Introduction
Mortality from traumatic hemorrhagic shock remains high despite optimal resuscitation efforts.Reference Krausz 1 - Reference Rossaint, Cerny and Coats 6 To improve trauma patient outcome, there has been a sustained effort to develop a hemoglobin-based oxygen carrier (HBOC) solution that could be used as a resuscitation fluid in the prehospital or military field setting.Reference Jahr, Walker and Manoochehri 7 - Reference Freilich, Pearce and Pitman 10 The development of such a solution requires the use of clinical trials that demonstrate the effects of a potential resuscitation fluid.Reference Riou, Landais and Vivien 11 - Reference George 13 In order to assess the efficacy of an HBOC solution in traumatic hemorrhagic shock, the study population must demonstrate improved survival with use of the study fluid as compared to use of standard therapies. Optimally, the ability to detect the efficacy of a new therapy requires study subjects with predicted mid-range mortality and Trauma and Injury Severity Score (TRISS) survival predictions of 40 to 60%.Reference Riou, Landais and Vivien 11
Both Diaspirin Cross-Linked Hemoglobin (DCLHb, Baxter Healthcare Corporation, Chicago, Illinois USA) and PolyHeme (Northfield Laboratories Inc., Evanston, Illinois USA) have been studied in the prehospital setting utilizing physiologic criteria indicative of hypoperfusion as the entry criteria.Reference Kerner, Ahlers, Veit, Riou, Saunders and Pison 14 - Reference Moore, Lavoie and Turgeon 15 A previously proposed prehospital traumatic hemorrhagic shock study of HBOC-201 (Biopure Corporation, Cambridge, Massachusetts USA) suggested the use of an RTS stratum of 1-3.99 as a proposed entry criterion.Reference Mongan, Moon-Massat, Rentko, Mihok, Dragovich and Sharma 16 - Reference Jahr, Mackenzie, Pearce, Pitman and Greenburg 19 Use of RTS as a study entry criterion allows for ease of prehospital calculation using three clinical variables that can be rapidly assessed: Glasgow Coma Scale (GCS), systolic blood pressure (SBP), and respiratory rate (RR).Reference Champion, Sacco, Copes, Gann, Gennarelli and Flanagan 20 RTS has been examined with regard to predicting mortality in trauma patients, injury severity, outcome of poly-traumatized patients, and length of hospital stay. However, no studies have examined RTS as a study entry criterion, nor has any traumatic hemorrhagic shock clinical trial specifically utilized RTS as an entry criterion.Reference Gabbe, Cameron and Finch 21 - Reference Ahmad 25
The purpose of this study was to compare the proposed RTS 1-3.99 range with other RTS ranges in order to determine which RTS range would identify a population of blunt and penetrating trauma patients with predicted and actually mortality rates near 50%. Additionally, receiver operator curve (ROC) calculations compared the composite RTS score and its component variables (GCS, SBP, RR) to understand which of these four variables best predicted 28-day mortality.
Methods
Data for this analysis of RTS stratifications comes from the paired, multi-center, randomized, single-blinded, normal saline-controlled, phase III clinical efficacy and safety studies of DCLHb in severe traumatic hemorrhagic shock. The US study involved 98 patients enrolled in the efficacy trial in 17 US trauma centers from February 1997 through January 1998; the European Union (EU) study enrolled 121 patients in four Belgian, 17 French, and 11 German trauma centers from July 1997 through May 1998.Reference Kerner, Ahlers, Veit, Riou, Saunders and Pison 14 , Reference Sloan, Koenigsberg and Gens 26 Inclusion criteria required that patients have hemorrhage and proven hypoperfusion (SBP <90 mm Hg and HR >120 beats/min, SBP <90 mm Hg and HR <60 beats/minute, or base deficit >15mEq/L). Patients excluded from the studies were those with traumatic brain injury, patients with imminent death, patients whose injury occurred more than four hours prior to infusion, minors, and pregnant women. The patients from the two traumatic hemorrhagic shock clinical trials were utilized for this analysis because they most accurately represented the population of patients that may be studied in future resuscitation trials using hemoglobin-based solutions.
The data for the current retrospective database analysis study came from the original datasets provided by Baxter Healthcare from the US and EU studies. The RTS was calculated with entry GCS, SBP, and RR, utilizing the formula RTS = 0.9368(GCS) + 0.7326(SBP) + 0.2908(RR), using codified variables in the equation (www.trauma.org/archive/scores/rts.html).Reference Champion, Sacco, Copes, Gann, Gennarelli and Flanagan 20 All SBP measurements are in millimeters of mercury (mm Hg) and all RR measurements are given in breaths per minute (breaths/min). Final patient survival status (lived vs. died) was based on all-cause 28-day mortality. The analyses included three patients for whom 28-day mortality status was unknown, with the assumption that they were alive at 28 days.
Statistical analysis of the RTS data included mean and standard deviation for age, Injury Severity Score (ISS), RTS, GCS, entry SBP and RR, and the TRISS-predicted survival.Reference Boyd, Tolson and Copes 27 Analysis of variance, Chi-square, and two sample mean and proportion tests of significance were used at the P < .05 significance level. Receiver operator curve (ROC) analysis compared the capability of the composite RTS and its component variables in predicting 28-day mortality, using IBM SPSS Statistics v20.0 (IBM Corporation, Armonk, New York USA) and Epi Info StatCalc v3.5.1 (Centers for Disease Control and Prevention, Atlanta, Georgia USA).
Initially, the RTS 1-3.99 stratum proposed in the HBOC-201 RESUS clinical trial protocol was analyzed, with subsequent analyses of strata that were higher in value (RTS 2-4.99), wider in value (1-4.99), and both higher and wider in RTS value (2-5.99). Because the RTS 2-5.99 stratum had optimal TRISS-predicted and actual mortality rates, it was further analyzed in an effort to better understand if this range would best identify patients that would make the at the time pending HBOC-201 RESUS study and other traumatic hemorrhagic shock clinical trials feasible.
The protocols used in the US and EU clinical trials were approved by the Institutional Review Board (IRB) of each participating institution prior to the enrollment of any subjects. Trials were conducted in compliance with all regulations for good clinical trials and practice. The US study was conducted under federal regulations governing emergency research with an exception to informed consent. The current analysis of the data was conducted with IRB approval.
Results
Included in the study were 208 patients (95%) from the two DCLHb clinical trials for whom a valid RTS score was available. Mortality and treatment group did not differ in the 11 patients for whom the RTS was not available.
The mean age of study patients was 37 (SD = 14) years; 65% of the patients sustained a blunt injury, 49% received DCLHb resuscitation, and 57% were studied in the European Union (Table 1). The mean ISS was 31 (SD = 18), RTS was 5.62 (SD = 1.8), GCS was 10.4 (SD = 4.8), SBP was 76 (SD = 19), and RR was 20 (SD = 7). The TRISS-predicted survival rate for the entire study group was 66% (SD = 35%). This gave a TRISS-predicted mortality rate of 34% which did not differ from the actual mortality rate of 35%. Aside from a slightly higher entry SBP in the US patients as compared to EU patients (79 vs. 73, P < .024), there were no significant differences based on study site.
