Significant outcomes
-
∙ In our study, 12.40% of mothers were at increased risk of postpartum depression (PPD) in the second month after childbirth.
-
∙ Medical risk factors of PPD were premenstrual syndrome before pregnancy and family history of psychiatric disorders.
-
∙ Psychological risk factors of PPD were a high score in neuroticism scale [the Neo Five-Factor Inventory (NEO-FFI) Personality Inventory] and a high score (>12 points) in the Edinburgh Postnatal Depression Scale (EPDS) in the first postnatal week.
Limitations
-
∙ The lack of any validated procedure to identify a major depressive disorder is the main limitation of this study.
-
∙ The sample of patients is too small, highly selected, and relatively homogenous.
-
∙ The final multivariate logistic regression model explained solely 32.6% of the dependent variable.
Introduction
The prevalence of depression after childbirth has been estimated to range from 6.5% to 19.2% during the first 3 months after delivery, and from 6.5% to 12.9% during the first year (Reference Gavin, Gaynes and Lohr1,Reference Myers, Aubuchon-Endsley and Bastian2). Postpartum or PPD may lead to significant adverse effects for both the mother and the newborn (Reference Myers, Aubuchon-Endsley and Bastian2,Reference Deave3) so dealing with it (diagnosis, treatment, and follow-up) requires particular attention. The proper diagnosis of PPD is not an easy task. The ICD-10 does not recognise PPD as a separate disorder, but places it among mental and behavioural disorders associated with puerperium, not elsewhere classified: F53 (4). Likewise, the only relevant entry in the DSM-5 is ‘Major Depressive Disorder with Peripartum Onset’ (5).
Various reasons for PPD have been suggested in the literature, though the data are inconsistent. Myers et al. (Reference Myers, Aubuchon-Endsley and Bastian2) report the following risk factors with acceptably strong evidence: unemployment, preterm or low birth weight infant, poor health of the mother, a psychiatric history (of perinatal depression, depression, premenstrual syndrome/dysphoric disorder – PMS/PPD, anxiety, neuroticism, or vulnerability), marital status, poor quality of relationship, and poor social support.
The link between personality and depression is well known (Reference Clark, Watson and Mineka6–Reference Hakulinen, Elovainio and Pulkki-Raback8). The theory that there is an association between PPD and the personality trait of neuroticism is supported by a vast body of evidence (Reference Dudek, Jaeschke and Siwek9–Reference Verkerk, Denollet and Van Heck11). A Spanish study found that neuroticism, as measured by the Eysenck Personality Questionnaire, is a strong risk factor for PPD between 2 and 8 months after delivery (Reference Martin-Santos, Gelabert and Subira10). This same correlation was also found in a Polish study by Podolska (Reference Podolska, Bidzan and Majkowicz12), where neuroticism was measured with the NEO-FFI Personality Inventory. Social support plays a critical role in the development and treatment not only of depression, but of any postnatal mood disorders, whether this support is provided by a partner (Reference Pilkington, Whelan and Milne13) or other sources (Reference Gjerdingen, McGovern and Attanasio14).
In 1987, Cox et al. developed a screening test: the EPDS. When a patient is screened positive, she needs to be referred to a specialist for evaluation (Reference Cox, Holden and Sagovsky15). There is no consensus on the specific time of screening. Haran et al. suggest 4–6 weeks and 3–6 months or sometime between 6 and 12 months after delivery, but give no strict limits (Reference Haran, van Driel and Mitchell16). It has also been proposed to screen patients twice, with a 3-week break in between (Reference Cox, Holden and Sagovsky15,Reference v Ballestrem, Strauss and Kachele17). There are other tools that can be used to screen for PPD as well, such as the Patient Health Questionnaire (PHQ), the Postpartum Depression Screening Scale (PDSS), or the Beck Depression Inventory (BDI II) (Reference Myers, Aubuchon-Endsley and Bastian2,Reference Conradt, Manian and Bornstein18). The PDSS is a popular screening test with specificity and sensitivity for major depression in the range of 80–90% (Reference Myers, Aubuchon-Endsley and Bastian2,Reference Beck and Gable19). It is a 35-item Likert-type self-report tool (Reference Beck and Gable19). It was conceived due to limitations of BDI II in postnatal depression screening. The BDI is designed for general depression episode detection in both non-perinatal women and men (Reference Conradt, Manian and Bornstein18). It comprises of 21 items and its psychometric features (specificity and sensitivity) are 74% and 79.9%, respectively (Reference Myers, Aubuchon-Endsley and Bastian2). We may find statements that BDI is not a proper tool for diagnosing PPD due to the fact that it examines somatic symptoms typical of puerperium. Therefore, we may have more false positive diagnoses (Reference Conradt, Manian and Bornstein18,Reference Kammerer, Marks and Pinard20). In a paper comparing EPDS, PDSS short form, and PHQ-9, the authors state that EPDS is an accurate way of screening during the first 6 months (Reference Hanusa, Scholle and Haskett21). Another paper supports the use of EPDS, PDSS, and BDI II with urban, low-income mothers (Reference Chaudron, Szilagyi and Tang22).
