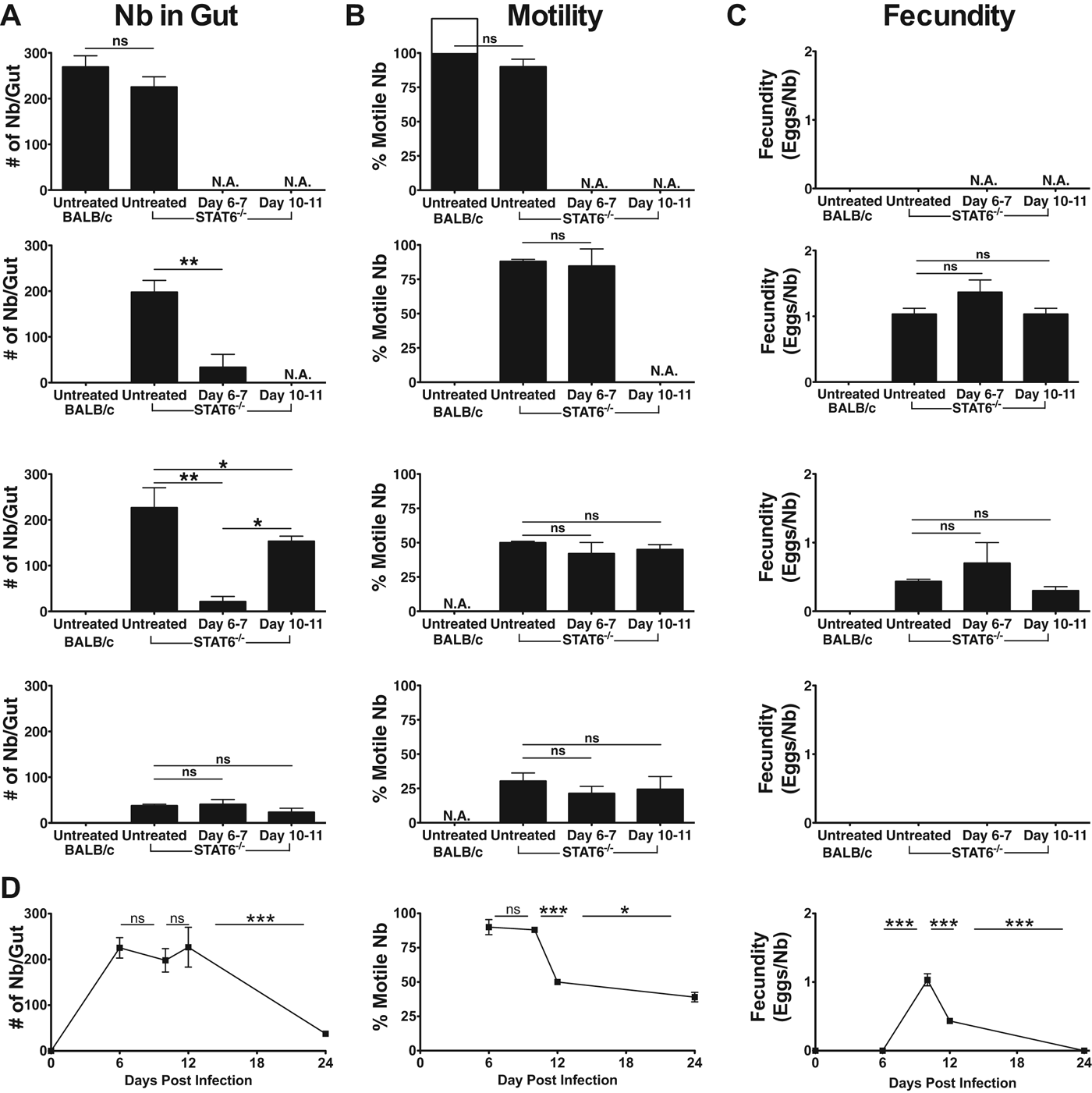
To determine whether N. brasiliensis become trapped in the mucosa in the absence of STAT6 the number of N. brasiliensis present in the gut was determined. Guts were then incubated overnight at 37 °C to determine the percentage of N. brasiliensis which were motile and able to migrate from the gut. On day 6 p.i. N. brasiliensis retained a high degree of motility with a minority of worms remaining in the gut of both WT and STAT6−/− mice (Fig. 2A, B and D). However, by day 12 p.i. the majority of N. brasiliensis resident in the gut of STAT6−/− mice were unable to migrate from the gut and remained trapped in the mucosa following primary infection (Fig. 2B and D). Mucosal trapping was also analysed after administration of the anthelmintic drug pyrantel pamoate which clears N. brasiliensis by inhibition of helminthic cholinesterase resulting in spastic paralysis and clearance by normal gut peristaltic motion (Aubry et al. Reference Aubry, Cowell, Davey and Shevde1970). STAT6−/− mice infected with N. brasiliensis were either left untreated or received 2 mg pyrantel pamoate on days 6 and 7 or days 10 and 11 p.i. Following drug treatment the numbers of N. brasiliensis resident in the gut were determined on days 10, 12 and 24 p.i. Drug treatment at days 6–7 effectively cleared the majority of worms, whereas treatment on day 10–11 led to a much less efficient clearance of worms (Fig. 2A) indicating that the majority of N. brasiliensis present in the gut of STAT6−/− animals at day 10–11 was largely unaffected by drug treatment. On day 6 p.i. when N. brasiliensis are susceptible to clearance by pyrantel pamoate very few N. brasiliensis were non-motile; however, this increased to > 50% on days 12 and 24, indicating that the failure of pyrantel pamoate to clear N. brasiliensis from the gut following treatment at day 10 was largely due to mucosal trapping of N. brasiliensis. Further, on day 24 N. brasiliensis were largely absent from the gut of untreated STAT6−/− animals indicating that there is a STAT6 independent mechanism of resistance occurring at a late phase during primary infection which is coincident with the induction of mucosal trapping (Fig. 2D). Additionally, N. brasiliensis fecundity was calculated by comparison of caecum egg counts with N. brasiliensis present in the gut (Fig. 2C and D). In