Table 1 Patient Demographics and Clinical Variables by Study Site
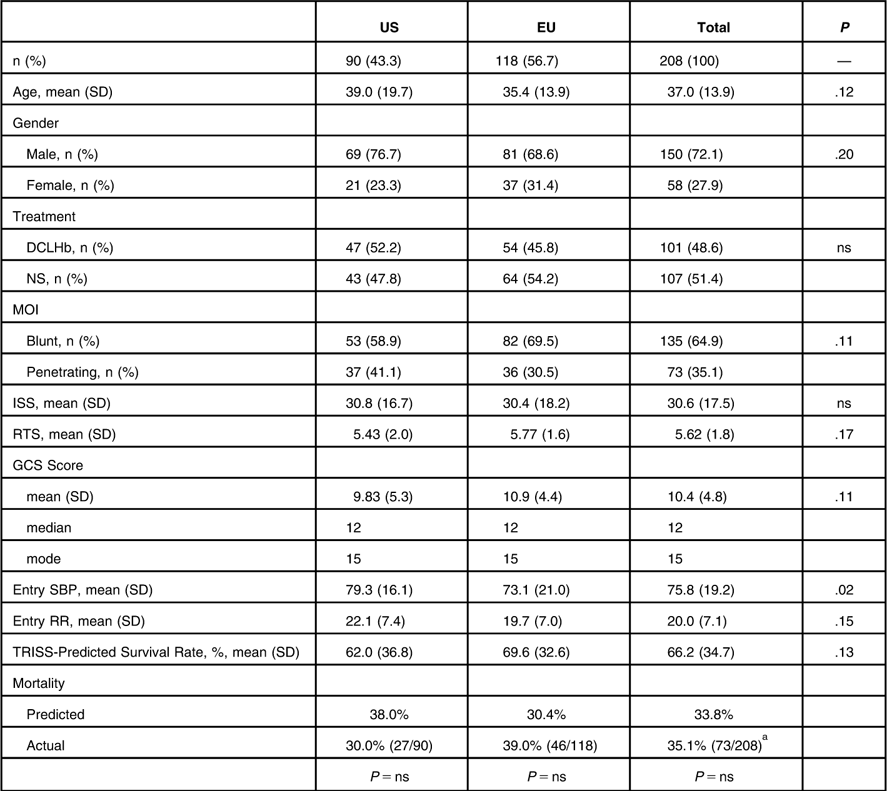
Abbreviations: DCLHb, Diaspirin cross-linked hemoglobin; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MOI, Mechanism of Injury; ns, not significant; NS, Normal Saline; RR, Respiratory Rate; RTS, Revised Trauma Score; SBP, Systolic Blood Pressure; TRISS, Trauma and Injury Severity Score.
a P = ns for US vs. EU.
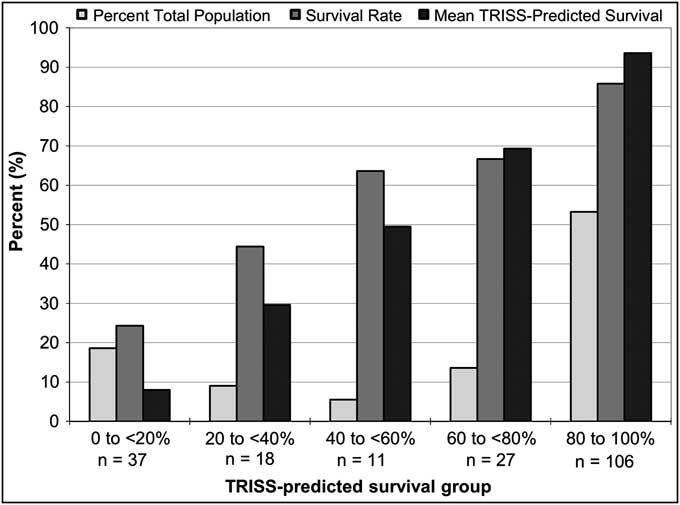
The only difference in demographics or clinical variables based on treatment with DCLHb was a 52% higher actual mortality rate in patients treated with DCLHb as compared to NS (P = 0.03) (Table 2). Of the 135 combined dataset patients (65%) who survived to 28 days, differences were observed based on patient outcome in patient age, mechanism of injury (MOI), ISS, RTS, GCS, DCLHb treatment, and TRISS-predicted survival (Table 3). Non-survivors had a lower RTS (4.61 vs. 6.17), a lower mean GCS score (7.96 vs. 11.8), and a lower TRISS-predicted survival rate of 40% vs. 79% (P < .001). The TRISS-predicted survival prediction distribution data demonstrated a bimodal patient distribution, with 106 patients (53%) in the 80-100% TRISS-predicted survival group and 37 (19%) in the 0-20% TRISS-predicted survival group, totaling 72% of the total patient population (lightest bars, Figure 1). Only 11 patients (5.5%) fell into the mid-range mortality range of 40-60% predicted survival. Actual and TRISS-predicted survival within all of the TRISS-predicted survival strata were comparable except in the 0-20% predicted survival stratum, in which actual survival was higher than the TRISS-predicted survival rate.
Table 2 Patient Demographics and Clinical Variables by Treatment Group

Abbreviations: DCLHb, Diaspirin cross-linked hemoglobin; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MOI, Mechanism of Injury; NS, Normal Saline; RR, Respiratory Rate; RTS, Revised Trauma Score; SBP, Systolic Blood Pressure; TRISS, Trauma and Injury Severity Score.
a P = .028 for DCLHb vs. NS.
Table 3 Patient Demographics and Clinical Variables by Outcome

Abbreviations: DCLHb, Diaspirin cross-linked hemoglobin; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MOI, Mechanism of Injury; ns, not significant; NS, Normal Saline; RR, Respiratory Rate; RTS, Revised Trauma Score; SBP, Systolic Blood Pressure; TRISS, Trauma and Injury Severity Score.
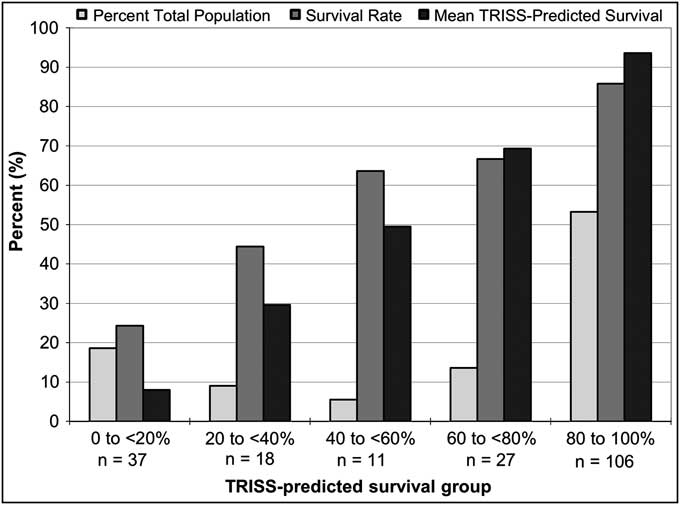
Figure 1 Distribution of All Patients by TRISS-predicted Survival in the US and EU DCLHb Clinical Trials (n = 199)
Patients were stratified into different RTS ranges in order to determine which subgroup provided the greatest number of traumatic hemorrhagic shock patients with predicted and actual mortality rates near 50%. The stratifications included the initial RTS range of 1-3.99, a higher range (2-4.99), a wider range (1-4.99), and a higher and wider range (2-5.99). The RTS 2-5.99 sub-group provided the largest number of patients (n = 79, 38%), the greatest proportion of penetrating injury patients (24%), the highest mean RTS (3.99, SD = 1.1), the highest mean GCS (5.85, SD = 3.5), the highest TRISS-predicted survival rate (38%, SD = 30%), and the actual mortality rate (53%) closest to the desired 50% mortality rate (Table 4). Predicted and actual mortality rates did not differ within any of the RTS strata subgroups.