Aims of the study
So far, there are few available studies that have combined personality, social support, medical, and sociodemographic data in the context of possible postpartum mood disorders. The purpose of our study was to identify risk factors for PPD.
Materials and methods
Participants
A cross-sectional study was designed. Women who gave birth between May 2013 and June 2014, and who stayed at the postpartum ward were invited to join the study. A group of 567 subjects agreed to fill in a questionnaire, which they subsequently received. They were asked to return forms to informed medical staff before discharge from the hospital. Eligible participants were mothers who were 18 or more years old, and who had live birth. They were informed about the purpose and structure of the study, and each provided written consent. They subsequently received questionnaires in the fourth week after delivery, via e-mail or post. We included patients of 3 weeks of puerperium (therefore in their fourth postnatal week) in the study as this period is rarely investigated due to the time criterion for PPD in DSM-5 (4 weeks) and ICD-10 (6 weeks), whereas postpartum maternal blues should last not longer than 14 days (Reference Henshaw23,Reference Meltzer-Brody24). We believe this time frame should not be omitted. This specific time was chosen for the research because of the potentially strong effect of PPD on the development of the child, so we decided to explore it as soon as possible (Reference O’Hara25,Reference Talge, Neal and Glover26). To improve retention, we sent two consecutive emails, one text, and one letter with a questionnaire in each phase, whenever contact information was provided. The time limit for response was 4 weeks after receiving the questionnaire. We assumed that our examination took place in the second postnatal month, although it was exactly between 21 and 49 days. We used following exclusion criteria: death of a newborn child, illness of a child requiring special parental care (genetic or surgically treated), preterm labour earlier than 32 weeks of pregnancy, a neurological or other severe illness of the mother, stressful life events at any time during the study (death of a child or parent, divorce/marital separation, job loss, serious illness of a member of the family). The survey did not focus on such specific circumstances. Due to the number of patients and different ways of withdrawing from the study (an oral refusal, a questionnaire left empty or partially filled, not returning the questionnaire), the exact number of questionnaires sent is not known. Overall, 424 patients returned two questionnaires which represents a 74.8% participation rate, and 387 women satisfied all criteria and were included in the study for this paper, which is a rate of 68.4%. Further research in the third and sixth months after delivery is planned for this project.
Women who were identified as being at risk of PPD (EPDS>12 or thoughts of harming themselves) were advised to visit a psychiatrist, an obstetrician or a psychologist immediately for prompt diagnosis. Both included and excluded patients received this information.
Psychometric measures
The questionnaire used in the study contained the EPDS, questions on social and medical status, and standardised psychological scales: the NEO-FFI Personality Inventory, the Berlin Social Support Scales (BSSS). The investigators carried out a meticulous medical interview on admission, and studied all available medical documentation which a patient is obliged to provide. We have focussed on information concerning the patients’ psychiatric history, including any medication or treatment, family history, and current psychiatric symptoms.