all 3 STAT6−/− groups fecundity peaked at day 10, with egg counts diminishing from day 10 to day 12 and with no eggs detected at 24 days p.i. Interestingly, the decrease in egg counts on day 12 corresponded directly with the increase in mucosal trapping observed, suggesting that mucosal trapping of N. brasiliensis inhibits its ability to reproduce effectively or alternatively that the eggs produced by N. brasiliensis may become trapped in the mucosal layer, inhibiting their migration through the digestive tract and thus effectively disrupting their life cycle. Because pyrantel pamoate is efficacious on living worms and can influence gut physiology, it is important to point out that we observed similar proportions of motile N. brasiliensis at days 12 and 24 in treated and untreated animals, indicating that it is unlikely that the lack of responsiveness observed is indicative of a subpopulation of senescent or metabolically compromised worms in the intestine of treated animals. Thus we see here that N. brasiliensis can be cleared early during infection by drug treatment whereas at days 10–12 during infection a significant proportion of N. brasiliensis become trapped in the gut, such that they cannot be cleared by normal peristaltic motion.

Fig. 2. Mucosal trapping of Nippostrongylus brasiliensis occurs in the absence of STAT6−/−. Balb/c and STAT6−/− mice were inoculated with N. brasiliensis (600 larvae) via i.d. injection (primary infection). Following primary infection STAT6−/− mice were treated with pyrantel pamoate on days 6 and 7 or days 10 and 11, or were left untreated. At the time points indicated after infection gut tissues were isolated and processed. (A) Guts were dissected longitudinally and numbers of N. brasiliensis present in each gut were counted under a dissecting microscope. (B) Guts were then incubated overnight in PBS at 37 °C and the number of N. brasiliensis able to migrate from the gut was determined. (C) Number of eggs/caecum was also determined. (D) Time-course analysis of data from (A), (B) and (C) for untreated STAT6−/− mice. Data points shown indicate mean±s.e. from 3 individual animals and are representative of 2 individual experiments. Statistical significance determined by (A–C) ANOVA and Tukey multiple comparison post-test, or (D) by Student's t-test; * P < 0·05, ** P < 0·01 and *** P < 0·001.