Table 4 Clinical Data Based on RTS Strata from the Two DCLHb Traumatic Hemorrhagic Shock Clinical Trials
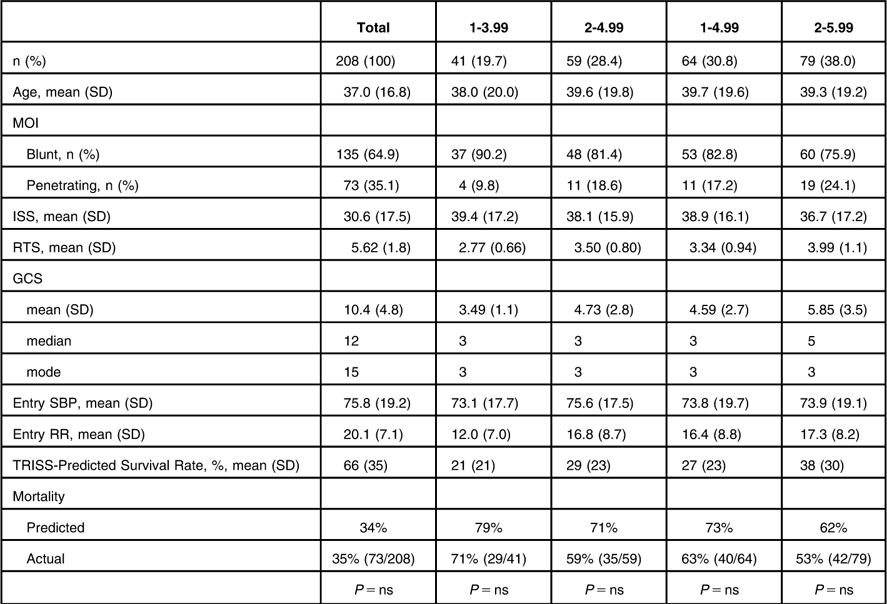
Abbreviations: DCLHb, Diaspirin cross-linked hemoglobin; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MOI, Mechanism of Injury; ns, not significant; RR, Respiratory Rate; RTS, Revised Trauma Score; SBP, Systolic Blood Pressure; TRISS, Trauma and Injury Severity Score.
Unlike the overall patient population, the RTS 2-5.99 subgroup did not show a bimodal TRISS-predicted survival curve (white bars, Figure 2). In this subgroup, 39% of the patients were in the 0-20% TRISS-predicted survival stratum and 71% were evenly distributed through the other strata. As such, the percentage of patients in the 20%-100% TRISS-predicted survival stratum significantly decreased upon isolation of the RTS 2-5.99 patients as compared to the total patient population (61% vs. 81%, P < .001). In the RTS 2-5.99 subgroup, 47% of patients had a TRISS-predicted mortality between 20% and 80%, while in the total patient population only 28% had a predicted survival within this range (P < .004).
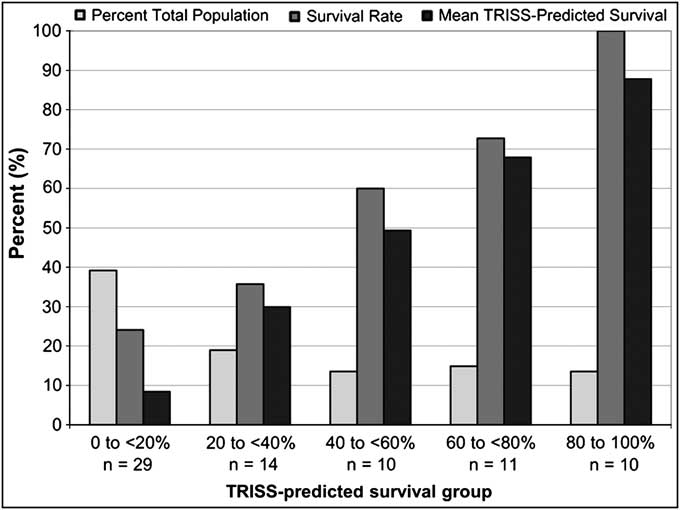
Figure 2 Distribution of RTS 2-5.99 Patients by TRISS-predicted Survival in the US and EU DCLHb Clinical Trials (n = 74)
Due to its favorable mortality characteristics, further analysis of the RTS 2-5.99 subgroup was performed. United States patients in this RTS 2-5.99 subgroup had a lower mortality rate than EU patients (39% vs. 65%, P < .02) (Table 5). Patients in the RTS 2-5.99 subgroup who survived their trauma were more likely to have sustained penetrating trauma (43% vs. 7.1%), had a lower mean ISS (28 vs. 45), a higher RTS (4.33 vs. 3.68), and a higher TRISS-predicted mean survival rate (55% vs. 21%) as compared to non-survivors in this subgroup (P < .01). In the 2-5.99 RTS subgroup, there was no difference in age, gender distribution, DCLHb treatment, GCS, SBP, or RR between patients who survived and expired by 28 days.
Table 5 Demographics and Clinical Variables by Outcome in RTS 2-5.99 Patients
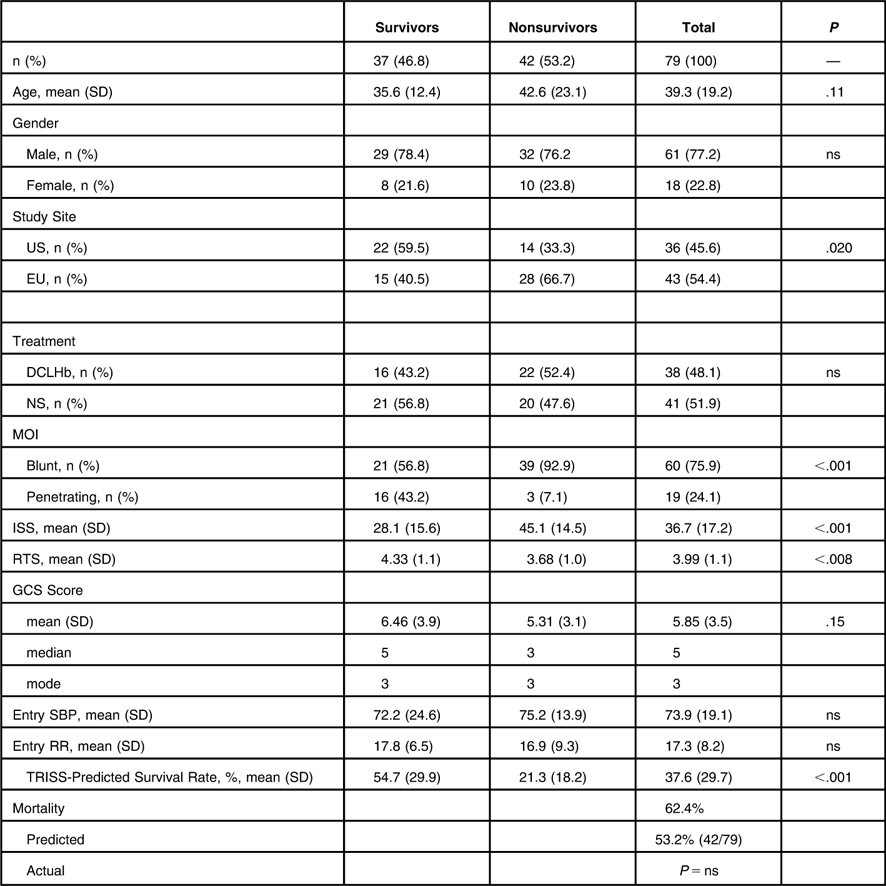
Abbreviations: DCLHb, Diaspirin cross-linked hemoglobin; GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MOI, Mechanism of Injury; ns, not significant; NS, Normal Saline; RR, Respiratory Rate; RTS, Revised Trauma Score; SBP, Systolic Blood Pressure; TRISS, Trauma and Injury Severity Score.
When comparing RTS 2-5.99 patients based on mechanism of injury, penetrating trauma patients had a lower mean ISS (21 vs. 42), a higher mean RTS (4.67 vs. 3.77), a higher mean GCS (7.89 vs. 5.20), and a higher mean TRISS-predicted survival rate (62% vs. 29%) as compared to blunt trauma patients in this RTS stratum (P < .003) (Table 6). Blunt injury patients in this RTS stratum had a 1.9x higher predicted mortality rate (71% vs. 38%), and a 4.1x higher actual mortality rate (65% vs. 16%) as compared to penetrating trauma patients (P < .001). Predicted and actual mortality in this RTS 2-5.99 stratum did not differ in either penetrating or blunt patient subgroups.
Table 6 Demographics and Clinical Variables by Mechanism of Injury in RTS 2-5.99 Patients

Abbreviations: GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MOI, Mechanism of Injury; ns, not significant; RR, Respiratory Rate; RTS, Revised Trauma Score; SBP, Systolic Blood Pressure; TRISS, Trauma and Injury Severity Score.
a P = ns for Blunt vs. Penetrating.