The EPDS consists of 10 questions, and between 0 and 30 points may be scored. The tool does not include any items concerning fatigue or changes in sleep, appetite, or libido, as these may be natural shortly after childbirth (Reference Eberhard-Gran, Slinning and Rognerud27). Thirteen, twelve, or ten points is most often taken as the cut-off score for identifying risk of PPD (Reference Myers, Aubuchon-Endsley and Bastian2,Reference Haran, van Driel and Mitchell16) or probable or major depression (Reference Gavin, Gaynes and Lohr1), and in this study we assumed a score of 13 as the cut-off point. This limit yields satisfactory sensitivity and specificity: 84.2–93.9% and 75.2–76.7%, respectively. The Cronbach’s α is reported as 0.87–0.88% (Reference Dennis28). The Personality Inventory NEO-FFI test consists of five dimensions: neuroticism (N), extraversion (E), openness to experience (O), agreeableness (A), and conscientiousness (C). The raw points are converted into a standardised 10-point scale. As required, individual forms were purchased specifically for use in the study. Psychometric values were proven to be satisfactory, that is, internal consistency coefficients of subscales≥0.70 (Reference Zawadzki, Strelau, Szczepaniak and Śliwińska29). The BSSS measures the following parameters of social support: perceived available support, need for support, support seeking, currently received support, and protective buffering. Emotional, informative, instrumental support, and satisfaction were subscales found in some of the scales above. The final result is the arithmetical mean of a scale or subscale. Its psychometric features such as Cronbach’s α (0.71–0.90) and validity are of satisfactory level (Reference Łuszczyńska, Mazurkiewicz and Kowalska30). Other data assessed by the patient (quality of feeding the baby) were measured on a 10-point scale similar to the Numerical Rating Scale used for pain. The second questionnaire consisted of EPDS II and questions concerning medical and social status.
Statistics
A statistical analysis was conducted using STATISTICA 12.0 software and it involved calculation of descriptive and inferential data. For testing statistical hypothesis, a two-tailed critical region was assumed. Qualitative characteristics are shown as frequencies (%), whereas quantitative features as arithmetical means with measures of statistical dispersion: a range between maximum and minimum, and standard deviation. We used the following tests: the Student’s t-test and the Mann–Whitney U-test (continuous variables), the χ2 test (categorical variables), the test for significance of two means. A one-way analysis of variance (ANOVA) was performed to check if there are any statistically significant differences between the means of EPDS found separately in each of the 4 investigated postnatal weeks. Further predictive analysis was involved. Univariate and multivariate logistic regression were used to investigate risk factors for the disorder in question. We studied the relationship between one dependent binary variable (presence or not of risk of PPD) and one or more independent variables. The risk of PPD was assessed on the basis of the odds ratio (OR). When necessary, preliminary analyses were conducted to assess normality, linearity, and homoscedasticity. A two-tailed p<0.05 was assumed to be the threshold of statistical significance.
Ethics
The study was approved by the institutional Board of Ethics (NKEBN/531/2011-2012). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
The patients responded to the second questionnaire an average of 29.49 (range: 21–49, SD 6.47) days after childbirth. The average mother was 30.37-years old (range: 19–46). These women gave birth in the 39.15th week of pregnancy (range: 32.3–42.0) on average, most often by vaginal birth (69.77%). The most common place of residence in our sample was a city with >100 000 habitants (69.77%). Most of these women had higher education (79.07%) and were employed (90.70%). They were most often married (81.14%) and primiparous (56.85%). The majority were breastfeeding: 82.17% in the first week and 86.30% in the fourth week after delivery. Two patients (0.52%) had a current psychiatric disorder, 7.49% of them had positive psychiatric history (most often an episode of major depressive disorder or anxiety disorder), and 6.54% had a positive family history. Few patients (4.39%) had been administered psychiatric medication (sertraline, citalopram, fluoxetine, venlaxetine, alprazolamum among them) and none were taking any at the time.
In the first week after childbirth, patients scored an average of 7.84 points on EPDS I, with a range of 0–20 points, and an average of 7.16 (range: 0–26) in the fourth week (EPDS II). We analysed the results of EPDS II depending on time of response (Table 1).
Table 1 Results of the Edinburgh Postnatal Depression Scale in separate postnatal weeks

A one-way between-subjects ANOVA was conducted to compare the effect of time of response on the EPDS results using 3, 4, 5, and 6 weeks as conditions. There was no significant effect of time of response on results of the EPDS at p<0.05 for the four conditions [F(3,247)=0.9817, p=0.402]. Notably, patients responding in the fourth week were a majority (31.7%). There were 12 patients who responded too late [range: 51–67, mean 57.41 (SD 5.76) days after a delivery]. Thus, they were excluded from the statistical analysis. They received 5.33 (SD 3.20) points in EPDS II on average.