Adaptive immune responses and mucosal trapping
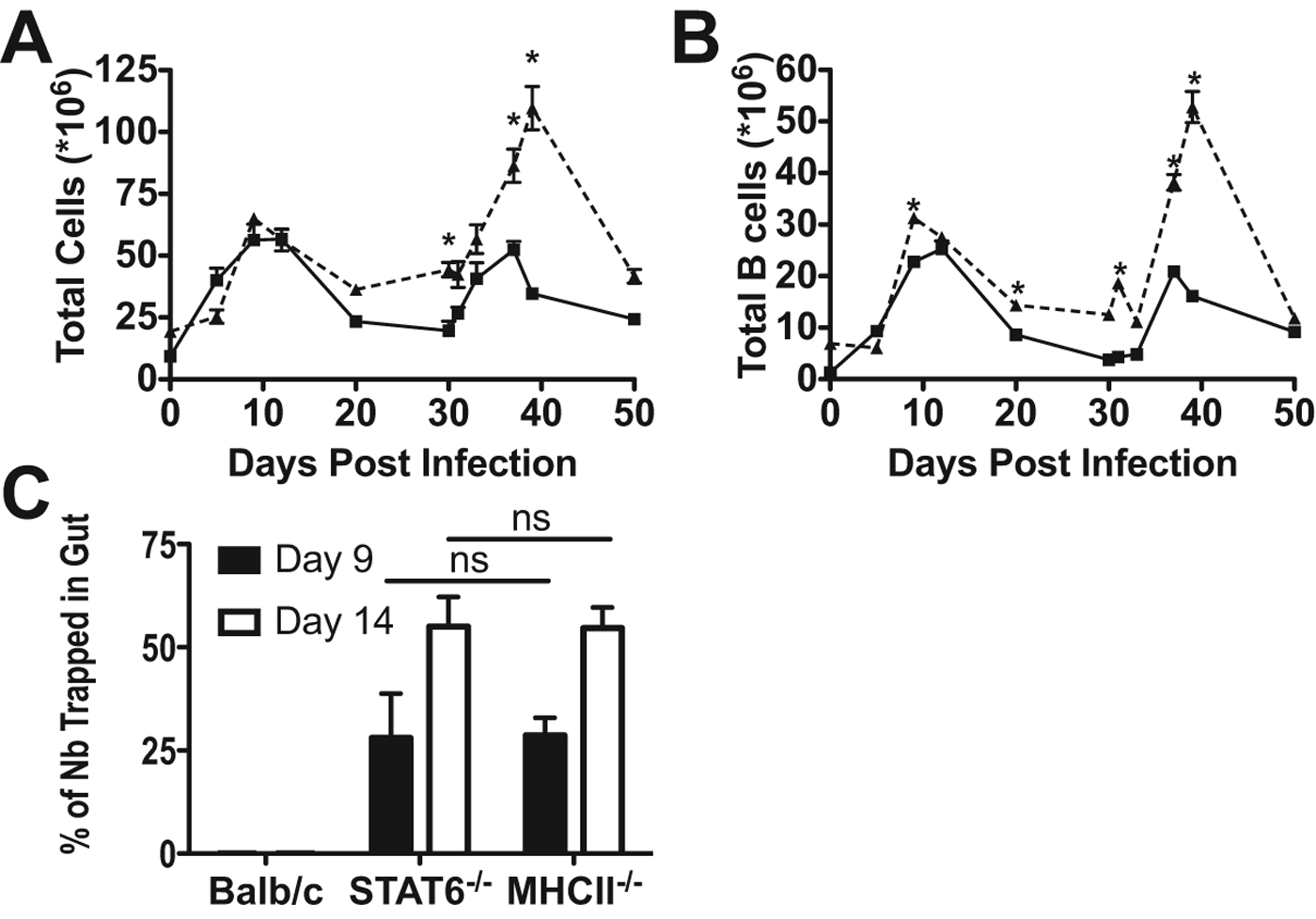
As we have previously described a STAT6-independent expansion of CD4+ T cells (van Panhuys et al. Reference van Panhuys, Tang, Prout, Camberis, Scarlett, Roberts, Hu-Li, Paul and Le Gros2008), we next studied the adaptive immune response during infection by analysing the expansion of B cells present in the mesenteric lymph node following primary and secondary infection with N. brasiliensis. Following primary infection with N. brasiliensis total mesenteric lymph node cell numbers (Fig. 3A) and B cell numbers (Fig. 3B) increased in WT and STAT6−/− animals with similar kinetics peaking at day 10 p.i. Following primary infection and clearance, total cell, CD4+ and B cell numbers decreased in both WT and STAT6−/− animals. However, cell numbers were maintained at a higher level in STAT6−/− animals most likely due to the incomplete clearance of N. brasiliensis by pyrantel pamoate. Upon secondary infection, WT animals rapidly cleared N. brasiliensis (Fig. 1D and E), whereas N. brasiliensis clearance in STAT6−/− was more delayed. The speed of clearance is reflected in the secondary response observed, with the WT peak response occurring on days 5–7, whereas 3-fold greater cell numbers were observed in STAT6−/− animals with the peak of response occurring at day 9 p.i. As there is a significant adaptive immune response to N. brasiliensis infection in the absence of STAT6, we wanted to determine whether the adaptive response was required for mucosal trapping. As CD4+ cells have previously been found to be required for expulsion of N. brasiliensis (Urban et al. Reference Urban, Noben-Trauth, Donaldson, Madden, Morris, Collins and Finkelman1998), mucosal trapping was assessed following primary infection in STAT6−/− and MHCII−/− animals (Fig. 3C). On day 9 p.i. a comparable proportion of N. brasiliensis were found to be trapped in the gut mucosa, and the proportion of trapped N. brasiliensis increased in both groups on day 14, indicating that MHCII expression, CD4+ activation and associated B cell responses are not required for the mucosal trapping of the N. brasiliensis.