The RTS 2-5.99 subgroup patients had a higher RTS (3.99 vs. 2.77), a higher GCS (5.85 vs. 3.49), a higher RR (17 vs. 12), and a higher mean TRISS-predicted survival rate (38% vs. 21%) as compared to the RTS 1-3.99 subgroup patients (P < .02). Among survivors, the RTS 2-5.99 subgroup patients had a higher RTS (4.34 vs. 3.05), a higher GCS (6.46 vs. 3.75), and a higher TRISS-predicted survival rate (55% vs. 32%; P < .03). Among non-survivors, the RTS 2-5.99 subgroup patients had a higher RTS (3.68 vs. 2.65), and a higher GCS (5.31 vs. 3.38) as compared to the RTS 1-3.99 subgroup (P < .002).
Because GCS <5 patients have been shown to confound the ability of traumatic hemorrhagic shock clinical trials to determine therapy efficacy, further analyses of the RTS stratum with the exclusion of GCS <5 patients was conducted. The RTS 2-5.99 subgroup distribution with the exclusion of GCS <5 patients showed 18% of patients in the 40%-60% TRISS-predicted survival subgroup, displaying a uniform distribution across all TRISS-predicted survival strata (Figure 3). Without the GCS <5 patients, there was no significant increase in the distribution of patients with TRISS-predicted survival rates above 20% as compared to the RTS 2-5.99 subgroup with GCS <5 patients included (72% vs. 61%, P = ns).
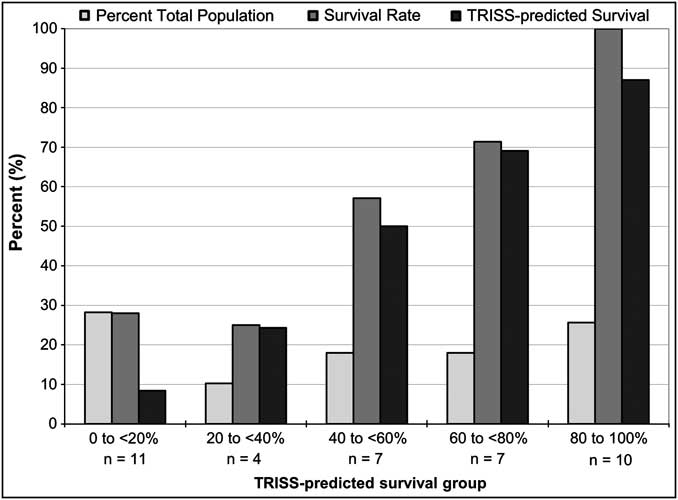
Figure 3 Distribution of RTS 2-5.99 Patients without GCS <5 Patients by TRISS-predicted Survival in the US and EU DCLHb Clinical Trials (n = 39)
Exclusion of GCS <5 patients from the RTS 2-5.99 subgroup caused the removal of 48% of this population, leaving 41 patients eligible for potential inclusion (20% of the total population of 208 patients) (Table 7). Without GCS <5 patients, the RTS (4.73 vs. 3.99) and the GCS (8.41 vs. 5.85) were higher when compared with the complete RTS 2-5.99 subgroup (P < .001). TRISS-predicted survival increased to 46% from 38%, and the actual mortality dropped to 49% from 53% with the removal of the GCS <5 patients (P = ns). Excluding GCS <5 patients caused the removal of 83% of patients from the RTS 1-3.99 subgroup, leaving only seven patients (3.4% of the total population).
Table 7 Patient Demographics and Clinical Variables Including and Excluding GCS <5
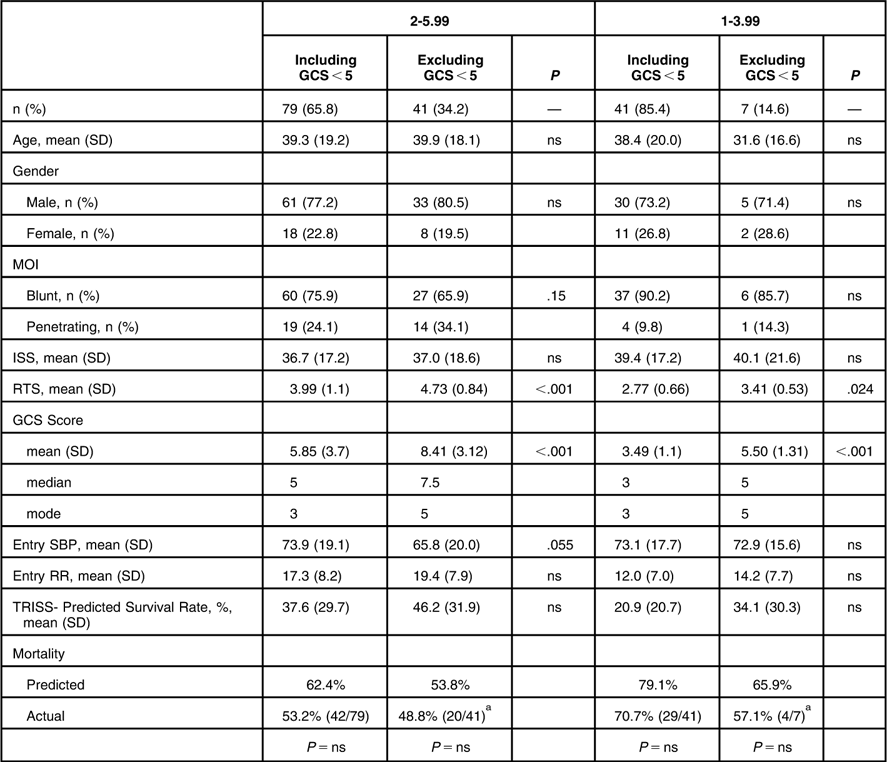
Abbreviations: GCS, Glasgow Coma Scale; ISS, Injury Severity Score; MOI, Mechanism of Injury; ns, not significant; RR, Respiratory Rate; RTS, Revised Trauma Score; SBP, Systolic Blood Pressure; TRISS, Trauma and Injury Severity Score.
a P = ns for Including GCS <5 vs. Excluding GCS <5.
In the RTS 2-5.99 subgroup, removal of GCS <5 patients resulted in a 55% reduction in the number of blunt trauma patients and only a 26% reduction in eligible penetrating trauma patients (Table 6). TRISS-predicted and actual mortality rates did not differ with the exclusion of these GCS <5 in either mechanism of injury group. Without GCS <5 patients, there was a 21% increase in RTS (3.77 to 4.57), as well as a 51% increase in GCS (5.20 to 7.85) in blunt trauma patients. A similarly large increase in RTS and GCS was not seen in penetrating trauma patients, such that the removal of GCS <5 patients caused the RTS and GCS to be similar in both mechanism of injury subgroups.
An ROC analysis was performed in order to determine if the individual components of the RTS would more successfully predict 28-day mortality in these traumatic hemorrhagic shock patients. In the total patient population (Figure 4a) and in the RTS 2-5.99 subgroup, the RTS and GCS were equally predictive of mortality as measured by area under the curve (AUC). Although the removal of GCS <5 patients led to a slight decline in predictive power of the composite RTS and its individual components, the GCS and RTS still remained the best predictors of mortality in the overall patient population (Figure 4b) and in the RTS 2-5.99 subgroup.