A risk of PPD was found in 48 patients in the second postnatal month, which is a prevalence of 12.40%. These patients had significantly lower scores than in the first test in week 1 (p=0.00). In comparison with a group of patients not at risk of PPD, the women with probable PPD had higher scores on EPDS II [15.94 (SD 3.12) vs. 5.91 (SD 3.38), respectively, p=0.00]. It should be pointed out that the same trend was observed when the results of EPDS I were adjusted to risk from EPDS II: 11.66 (SD 4.88) versus 7.30 (SD 3.98), respectively, p=0.00. When the cut-off point in the EPDS is put at 10 points, the prevalence of risk is 28.87% (112 subjects). Both EPDS I and EPDS II positive answers were found in 22 patients, which is 5.67%.
Women who were lost in follow-up (n=136, including those responding too late) less frequently had higher education (64.71% vs. 85.82%, p<0.001), were more likely to be unemployed (19.85% vs. 9.28%, p=0.002), to have smoked during the pregnancy (17.65% vs. 9.54%, p=0.01), and to have had a caesarean section (38.24% vs. 28.87%, p=0.04). The patients who failed to respond were less likely to have taken a prenatal class (27.41% vs. 47.42%, p=0.001) and to exclusively breastfeed in a hospital after the delivery (30.91% vs. 53.74%, p=0.002). There was no difference between points scored in EPDS I [8.32 (SD 4.34) vs. 7.82 (SD 4.34), p=0.25].
Mothers with probable PPD in the second month after delivery more often had a history of any psychiatric disorder and of premenstrual syndrome. They scored more than either 12 points or 9 points soon after the delivery (in the first week) more often. They were less satisfied with feeding the newborn in the fourth week after delivery. They rarely breastfed exclusively in the first week. No other significant differences were found. The significant risk factors for PPD were a history of any psychiatric disorder, PPD, and premenstrual syndrome, as well as risk of PPD in the first week of puerperium (for EPDS>12 and for EPDS>9). Low satisfaction with their quality of feeding the baby may be linked with risk of PPD, as well as lack of exclusive breastfeeding (Table 2).
Table 2 Sample characteristics of women at and not at risk of postpartum depression (PPD) based on an Edinburgh Postnatal Depression Scale (EPDS) cut-off score of 13
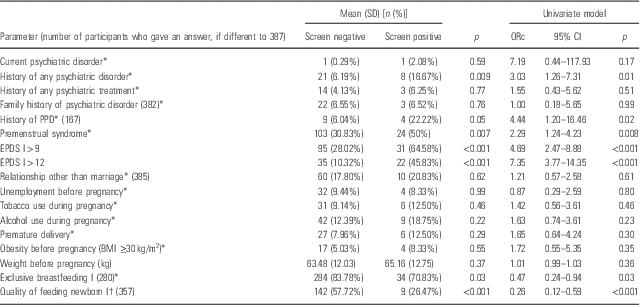
Odds ratio from logistic univariate regression.
I, in the first week of puerperium; II, in the fourth week of puerperium; BMI, body mass index; ORc, crude odds ratio; CI, confidence interval.
* Yes/no answer.
† Self-assessment on a scale from 0 (worst ever) to 10 (best ever).
Women who were more likely to experience PPD presented a significantly higher level of neuroticism, and lower levels of extraversion, openness to experience, and conscientiousness. A high level of neuroticism increased the risk of PPD, whereas extraversion, openness to experience, and conscientiousness were found to be protective factors in univariate logistic regression only (Table 3).
Table 3 Comparison of Neo Five-Factor Inventory (NEO-FFI) personality features of women at and not at risk of postpartum depression based on an Edinburgh Postnatal Depression Scale (EPDS) cut-off score of 13

Odds ratio from univariate logistic regression.
A, agreeableness; C, conscientiousness; CI, confidence interval; E, extraversion; N, neuroticism; O, openness to experience; ORc, crude odds ratio.
Patients at risk of PPD also showed lower scores in terms of social support dimensions (which implies that good social support would be a protective factor against depression): perceived available, perceived emotional, perceived instrumental, currently received, currently received emotional, informative, and instrumental. They were less satisfied with the social support they were receiving and had higher results on a buffering protective scale. The factors affecting probable PPD are all dimensions of social support, except for need for support and support seeking (Table 4).
Table 4 Comparison of social support (BSSS) of women at risk and not at risk of postpartum depression based on an Edinburgh Postnatal Depression Scale cut-off score of 13

Odds ratio from univariate logistic regression.
BSSS, Berlin Social Support Scales; CI, confidence interval; ORc, crude odds ratio.