Fig. 3. STAT6-deficient animals have heightened secondary responses to Nb. Balb/c (solid line) and STAT6−/− (dashed line) mice were inoculated with N. brasiliensis (600 larvae) via i.d. injection (primary infection), STAT6−/− animals were treated on days 9 and 10 p.i. with the anthelmintic drug pyrantel pamoate to eliminate N. brasiliensis from the gut. After 30 days, secondary i.d. infections (600 L3) were administered. At the time points indicated after infection mice were culled and mesenteric lymph nodes removed. (A) Cell numbers were determined using a haemocytometer and Trypan blue exclusion. (B) Total B cell numbers were determined by flow cytometry following staining for CD45, CD4 and B220. Data points shown indicate mean±s.e. from 3 individual animals. (C) Balb/c, STAT6−/− and MHCII−/− mice were inoculated with N. brasiliensis as in (A). At the time points indicated after infection gut tissues were isolated and processed. Data points shown indicate mean±s.e. from 3 individual animals. Statistical significance determined by Student's t- test (A and B) or ANOVA and Tukey multiple comparison post-test (C); * P < 0·05, ** P < 0·01 and *** P < 0·001.
Innate effector responses and mucosal trapping
As the adaptive arm of the immune system does not seem to play a role in the mucosal trapping of N. brasiliensis we investigated the possibility that the intestinal mucosa and associated innate cell types could be directly responsible for trapping. MUC2, ST3GalIV, Intelectin-2 (Soga et al. Reference Soga, Yamauchi, Kawai, Yamada, Uchikawa, Tegoshi, Mitsufuji, Yoshikawa and Arizono2008; Takeda et al. 2010), Relm-β (Herbert et al. Reference Herbert, Yang, Hogan, Groschwitz, Khodoun, Munitz, Orekov, Perkins, Wang, Brombacher, Urban, Rothenberg and Finkelman2009) and delayed goblet cell hyperplasia (Horsnell et al. Reference Horsnell, Cutler, Hoving, Mearns, Myburgh, Arendse, Finkelman, Owens, Erle and Brombacher2007) have all previously been reported to be involved in pathways important for the clearance of N. brasiliensis. To test whether these components were associated with mucosal trapping we initially analysed the expression of ST3GalIV, Intelectin and Relm-β by real-time PCR (Fig. 4A). Levels of the mucosal sialyltransferase ST3GalIV were found to be upregulated in WT epithelial cells on day 7 p.i. and remained high until day 14, whereas STAT6−/− cells exhibited a small increase in expression which was determined to be non-significant. HID-AB2.5 histochemistry was additionally used to directly examine levels of highly sulphated mucin and weakly acidic mucin such as sialylated mucin: sulfomucin (Fig. 4B). In WT mice, virtually all the goblet cells contained sulphated mucin and, following infection, the number of goblet cells with weakly acidic mucin increased significantly in the crypt epithelium of WT mice, while in the villus epithelium the majority of goblet cells were those containing sulfomucin. A proportion of goblet cells in the crypt epithelium and lower (neck) part of the villi contained both sulfo- and weakly acidic mucin (coloured dark brown and blue). In STAT6−/− mice, weakly acidic mucin-containing goblet cells also appeared after infection, but the numbers were significantly smaller than those in WT mice (Table 1). In BALB/c and STAT6−/− mice, the appearance (or non-appearance) of weakly acidic mucin containing sialomucin during infection parallels the levels of ST3GalIV gene transcription. Expression of Intelectin-2 mRNA was found to follow a pattern of expression similar to ST3GalIV with WT animals having an increase of expression on days 7 and 14, whereas STAT6−/− animals exhibited a small, but non-significant increase following infection. It should also be noted that WT animals expressed higher levels of Intelectin-2 on day 14, even though N. brasiliensis were expelled on day 9.