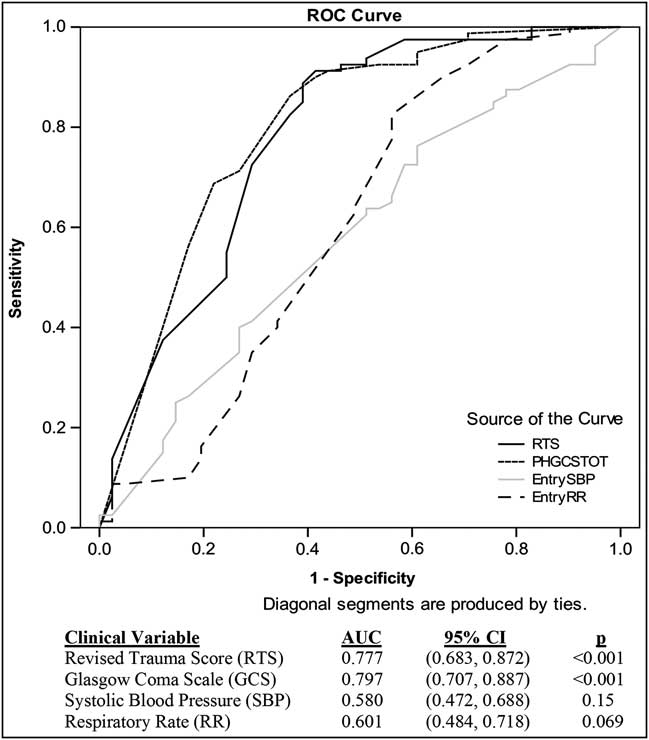
Figure 4a ROC Curve of RTS Clinical Variables on 28-day Mortality for All Patients
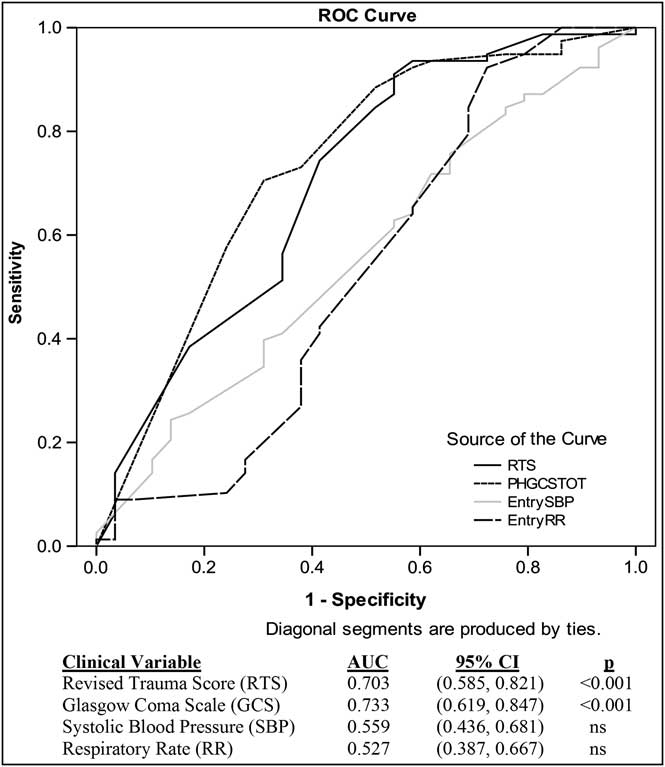
Figure 4b ROC Curve of RTS Clinical Variables on 28-day Mortality for All Patients Excluding GCS <5
Discussion
There has been a decades-long search for an acceptable hemoglobin-based oxygen carrier that could be used to improve the resuscitation of traumatic hemorrhagic shock patients.Reference Winslow 8 , Reference Moore, Johnson, Cheng, Masuno and Banerjee 28 - Reference Alayash, D'Agnillo and Buehler 30 The most important clinical trials that have tested hemoglobin-based oxygen carriers for this indication are the two DCLHb clinical trials and the recently published US prehospital clinical trial of PolyHeme, a polymerized hemoglobin solution.Reference Moore, Johnson, Moore and Moore 9 , Reference Kerner, Ahlers, Veit, Riou, Saunders and Pison 14 , Reference Sloan, Koenigsberg and Gens 26 The Naval Medical Research Center (NMRC) attempted to receive FDA approval to conduct RESUS, another prehospital traumatic hemorrhagic shock clinical trial using HBOC-201.Reference Freilich, Pearce and Pitman 10 - Reference Freilich 17
One of the most important aspects of any proposed clinical blood substitution trial is the inclusion of traumatic hemorrhagic shock patients who can benefit from the therapy being tested.Reference Peelen, Peek, de Jonge, Scheffer and de Keizer 31 Also critical is the inclusion of patients whose mortality risk will maximize the likelihood that any potential benefit of the tested hemoglobin-based oxygen carrier can be demonstrated with the study that includes a reasonable number of patients. A mid-range mortality risk near 50% might optimally make any beneficial treatment effects apparent by improving survival as a result of receiving the test solution or therapy. Finding a population of patients with this mid-range mortality risk has been difficult, since most traumatic hemorrhagic shock patient populations have demonstrated a bimodal mortality risk distribution, including patients with both extremely low and extremely high mortality risk.Reference Riou, Landais and Vivien 11 This finding suggests that conducting a clinical trial could be difficult because of the limited number of patients whose mortality risk is actually mid-range as a result of moderate injury severity and physiologic derangement at the time of study inclusion.Reference Atroshi and McCabe 32 Although some suggest that clinical trial inclusion criteria should result in the study of a large patient population with a wide range of mortality risk, this approach could obscure the effects of the HBOC being studied if the greatest proportion of patients studied do not have a mid-range mortality and therefore, could not benefit from the therapy being tested.Reference Buyse 33 , Reference McMahon 34
The NMRC clinical trial of HBOC-201 (RESUS) proposed the use of the RTS for the prehospital identification of traumatic hemorrhagic shock patients who might optimally be studied with the ability to identify patients of uniform mortality risk.Reference Freilich 17 The RTS was proposed as the inclusion criteria because it can be quickly calculated by paramedics in the prehospital setting with the use of systolic blood pressure (SBP), respiratory rate (RR) and the Glasgow Coma Scale (GCS) score only.Reference Champion, Sacco, Copes, Gann, Gennarelli and Flanagan 20 The RTS has been studied for its ability to predict outcome.Reference Gabbe, Cameron and Finch 21 - Reference Esme, Solak and Yurumez 23 Specifically, Lichtveld determined that along with advanced age, the triage RTS is the single strongest predictor of in-hospital mortality.Reference Lichtveld, Spijkers, Hoogendoorn, Panhuizen and van der Werken 24 Ahmad found in poly-traumatized patients that an RTS of 6 indicated a 50% mortality risk.Reference Ahmad 25 In a study of clinical factors leading to over-triage and under-triage of trauma patients, Rehn et al found that a triage-RTS <12 significantly reduced the risk for under-triage of severely injured patients.Reference Rehn, Eken, Kruger, Steen, Skaga and Lossius 35 However, Giannakopoulos and co-authors found that this same cut-off for trauma patients may not be adequate for helicopter emergency medical services cancellations as it could lead to up to 17% under-triage of major trauma patients.Reference Giannakopoulos, Saltzherr and Lubbers 36 Other scores have been examined for their ability to predict mortality, such as the Mechanism, Glasgow Coma Scale, Age, and Arterial Pressure (MGAP) developed by Sartorius et al.Reference Sartorius, Le Manach and David 37 The MGAP was found to be as sensitive as and more specific than the triage-RTS and RTS. Despite extensive study of the RTS as an outcome predictor, it has not been proposed or studied as an entry criterion for any traumatic hemorrhagic shock clinical trials.
The clinical efficacy trials of DCLHb provide an excellent setting for a retrospective review of the utility of the RTS in attempting to isolate a subgroup of patients with midrange mortality. Combining the two DCLHb clinical trial populations is reasonable because although they come from two different clinical settings, they are otherwise similar to other traumatic hemorrhagic shock studies.Reference Moore, Johnson, Moore and Moore 9 , Reference Sperry, Ochoa and Gunn 38 - Reference Dutton, Mackenzie and Scalea 40 This combined patient population is sufficiently large to allow for the study of how RTS ranges of different values could influence the enrollment of patients in future HBOC clinical trials. Additionally, there are no significant differences in demographic variables between these two study populations.