In order to assess the influence of most important aforementioned data on the risk of PPD, a multivariate logistic regression analysis was performed. A model was proven to be significant (χ2=91.068, df=24, p<0.001), and all predictors explained 32.6% of the dependent variable (pseudo R 2=0.326). Significant predictors are as follows: a positive result on the EPDS (>12 points) in the first week of puerperium, a history of premenstrual syndrome, a history of any family psychiatric disorder, and personality trait neuroticism. Due to the fact that the 95% confidence interval is wide for both positive results of EPDS in first week, and a history of premenstrual syndrome, the associations should be interpreted with caution (Table 5).
Table 5 The major determinants of risk of postpartum depression (screened positive in EPDS II) as determined by multivariate logistic regression model

Model fully adjusted.
I, in the first week of puerperium; BSSS, Berlin Social Support Scales; CI, confidence interval; EPDS I, Edinburgh Postnatal Depression Scale used in the first week of puerperium with score more than 12 points; EPDS II, Edinburgh Postnatal Depression Scale used in the fourth week of puerperium; NEO-FFI, the Neo Five-Factor Personality Inventory; ORa, adjusted odds ratio.
The results were as follows: χ2=91.068, df=24, p<0.001, Pseudo R 2=0.326.
* Yes/no answer.
† Self-assessment on a scale 0–10.
Discussion
Puerperium is a time when we should screen patients for PPD. This level of access up to several weeks after delivery is due to the organisation of the Polish healthcare system. New mothers are invited to see a gynaecologist 6 weeks after delivery. This visit should include an examination of their mental health, as it is rarely possible to set up a separate visit with a psychiatrist (Reference Haran, van Driel and Mitchell16,Reference Austin31). We ought to be especially aware of which patients remain at risk of PPD, and this study shows what we would pay attention to.
Personality traits such as high neuroticism, low extraversion, and conscientiousness are associated with postpartum disorders (Reference Hakulinen, Elovainio and Pulkki-Raback8,Reference Dudek, Jaeschke and Siwek9,Reference Podolska, Bidzan and Majkowicz12,Reference Maliszewska, Swiatkowska-Freund and Bidzan32,Reference Maliszewska, Bidzan and Swiatkowska-Freund33). According to our results, neuroticism is the strongest predictive factor for establishing the probability of PPD, as it was significant in a multivariate logistic regression model. A high level of neuroticism means greater vulnerability to the experience of negative feelings. Such people will have more difficulty adapting to stressful events. The early stage of maternity is a stressful event (Reference Kudo, Shinohara and Kodama34,Reference Ahn and Corwin35). It is believed that this association between personality and depression is bidirectional and there are other explanatory theories (Reference Hakulinen, Elovainio and Pulkki-Raback8,Reference Klein, Kotov and Bufferd36). In clinics, it is hard to assess a patient’s personality and degree of neuroticism without specific tools and knowledge.
Premenstrual syndrome or premenstrual dysphoric disorder appeared to be important risk factor. These findings are in line with the literature. It shows the importance of emotional and physical symptoms specific to stages of the reproductive cycle of women. Moreover, it suggests a common aetiology. Presently, the theory of fluctuations of sex hormones is dominant (Reference Buttner, Mott and Pearlstein37,Reference Lee, Yi and Ju38). There is no simple answer to the question of whether premenstrual syndrome leads to the onset of PPD or whether they are both the result of some common cause.
The result of the EPDS in the first week of puerperium is another risk factor. A score above 12 at this time suggests a possible affective disorder has already appeared. The patient may have had depressive symptoms perinatally, before delivery, or even before pregnancy, and will remain vulnerable to mental disorders (Reference Myers, Aubuchon-Endsley and Bastian2,Reference Dennis28). Baby blues commonly occurs around the time of the first examination, and this mild condition, typical to the postnatal period (with a prevalence of around 40–80%) (Reference O’Hara and McCabe39), may have been present. However, a positive result of EPDS I, as was the case for 57% of patients, is not the same as maternal blues, as this is not the tool to make such a diagnosis and this disorder may actually be overestimated (Reference Matthey40). It should be noted that an even lower cut-off score of 10 in EPDS yields an over four times greater chance for PPD in a univariate model. We must focus on such patients and give them special attention. Another implication is the need for screening for mood disorders in hospital soon after delivery – we may find a history of psychiatric disorder or PPD. This can help identify patients suffering or prone to suffer from similar mental disorders.