Fig. 4. Gut mucosal response in the absence of STAT6 Balb/c and STAT6−/− mice were inoculated with N. brasiliensis (600 larvae) via i.d. injection. At the time points indicated after infection gut tissues were excised. (A) Total gut cell production of ST3GalIV, Intelectin and Relm-β were determined by real-time PCR. Data points shown indicate mean±s.e. from 3 individual animals. At day 9 p.i. (B) the HID-AB2.5 method was used to differentiate highly sulphated mucin and weakly acidic mucin such as sialylated mucin: sulfomucin coloured dark brown and weakly acidic mucin blue in gut epithelia. (C) Jejunal sections stained with periodic acid-Schiff (PAS) reaction and haematoxylin. On day 14 p.i. (D) representative cross-sections of N. brasiliensis (indicated by arrows) in tissue sections stained with PAS were taken. Representative micrographs shown are from 3 individual animals per time point. Statistical significance determined by Student's t-test; * P < 0·05, ** P < 0·01 and *** P < 0·001.
Table 1. Goblet cell index (number of goblet cells in villus/total number of epithelial nuclei in villus)
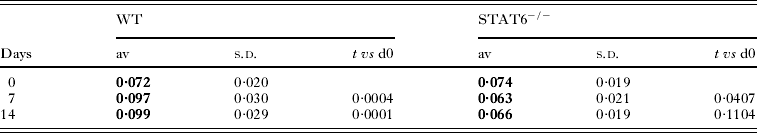
Levels of Relm-β were found to be increased over baseline at all time points examined, with expression peaking in WT animals on day 7, whereas peak expression in STAT6−/− animals was significantly later, on day 14, indicating both STAT6-dependent and independent mechanisms of regulation. During infection goblet cells in WT mice showed not only hyperplasia, but also swelling of the ‘goblet’ and secretion of PAS-positive mucus (Fig. 4C). In STAT6−/− mice, especially 14 days p.i., mild goblet cell hyperplasia occurred, centred in the crypt epithelium, but the extent was significantly lower than that observed in WT mice. Additionally, we noted that initial goblet cell levels in WT and STAT6−/− mice were equivalent but, following infection, the ratio of goblet cells to epithelial cells was found to increase in WT mice and decrease in STAT6−/− animals (Table 1). Cross-sections of gut epithelia containing N. brasiliensis were stained with PAS and at day 7 p.i., no clear association between goblet cells and N. brasiliensis was detected. However, by day 14 N. brasiliensis were found to be associated with a significant number of PAS-positive goblet cells, additionally indicating that they were found deeper in the villi as the majority of PAS-positive cells in STAT6−/− animals were found to be in the villus crypts. This finding indicated that mucosal trapping correlates with the increased expression of Relm-β in the goblet cells present in the villus crypts.