The population being studied in this RTS as inclusion criterion analysis is significant in that all of the patients who were included in the studies were hypotensive per the entry criteria, such that the SBP component of the RTS would be similar for all patients in this analysis. As such, the relative importance of the GCS score, which already is weighted more heavily in the calculation of the RTS, is the most important variable in the mortality prediction capabilities of different RTS ranges.Reference Champion, Sacco, Copes, Gann, Gennarelli and Flanagan 20 The only other important attribute of this population is the overall mortality rate, which was comparable to the TRISS-predicted mortality rate. Of note is the fact that the TRISS survival predictions for this 208 patient population also generated a bimodal distribution, with larger numbers of patients with very low and very high mortality risk, similar to Riou's observations.Reference Riou, Landais and Vivien 11 Because the TRISS-predicted and actual mortality rates were for the most part comparable in these studies, it suggests that the TRISS-prediction model is useful when attempting to identify an optimal patient population for study.
The RTS values, GCS scores, and TRISS predictions were all correlated with mortality, establishing that this dataset could be used in the search for an optimal RTS range that could identify a mid-range mortality risk population for future traumatic hemorrhagic shock studies. The RTS entry criterion range of 1-3.99 generated a subgroup whose mortality rate was twice that of the overall population's mortality rate. This finding reflects the desire to study a population with a sufficiently high mortality rate to allow for assessment of treatment efficacy. This study examined RTS ranges that were higher (2-4.99), wider (1-4.99), and higher and wider in value (2-5.99). There was no scientific basis for the selection of these specific RTS strata other than the assumption that higher and wider RTS strata ranges would identify more patients with a uniform, mid-range mortality risk, as well as a larger number of patients eligible for study.
The RTS range of 2-5.99 was studied further because of its optimal mortality risk, which included a 62% predicted and 53% actual mortality rates. This population also included the largest percentage of patients and the largest proportion of penetrating trauma patients as compared to other strata that were examined. Of note in the RTS 2-5.99 population is the observation that the mean GCS was 5.8 and the median GCS was five. Although the GCS scores for these RTS 2-5.99 patients suggest significant traumatic brain injury, this is still the preferred stratum because all of the other proposed RTS strata in this study had a median GCS score of three, which signifies the potential inclusion of a greater numbers of GCS <5 patients, who are known to undermine the efficacy detection capabilities of traumatic hemorrhagic shock clinical trials.Reference Moore, Johnson, Moore and Moore 9 , Reference Sloan, Koenigsberg and Gens 26 , Reference Moore, Moore and Fabian 41 , Reference Chamoun, Robertson and Gopinath 42 The proposed optimal RTS 2-5.99 range was compared to the initially proposed RTS 1-3.99 range with the finding that the TRISS-predicted survival in the RTS 2-5.99 stratum was nearly two times higher as well as much closer to the desired 50% mortality risk.
The RTS 2-5.99 patient subgroup was analyzed based on mechanism of injury in order to determine if this stratum might be more useful with blunt or penetrating trauma patients. A difference would suggest the need for separate traumatic hemorrhagic shock clinical trials based on mechanism of injury. Blunt trauma patients in the RTS 2-5.99 stratum were found to be more severely injured than the penetrating trauma patients. Consequentially, blunt trauma RTS 2-5.99 patients had a four-fold higher mortality rate as compared to the penetrating trauma patients in the same RTS range. Despite the outcome difference in patients with blunt and penetrating trauma mechanisms, the overall conclusions from this study regarding the use of the RTS are still sound. However, any future studies of how the RTS might be used as an entry criterion for traumatic hemorrhagic shock clinical trials should optimally analyze blunt and penetrating injury patients independently.
When analyzing the RTS 2-5.99 patients with regard to treatment group, there were no observed differences with regard to RTS, GCS, ISS, SBP, RR, TRISS-predicted survival, or actual mortality rates. As such, DCLHb treatment effects did not render the RTS entry criteria stratum analysis invalid.
Because it is known that GCS <5 patients might best be excluded from traumatic hemorrhagic shock clinical trials due to the potential for including severely head-injured patients, and because the recently completed PolyHeme study excluded GCS 3 and 4 patients, and because the RESUS study intended to exclude these patients, further analysis examined both RTS strata with the exclusion of GCS <5 patients.Reference Moore, Johnson, Moore and Moore 9 Excluding GCS <5 victims resulted in a remaining population that included half of the RTS 2-5.99 population (one-fifth of the overall 208 patient population). This exclusion also was noteworthy because although it removed over half of the blunt trauma patients, it only removed around a quarter of the penetrating trauma patients in this stratum, leading to a more equal distribution of blunt and penetrating trauma patients in the RTS 2-5.99 stratum (66% and 34%, respectively). The GCS characteristics of this refined RTS 2-5.99 population also were enhanced in that the mean GCS increased to 8.4, and the median GCS was in the 7-8 range. As such, the RTS 2-5.99 stratum without GCS <5 patients perhaps optimally identifies trauma patients whose GCS score suggests altered mental status as a result of traumatic hemorrhagic shock as opposed to the presence of concomitant severe traumatic brain injury. When the GCS <5 patients are excluded, the mortality rate of the RTS 2-5.99 stratum approaches the desired midrange mortality risk, with predicted TRISS survival of 46% and actual mortality of 49%.
When the RTS 2-5.99 stratum without the GCS <5 patients was analyzed based on mechanism of injury, it was noted that the majority of excluded patients came from the blunt subgroup. This reflects the fact that most of the GCS <5 patients were blunt trauma patients with concomitant significant closed head injury. Once these GCS <5 patients were removed, the RTS and GCS were comparable in both blunt and penetrating subgroups; this finding suggests that a study utilizing RTS 2-5.99 patients would not necessarily require two separate studies, given the similar RTS and GCS characteristics of the blunt and penetrating subgroups.
Nearly half of the patients in the RTS 2-5.99 stratum without GCS <5 had a predicted survival between 20 and 80% which is similar to the range observed in the RTS 2-5.99 subgroup that included GCS <5 patients. This stable TRISS-predicted survival rate suggests that the RTS 2-5.99 stratum selects a patient population with mid-range mortality risk, even if patients with severe traumatic brain injury are excluded. The overall distribution of patient in the RTS 2-5.99 stratum without GCS <5 patients demonstrates nearly one quarter of patients in each of the extremely low and extremely high survival risk groups, a finding that suggests that this distribution may be the best that can be achieved when conducting a traumatic hemorrhagic shock study with an undifferentiated population of trauma patients.
The ROC data showed that GCS was similar to RTS in its ability to predict mortality in the traumatic hemorrhagic shock patient, and that the SBP and RR values were not adequately predictive of outcome. This finding does not suggest that SBP is not a useful entry criterion for a traumatic hemorrhagic shock study; rather SBP simply is not predictive of outcome because most of the patients from these two trauma studies had similar hypotensive SBPs at the time of study entry per the inclusion criteria. When using the RTS as an entry criterion, patients will be enrolled whose shock compensatory mechanisms create a similar physiologic state, as measured by GCS, SBP, and RR, and whose TRISS-predicted survival is related primarily to injury severity. When the GCS <5 patients were removed and the 28-day mortality ROC analysis was repeated, it was observed that the sensitivity and specificity of each component and the composite RTS decreased slightly, with GCS and RTS remaining the best predictors of mortality. This drop in predictive power with the removal of GCS <5 patients indicates the importance of GCS scores of less than five in predicting 28-day mortality.
Limitations
One limitation of this study is the small patient population (208) that resulted from the early termination of the DCLHb efficacy trials. If a larger trauma population from an HBOC clinical trial such as the PolyHeme clinical trial was used for this type of analysis, it might be possible to generalize this data to a larger population of trauma patients who could be enrolled in future traumatic hemorrhagic shock clinical trials. Another possible limitation is the fact that the RTS strata were chosen empirically and not based on previously established optimal RTS strata. This reflects the fact that there is limited data relating RTS to mortality prediction at the time of initial medical contact, aside from the Lichtveld data, which does not specifically identify clinically useful strata.Reference Lichtveld, Spijkers, Hoogendoorn, Panhuizen and van der Werken 24
Future research should examine what could be optimal entry criteria using larger traumatic hemorrhagic shock clinical trial databases in order to establish what might be the optimal RTS range, and whether the use of the RTS as an entry criterion is actually better than the traditional use of SBP <90 mm Hg as a measure of critical hemorrhage and the need for optimal traumatic hemorrhagic shock resuscitation. Additional work should examine the effect of excluding patients with low GCS scores on the conduct of traumatic hemorrhagic shock clinical trials, both with regard to what type of patients will be studied and how the exclusion of these patients will affect patient enrollment and the ultimate feasibility of the clinical trial.