Our findings about importance of social support in the context of risk of PPD is consistent with other reviews (Reference Pilkington, Whelan and Milne13,Reference Pilkington, Milne and Cairns41). Although most scales were proven significant in univariate models, none was significant in the multivariate model. A dyadic support interaction may influence coping with stressful situations, and, in turn, a patient’s mental health in general. The importance of a good-quality relationship should be highlighted (Reference Eberhard-Gran, Slinning and Rognerud27,Reference Kaźmierczak, Kiełbratowska and Karasiewicz42,Reference Rosand, Slinning and Eberhard-Gran43).
A positive family history was proven to be significant, although it seems to have a minor influence on the risk of PPD. However, it was a general (yes/no) question as patients often could not give more specific information on nature of the disorder of a relative. In contrast, a personal history of psychiatric disorder and psychiatric treatment had no effect in the final model, although the first condition increased the risk of PPD over three times in a univariate model (crude OR=3.30, p=0.01). This outcome is not consistent with the literature, where a history of depression or anxiety is usually emphasised in this context (Reference Myers, Aubuchon-Endsley and Bastian2,Reference Henshaw23). The potential reasons of this inconsistency are a homogeneity of the studied group, patients who did not provide all appropriate information, or a statistical error. Therefore, this result should be interpreted with caution.
Depressive cognitive schemas may lead to negative thinking, that is, greater elaboration on negative information (Reference Gotlib and Joormann44). Thus, the subjective evaluation, especially of negative events, is worse. In this study, this pattern may apply to the self-assessment of social support (although measured by a separate tool, it is actually assessed by the patient). Therefore, we may not exclude a reciprocal relationship between these variables and postnatal depression to distinguish them as co-existing symptoms (rather than risk factors).
Our study has some clinical implications. A simple question about premenstrual syndrome or family psychiatric history is easy to use in a routine examination. Primary care anamnesis may lead to identifying a person’s potential risk of mood disturbance. We may find interesting results by comparing two consecutive EPDS tests (Reference Eberhard-Gran, Slinning and Rognerud27).
This paper presents interesting findings for a European country that has not been well represented in PPD literature. The sample was a somewhat meaningful one, and the findings are interesting. We established a prevalence rate for EPDS-defined PPD immediately following childbirth and about 1 month later. All of these data will be useful for future reviews and meta-analyses. The same can be said for the study of risk factors.
Mental state was assessed using the EPDS. The lack of confirmation by another validated tool or a full diagnosis by a psychiatrist or psychologist is the main limitation of this study. Although the EPDS was administered twice (after delivery and 3 weeks later), it yielded only 22 twice-positive patients, which is not a good number for statistical analysis. As the research was begun early (in the fourth week after delivery), although the actual time of participation was 4.5 weeks after delivery, conclusions should be made cautiously. The highly selected and relatively homogenous sample, along with the modest response rate, may be a threat to generalisability. The study should be repeated for full confidence in the results. Poland needs validation research to evaluate a cut-off point. In a properly designed future study, the EPDS should be used twice with a 3-week break, at a few separate time points during the first year of motherhood. The Structured Clinical Interview for DSM-IV Axis I Disorders, a semi-structured interview for making major DSM-IV Axis I diagnoses, would be a good choice as a comparison method. Moreover, we established the rate of PMS/PPD with a simple question about its presence in a patient’s life. There are specific tools for the diagnosis of premenstrual syndrome and these should be also used in such a study. We should emphasise that further research at 3 and 6 months after delivery is being carried out and will be reported.
We must be aware of the limitations of self-report measures. Such instruments are cost-effective and easy to use, not only during an examination but for scientific purposes as well. Quantitative results with sharp cut-off scores easily divide patients into two groups: those suffering and those not suffering from a disorder. However, this is based on the reliability of patients, which is unknown (Reference Stuart, Pasco and Jacka45). Each particular case requires an individual assessment, as patients may be misdiagnosed in screening programme. Therefore, these tools are useful for population screening, but are only vague indicators of an actual episode of depression, which requires clinical confirmation (Reference Myers and Weissman46). These tests may be validated and compared with a gold standard, that is ICD-10 or DSM-5 criteria (Reference Bergant, Nguyen, Heim, Ulmer and Dapunt47–Reference Guedeney and Fermanian49).