Protective responses to secondary infection
As we had previously observed a partially protective response following secondary infection in STAT6−/− animals, we examined whether the reduction in the number of motile N. brasiliensis observed was due to an increase in early mucosal trapping. On days 7 and 14 post primary and secondary infection a similar proportion of N. brasiliensis were found to be trapped in the mucosa (Fig. 5A). However, the total number trapped following secondary infection was greatly reduced in comparison to primary infection (Fig. 5B), indicating that the partially protective response seen post secondary infection occurs independent of mucosal trapping. As a significant B cell response was found to be coincident with secondary infection (Fig. 3B) this suggested that antibody-mediated immune damage may be responsible for early clearance of N. brasiliensis. To determine circulating Ab concentrations we used the FBA to determine serum levels of IgG1 (Fig. 5C) and IgG2a (Fig. 5D) following infection; serum IgE was not analysed as we have previously shown STAT6 is required for IgE production in this system (van Panhuys et al. Reference van Panhuys, Tang, Prout, Camberis, Scarlett, Roberts, Hu-Li, Paul and Le Gros2008). On day 0 WT animals exhibited a higher basal IgG1 level in comparison to STAT6−/− animals and upon infection levels of IgG1 increased in both strains with WT levels of IgG1 increasing until day 30. In contrast, STAT6−/− animals had a peak response on day 12 corresponding to peak mesenteric B cell numbers (Fig. 3C) and possibly indicating a defect in IgG1 producing plasma cell differentiation. Basal levels of serum IgG2a were similar in both strains and increased markedly in STAT6−/− animals peaking on day 12, whereas WT animals exhibited a small increase in IgG2a levels that was maintained until day 30. Upon secondary infection (day 30) WT levels of both IgG1 and IgG2a showed an immediate decrease before IgG1 production resumed showing a significant increase at day 50 whereas IgG2a levels were largely unaffected. STAT6−/− animals additionally had an immediate decrease in IgG1, with levels then climbing to peak on day 9 before declining to near basal levels. IgG2a production, on the other hand, immediately increased peaking on day 7, and on day 50 levels continued to decline. Thus the kinetics of IgG2a induction in STAT6−/− animals can potentially explain the early partially protective effect seen in the gut following secondary infection especially as previous studies in other model systems have indicated that N. brasiliensis is damaged by antibody release (Ogilvie and Hockley, Reference Ogilvie and Hockley1968; Dineen et al. Reference Dineen, Ogilvie and Kelly1973; Love et al. Reference Love, Ogilvie and McLaren1975; Jacobson et al. Reference Jacobson, Reed and Manning1977).

Fig. 5. Partial protection during secondary infection is independent of mucosal trapping. Balb/c (solid line) and STAT6−/− (dashed line) mice were inoculated with 600 N. brasiliensis via i.d. injection (primary infection). After 30 days, secondary i.d. infections were administered. Mucosal trapping was analysed by determining (A) per cent worms trapped in the gut and (B) absolute number of trapped worms at days 7 and 14 p.i. Fluorometric bead analysis of serum (C) IgG1 and (D) IgG2a at the time points indicated. Data points shown indicate mean±s.e. from 3 individual animals per time point. (A, B) Statistical significance determined by ANOVA and Tukey multiple comparison post-test, (C, D) or by Student's t test; * P < 0·05, ** P < 0·01 and *** P < 0·001.
Discussion
STAT6 signalling plays a key role in the expulsion of N. brasiliensis from host mice under normal circumstances (Urban et al. Reference Urban, Noben-Trauth, Donaldson, Madden, Morris, Collins and Finkelman1998, Reference Urban, Noben-Trauth, Schopf, Madden and Finkelman2001a; Finkelman et al. Reference Finkelman, Shea-Donohue, Morris, Gildea, Strait, Madden, Schopf and Urban2004), but it appears that additional innate pathways for parasite clearance exist which operate independently of both STAT6 and the CD4+ component of the adaptive immune response.
In this study, we demonstrated that in the absence of STAT6 signalling clearance of N. brasiliensis is decreased, but not abrogated as previously suggested (Urban et al. Reference Urban, Noben-Trauth, Donaldson, Madden, Morris, Collins and Finkelman1998, Reference Urban, Noben-Trauth, Schopf, Madden and Finkelman2001a; Finkelman et al. Reference Finkelman, Shea-Donohue, Morris, Gildea, Strait, Madden, Schopf and Urban2004) and instead a secondary mechanism which traps N. brasiliensis in the gut mucosa is employed to clear animals of infection. We observed that STAT6 was essential for the partially protective responses observed in the lung following secondary infection and complete gut protection. Interestingly, even in the absence of STAT6 signalling, we observed a degree of partial protection in the gut following secondary infection by an undetermined mechanism. Due to the speed of the partial protection observed it is likely that the mechanism involves an element of the adaptive immune system and may potentially be antibody mediated, as previous studies have indicated that N. brasiliensis may be damaged by antibody release (Ogilvie and Hockley, Reference Ogilvie and Hockley1968; Dineen et al. Reference Dineen, Ogilvie and Kelly1973; Love et al. Reference Love, Ogilvie and McLaren1975; Jacobson et al. Reference Jacobson, Reed and Manning1977) into the gut and circulating levels of IgG1 and IgG2a are not as dependent on IL-4/STAT6 signalling as IgE is (Le Gros et al. Reference Le Gros, Ben-Sasson, Seder, Finkelman and Paul1990). Here , we clearly saw a marked increase in the levels of IgG1 and IgG2a with the kinetics of IgG2a closely matching the early clearance of N. brasiliensis. Alternatively, as drug clearance with pyrantel pamoate was incomplete, it is possible that the small numbers of remaining N. brasiliensis are sufficient to induce an ongoing response which creates a mucosal environment that impedes their ability to establish themselves in the gut mucosa and leads to their direct clearance by peristalsis. Future studies are required to address this scenario, but it raises the possibility of engineering unique mucosal environments which could be protective against future infections.