Conclusion
Based on study analysis of the DCLHb clinical trial data, an RTS range of 2-5.99 is a more appropriate range for the identification of traumatic hemorrhagic shock patients whose injury severity is more closely associated with a mortality risk near 50% than that observed in the RTS 1-3.99 stratum. The RTS 2-5.99 subgroup allows for identification of patients whose survival distribution may be optimal for study because half of the patients have an intermediate mortality risk. The exclusion of GCS <5 patients refines the RTS 2-5.99 patient population by creating a smaller subgroup with higher GCS scores, moderate injury severity, and TRISS-predicted and actual mortality rates that are nearly identical to the desired 50% mortality risk that could optimally demonstrate the effects of a novel traumatic hemorrhagic shock therapy such as a hemoglobin solution. When studying RTS 2-5.99 patients with exclusion of GCS <5 patients, the similar distribution and predictive characteristics of blunt and penetrating patients may allow for both to be studied in one traumatic hemorrhagic shock clinical trial. Because the GCS score showed mortality prediction comparable to the RTS, any future clinical trial must consider the GCS score as an independent inclusion or exclusion criterion.
Abbreviations
- DCLHb:
-
Diaspirin Cross-Linked Hemoglobin
- EU:
-
European Union
- GCS:
-
Glasgow Coma Score
- HBOC:
-
hemoglobin-based oxygen carrier
- ISS:
-
Injury Severity Score
- NMRC:
-
Naval Medical Research Center
- ns:
-
not significant
- ROC:
-
receiver operator curve (analysis)
- RR:
-
respiratory rate
- RTS:
-
Revised Trauma Score
- SBP:
-
systolic blood pressure
- SD:
-
standard deviation
- TRISS:
-
Trauma and Injury Severity Score
- US:
-
United States
Appendix 1: United States (US) DCLHb Clinical Efficacy Trial
Lead Investigators: University of Illinois at Chicago, Chicago, IL: Edward P. Sloan, MD MPH FACEP, and Max D. Koenigsberg, MD FACEP.
Collaborating Centers, Number of Patients Enrolled (in parentheses), and Investigators: Albert Einstein Medical Center (5), Philadelphia, PA: William C. Dalsey, MD, Mark Kaplan, MD, and Pamela Taggart RN PhD; Allegheny University Hospitals (0), Philadelphia, PA: Thomas A. Santora, MD; Carolinas Medical Center (11), Charlotte, NC: Jeffrey Runge, MD, Lucinda A. Edwards, RN, and Michael A. Gibbs, MD; Christiana Medical Center (6), Newark, DE: Glen Tinkoff, MD, Patty McGraw, RN MS, and Robert O'Conner, MD; Cleveland Metro Health (3), Cleveland, OH: Rita K. Cydulka, MD, William F. Fallon, MD, and Brian Plaisier, MD; Hershey Medical Center (3), Hershey, PA: J Stanley Smith, Jr., MD, Robert N. Cooney, MD, and Margaret Shand, RN; Lehigh Valley Hospital (14), Allentown, PA: Mark D. Cipolle, MD PhD, Michael D. Pasquale, MD, and Wendy J. Robb, MSN RN CCRN; Memorial Medical Center of Georgia (5), Savannah, GA: M. Gage Ochsner, MD FACS, Frank E. Davis, MD FACS, and Joseph Rondina MD; Methodist Hospital of Indiana (9), Indianapolis, IN: George H. Rodman, Jr., MD, Charles Miralgia, MD, and Maureen Misinski, RN; Oregon Health Sciences Center (8), Portland, OR: Patrick H. Brunett, MD FACEP, James H. Bryan, MD PhD FACEP, and Colleen McDevitt, BA; Parkland Medical Center (3), Dallas, TX: David Provost, MD, Mary Jane Colpi, RN MS, and Russel Stoltzfus, RN; Palmetto Richland Memorial Hospital (7), Columbia, SC: Raymond P. Bynoe, MD FACS, Jay D. Hamm, BSN RN EMT-P, N. John Stewart, MD FACEP, Dave Amsden, PharmD, and Christine Walukewicz, RN, MSN; St. Anthony's Medical Center (1), Denver, CO: Thomas Wachtel, MD FACS, Ray Coniglio, RN MSN, and Lee Hemminger, RN MS NP; University of Louisville (9), Louisville, KY: Mary Nan S. Mallory, MD, Eddy Carillo, MD, Richard L. Miller, PhD DDS, and Ashlee Miller, RN; University of Maryland Medical Center (16), Baltimore, MD: David R. Gens, MD, Laura A. Joseph, MA, and Mehrunissa H. Owens, MA; University of Pittsburgh (3), Pittsburgh, PA: Andrew B. Peitzman, MD, Marilyn J. Borst, MD, and Randy J. Woods, MD; Vanderbilt University (7), Nashville, TN: John A. Morris, MD, and Judy Jenkins, MSN; Washington Hospital Center (2), Washington DC: J. Duncan Harviel, MD, Marion Jordan, MD, Dennis Wang, MD, Lisa Beylo, MT (ASCP), and Kristin Y. Brandenburg, RND, EMT.
Other Contributing Centers: Akron General Medical Center, Akron, OH: James A. Dougherty, MD FACEP, Lynn J. White, MS, and Farid Muakkassa, MD FACS; Allegheny University Hospitals, Pittsburgh, PA: Fred Harchelroad, MD FAAEM, and Kris Potts, CRNP; Almeda County Medical Center, Oakland, CA: M. Andrew Levitt, DO, Ed Portoni, and Eva Hirvela, MD; Ben Taub General Hospital, Houston, TX: Mathew J. Wall Jr., MD, Kenneth L. Mattox, MD, and Alex Mendez, MD; Christ Hospital, Oak Lawn, IL: Michele Holevar, MD MBA, Gary Merlotti, MD, and Sue Berry, RN; Cook County Hospital, Chicago, IL: Edward P. Sloan, MD MPH FACEP, John Barrett, MD, Kim Nagy, MD, and Steve Stapleton, RN; East Carolina University, Greenville, NC: Juan A. March, MD, Susan Copeland, and Paul Catrou, MD; Hartford Hospital, Hartford, CT: George A. Perdrizet, MD PhD, Donna Rescrol, RN, and Lenworth Jacobs, MD; Henry Ford Hospital, Detroit, MI: Terry Kowalenko, MD, Barry Dereczyk, RN BSN, and Emanuel P. Rivers, MD; Hurley Medical Center, Flint, MI: Pascal Nyachowe, MD, and Judy Mikhil, RN MSN; Illinois Masonic Medical Center, Chicago, IL: Richard Fantus, MD, and Sharon Ward, RN MS; UC Irvine Medical Center, Orange, CA: Mark Langdorf, MD; Jacobi Medical Center, Bronx, NY: Ronald Simon, MD; Kern Medical Center, Bakersfield, CA: Dennis Martinez, MD, and Kate Botner; Kings County Trauma Center, Brooklyn, NY: Patricia Ann O'Neill, MD, Richard Sinert, MD, Karen Sue Eisenberg, RN MPS, and Joan H. Howanitz, MD; Medical College of Virginia, Richmond, VA: Dennis C. Gore, MD, Sherry Lockhart, RN, and Heather Chibelski, RN; Mount Sinai Hospital, Chicago, IL: Les Zun, MD, and Annette Kinsela; Rockford Memorial Health System, Rockford IL: Dennis Uehara, MD, and Jeffrey Maves, RN; St. Francis Medical Center, East Peoria, IL: George Z. Hevesy, MD; Temple University Hospital, Philadelphia, PA: Michael Badellino, MD, and Robert Buckman, MD; Truman Med Center-West, Kansas City, MO: Steven Go, MD FACEP, Ginger Morse, RN, and Berna Sue Casper; Tulane University Medical Center, New Orleans, LA: Norman McSwain Jr., MD, and Ruth Ann Wanstrath; University of Cincinnati, Cincinnati, OH: Fred A. Luchette, MD, Richard D. Branson, BA RRT, and Kenneth Davis Jr., MD; University Medical Center, Las Vegas, NV: John J. Fildes, MD, Connie A. Clemmons-Brown, RN BSN, and Cindy Roehr; University Medical Center, Tucson, AZ: Harvey Meislin, MD and Cheryle Gomez, RN BSN; LA County/ USC Medical Center, Los Angeles, CA: George C. Velmahos, MD FACS FRCS FRCPS, and Raymond Tatevossian, BS.