Previous studies have suggested mechanisms of clearance of primary Nb infection such as changes in intestinal smooth muscle function (Zhao et al. Reference Zhao, Urban, Anthony, Sun, Stiltz, van Rooijen, Wynn, Gause and Shea-Donohue2008); it was recently shown (Zhao et al. Reference Zhao, Urban, Sun, Stiltz, Morimoto, Notari, Madden, Yang, Grinchuk, Ramalingam, Wynn and Shea-Donohue2010) that following infection by N. brasiliensis the mucosal epithelia up-regulated IL-25 in a STAT6-dependent fashion. Here IL-25−/− mice had diminished intestinal smooth muscle and epithelial responses and an impaired Th2 protective response, indicating that IL-25 plays a critical role in nematode infection-induced alterations in intestinal function that are important for protective immunity. Additionally, it has been suggested that intestinal epithelial cell secretion of RELM-β protects against N. brasiliensis infection (Herbert et al. Reference Herbert, Yang, Hogan, Groschwitz, Khodoun, Munitz, Orekov, Perkins, Wang, Brombacher, Urban, Rothenberg and Finkelman2009). It was shown that IL-4 and IL-13 protect against intestinal lumen-dwelling worms primarily by inducing intestinal epithelial cells to differentiate into goblet cells that secrete RELM-β, and that RELM-β inhibits the ability of worms to feed on host tissues during infection via a mechanism that does not require T or B cells, alternative macrophage activation, or increased gut permeability.
As the standard technique (Camberis et al. Reference Camberis, Le Gros and Urban2003) for analysis of N. brasiliensis numbers in the gut and the efficacy of protective responses relies upon opening the intestine longitudinally and incubating them in PBS at 37 °C it is likely that most studies have overlooked the possibility of a trapping mechanism for clearance of N. brasiliensis. However, as we routinely counted the number of N. brasiliensis present in the gut at the time of tissue harvest we were able to deduce that significant numbers become trapped and degraded in the gut mucosa in the absence of STAT6.
In order to investigate a potential mechanism we assessed the expression of mucosal response genes ST3GalIV (Soga et al. Reference Soga, Yamauchi, Kawai, Yamada, Uchikawa, Tegoshi, Mitsufuji, Yoshikawa and Arizono2008; Takeda et al. Reference Takeda, Hashimoto, Uchikawa, Tegoshi, Yamada and Arizono2010), Intelectin-2 (Voehringer et al. Reference Voehringer, Stanley, Cox, Completo, Lowary and Locksley2007) and Relm-β (Herbert et al. Reference Herbert, Yang, Hogan, Groschwitz, Khodoun, Munitz, Orekov, Perkins, Wang, Brombacher, Urban, Rothenberg and Finkelman2009), as previous studies had indicated that they were up-regulated in the epithelia in response to infection by N. brasiliensis. Intelectin-2 was dependent on STAT6 signalling for expression, which is not entirely surprising as during Hp infection, WT mice showed marked up-regulation of Intelectin 2, but SCID mice did not (Knight et al. Reference Knight, Pemberton, Robertson, Roy, Wright and Miller2004), indicating control by the adaptive response which we have determined to be redundant in mucosal trapping. Surprisingly, ST3GalIV was also found to be STAT6 dependent as it was previously determined that it could be up-regulated in athymic rats, indicating an innate mechanism of regulation in response to parasite infection. Relm-β expression was found to be markedly up-regulated on day 7 in WT animals with a significant decrease in expression on day 14 p.i., which correlates closely to the presence of N. brasiliensis and to previously characterized levels of plasma IL-4/IL-13 (Finkelman et al. Reference Finkelman, Morris, Orekhova, Mori, Donaldson, Reiner, Reilly, Schopf and Urban2000). Strikingly, in STAT6-deficient animals, an increase in Relm-β expression was detected on day 7, increasing to peak levels observed in WT animals on day 14 and indicating the presence of an IL-13/STAT6-independent mechanism not previously described. Levels of Relm-β expression seem to correlate with the degree of mucosal trapping observed and the reduction in numbers of N. brasiliensis present overall, suggesting that Relm-β may play a role in the mucosal trapping and clearance of N. brasiliensis. Relm-β has previously been described to influence mucus secretion in a model of inflammatory bowel disease (Krimi et al. Reference Krimi, Kotelevets, Dubuquoy, Plaisancie, Walker, Lehy, Desreumaux, Van Seuningen, Chastre, Forgue-Lafitte and Marie2008) where it was shown to increase MUC2 and M1/MUC5AC gene expression and directly participate in maintaining the mucosal defence barrier. While we did not detect a large increase in MUC2 activity by histochemistry it is possible that Relm-β exerts its mucosal trapping effects on other mucus-associated genes, thus causing the immobilization and degradation of N. brasiliensis. We saw in the case of WT animals that Relm-β expression was tightly controlled and was expressed in conjunction with the pattern of expression of IL-4 previously observed during N. brasiliensis infection. In addition to Relm-β acting to decrease feeding in N. brasiliensis (Herbert et al. Reference Herbert, Yang, Hogan, Groschwitz, Khodoun, Munitz, Orekov, Perkins, Wang, Brombacher, Urban, Rothenberg and Finkelman2009) it also has resistin-like effects on metabolism, causing decreased insulin sensitivity and, as such, needs to be tightly controlled since uncontrolled expression of Relm-β could lead to the induction of pro-inflammatory macrophages, atherosclerosis and metabolic syndrome (Asano et al. Reference Asano, Sakosda, Fujishiro, Anai, Kushiyama, Horike, Kamata, Ogihara, Kurihara and Uchijima2006). Curiously, type II inflammation seems to have an inbuilt control for this because, instead of inducing insulin resistance, IL-4/STAT6 signalling and infection with N. brasiliensis have recently been described to improve insulin sensitivity (Ricardo-Gonzalez et al. Reference Ricardo-Gonzalez, Red Eagle, Odegaard, Jouihan, Morel, Heredia, Mukundan, Wu, Locksley and Chawla2010) through the action of eosinophils (Wu et al. 2011) in inducing the alternative activation of macrophages in fatty tissue. Thus, in describing a mucosal trapping pathway which potentially kills through the induction of Relm-β in the absence of STAT6 we must keep in mind the potential for dysregulation of energy homoeostasis if the mucosal trapping pathway is to be of clinical relevance. Overall it appears that multiple strategies have been developed for the clearance of gut-dwelling parasites, each suited to specific classes of helminth which likely home to differential gut niches. Under normal conditions N. brasiliensis is readily cleared by changes in intestinal smooth muscle function (Zhao et al. Reference Zhao, Urban, Anthony, Sun, Stiltz, van Rooijen, Wynn, Gause and Shea-Donohue2008) in combination with Relm-β (Herbert et al. Reference Herbert, Yang, Hogan, Groschwitz, Khodoun, Munitz, Orekov, Perkins, Wang, Brombacher, Urban, Rothenberg and Finkelman2009) and does not rely on antibody-based damage as do the rejection of H. polygyrus (Liu et al. Reference Liu, Kreider, Bowdridge, Liu, Song, Gaydo, Urban and Gause2010) and T. spiralis (Carlisle et al. Reference Carlisle, McGregor and Appleton1991), or upon epithelial cell turnover as mediates T. trichuria (Cliffe et al. Reference Cliffe, Humphreys, Lane, Potten, Booth and Grencis2005) expulsion or mastocytosis as with T. spiralis (Urban et al. Reference Urban, Schopf, Morris, Orekhova, Madden, Betts, Gamble, Byrd, Donaldson, Else and Finkelman2000). However, we have demonstrated here that in the absence of normal signalling N. brasiliensis infection is able to be controlled by co-opting mechanisms usually required for the expulsion of other nematodes, indicating that there is a great degree of functional redundancy present in anti-nematode responses likely indicating a strong evolutionary pressure to maintain normal nutrient levels and metabolic homoeostasis.
FINANCIAL SUPPORT
N.V.P. was funded by the New Zealand Foundation for Research Science and Technology.