Data Monitoring Committee: Roger J. Lewis, MD PhD, (Chairman), Harbor-UCLA Medical Center, Torrance, CA; Donald Berry, PhD, Duke University, Durham, NC; Henry Cryer III, MD PhD, UCLA Medical Center, Los Angeles, CA; Norman Fost, MD MPH, University of Wisconsin Children's Hospital, Madison, WI; Ronald Krome, MD, Detroit Receiving Hospital/UHC, Detroit, MI; Geraldine Washington, PhD, Los Angeles Chapter NAACP, Los Angeles, CA.
Statistical Data Analysis Center: Department of Biostatistics and Informatics, University of Wisconsin, Madison, WI: Marian Fisher, PhD, Robin Bechhofer, Tom Cook, PhD, and Melissa Schultz, MS. Baxter Healthcare Corporation: Hemoglobin Therapeutics, Round Lake, IL: Robert Przybelski, MD, John Blue, PharmD, Cynthia Goldberg, MS, Kathleen Stern, PhD, Jaime Houghton, MS, Maulik Nanavaty, PhD, Timothy Estep, PhD, Michael Saunders MD, and Tom Schmitz, PhD.
Appendix 2: European Union (EU) DCLHb HOST Clinical Efficacy Trial
Lead Investigator: Ulrich Pison, MD
Collaborating Centers and Investigators
Spain
Doctor Alted, MD ( Principal Investigator, Hospital 12 de Octubre, Madrid)
Belgium
Docteur Todorov, MD, PhD ( Principal Investigator, CIU Hopital Ambroise Parè, Mons)
Docteur Vanderpas, MD ( Lab Coordinator, CIU Hopital Ambroise Parè, Mons)
Docteur Fox, MD (Principal Investigator, Centre Hospitalier Regional de Namur)
Docteur Decroix, MD (Study Co-Coordinator, Centre Hospitalier Regional de Namur)
Docteur Schtickzelle, MD (Principal Investigator, Hospital Civil de Charleroi)
Doctor Beaucourt (Principal Investigator, Universitair Ziekenhuis Antwerpen)
France
Professor Bouletreau, MD, PhD (Principal Investigator, Hospital Edouard Herriot, Lyon Cedex 03)
Professor Collombel, MD, PhD (Lab Coordinator, Hospital Edouard Herriot, Lyon Cedex 03)
Dr. Samii, MD (Principal Investigator, Centre Hospitalier Bicètre, Le Kremlin Bicètre)
Professor Mazière, MD, PhD (Lab Coordinator, Centre Hospitalier Universitaire Amiens Nord)
Professor Ossart, MD, PhD (Principal Investigator, Centre Hospitalier Universitaire Amiens Nord)
Professor Dabadie, MD, PhD (Principal Investigator, Centre Hospitalier Universitaire Pellegrin, Bordeaux)
Professor Bertrand, MD, PhD (Principal Investigator, Centre Hospitalier Universitaire St Etienne, Saint-Etienne)
Professor Coriat, MD (Principal Investigator, Groupe Hospitalier Pitiè-Salpètrière, Paris Cedex 13)
Docteur Guerrini, MD (Principal Investigator, Hopital A. Mignot, Le Chesnay)
Professor Chauvin, MD, PhD (Principal Investigator, Hopital Ambroise Parè, Boulogne Billancourt)
Docteur Bladier, MD (Lab Coordinator, Hopital Avicenne, Bobigny Cedex)
Docteur Delacoux (Lab Coordinator, Hopital Beaujon, Clichy Cedex)
Professor Marty (Principal Investigator, Hopital Beaujon, Clichy Cedex)
Docteur Bemer, MD (Principal Investigator, Hopital Bel Air, Thionville)
Professor Desmonts (Principal Investigator, Hopital Bichat, Paris Cedex 18)
Docteur Poussel, MD (Principal Investigator, Hopital Bon- Secours, Metz)
Docteur Stoessel, MD (Lab Coordinator, Hopital Bon- Secours, Metz)
Professor Freysz, MD, PhD (Principal Investigator, Hopital General/Hopital Bocage, Dijon Cedex)
Docteur Duvaldestin, MD (Principal Investigator, Hopital Henri Mondor, Crèteil)
Professor Goossens, MD (Lab Coordinator, Hopital Henri Mondor, Crèteil)
Professor Payen (Principal Investigator, Hopiatl Lariboisiere, Paris Cedex 10)
Docteur Rouvier, MD (Principal Investigator, Hopital Percy, Clamart)
Professor Cathala, MD, PhD (Principal Investigator, Hopital Purpan, Toulouse)
Docteur Adenet, MD (Principal Investigator, Hopital R. Salengro, Lille)
Professor Rousseaux, MD PhD (Lab Coordinator, Hopital R. Salengro, Lille)
Docteur Ducasse, MD (Principal Investigator, Hopital Rangeuil, Toulouse Cedex)
Docteur Pasteyer, MD (Principal Investigator, Hopital Raymond Poincarè, Garches)
Professor Feiss, MD, PhD (Principal Investigator, Hopital Universitaire Dupuytren, Limoges Cedex)
Germany
Professor Reinhart, MD, PhD (Principal Investigator, Universität Jena)
Professor Dick (Principal Investigator, Universität Mainz)
Professor Gotzen, MD, PhD (Principal Investigator, Universität Marburg)
Doktor Weinand, MD (Lab Coordinator, Klinikum Ludwigsburg)
PD Dr. Ellinger (Principal Investigator, Klinikum Mannheim)
OA Dr. Tappe, MD, PhD (Principal Investigator, Marienhospital Osnabrück)
Professor Regel (Principal Investigator, Medizinische Hochschule Hannover)
Professor Schmucker, MD, PhD (Principal Investigator, Medizinische Uni Lübeck)
Professor Röse, MD, PhD (Principal Investigator, Universität Magdeburg)
Dr. Sokolowski, MD (Lab Coordinator, Universität Magdeburg)
Professor Motsch (Principal Investigator, Universität Heidelberg)
Professor Unertl, MD, PhD (Principal Investigator, Universität Tübingen)
Professor Katz, MD (Lab Coordinator, Universität Giessen)
Professor Benad, MD, PhD (Principal Investigator, Universität Rostock)
Professor Schuff-Werner, MD, PhD (Lab Coordinator, Universität Rostock)
Dr. Bergner (Lab Coordinator, Universität Erlangen)
Professor Schüttler, MD, PhD (Principal Investigator, Universität Erlangen)
Professor Hergert (Principal Investigator, Klinikum Schwerin)
Professor Lestin (Lab Coordinator, Klinikum Schwerin)
EU Data Monitoring Committee: J. Bion, P. Ferdinande, A. Grootendorst, R. Little, C. Robertson, D. Spahn, D. Spiegelhalter, A. Webb. Baxter Healthcare Corporation: S. Holmstrom, D. Gerard, T. Reppucci, A. Morrison (at Nivelles Belgium), J. Blue, C. Goldberg, R. Przybelski, K. Stern, J. Houghton, R. Sperelakis, K. Wallace, J. Petty, D. Balma, B. Bottoms (at Round Lake, Ill., USA), P. Carli (SAMU 75 and Centre Hospitalo-Universitaire Necker-Enfants Malades, Paris)