Congenital heart defects (CHD) affect approximately up to 9 in every 1,000 live-born infants (Reference Hoffman and Kaplan24;Reference Rosano, Botto, Botting and Mastroiacovo36), account for 40 percent of deaths due to congenital anomalies (31), and one in thirteen infant deaths (Reference Boneva, Botto and Moore6). Although the diagnosis of heart defects may emerge following prenatal or newborn screening, too often serious defects are only recognized when an infant develops life-threatening symptoms of cardiovascular collapse. Timely recognition in the newborn period is vital to prevent death before definitive management can be initiated and the morbidity consequent on collapse. Whereas antenatal screening programs have the potential to identify CHD (Reference Bricker, Garcia and Henderson8), existing evidence suggests these programs have variable success in recognizing fetuses with serious CHD (Reference Acharya, Sitras and Maltau1). A UK-wide study in the mid-1990s found that a fetal diagnosis was made in only 23 percent of all affected pregnancies and 12 percent of affected live births (Reference Bull11).
Newborn screening by clinical examination is undertaken in many countries (Reference Hall23). In the United Kingdom, the cardiovascular component of the routine screening examination comprises observation for cyanosis, auscultation of the heart, and palpation of the femoral pulses. The performance of this program is not routinely evaluated; however, there is evidence to suggest that the detection rate of the clinical screening examination is poor (Reference Wren, Richmond and Donaldson43). A large, prospective UK study found that the newborn examination detected only 44 percent of CHDs (Reference Ainsworth, Wyllie and Wren2).
There is, therefore, likely to be a continued need to screen infants for serious life-threatening CHDs shortly after birth. Technological developments make the use of echocardiography and pulse oximetry in newborn population screening feasible, and they now merit further evaluation as newborn screening tests (Reference Arlettaz, Archer and Wilkinson4;Reference Bakr and Habib5;Reference de Wahl, Mellander, Sunnegardh, Sandberg and Ostman-Smith18;Reference Hoke, Donohue and Bawa25;Reference Koppel, Druschel and Carter28;Reference Reich, Miller and Brogdon33;Reference Richmond, Reay and Abu34).
The objective of this study was, therefore, to investigate the effectiveness, costs, and cost-effectiveness of adding pulse oximetry or screening echocardiography to the current strategy of clinical screening alone to inform future screening policy and research using a decision-analytic model to synthesize all available evidence. A subsidiary objective was to investigate which research priorities would be of greatest value in reducing uncertainty regarding future newborn screening policies by using value of information analysis.
METHODS
Objectives of Newborn Screening
The evaluation of newborn screening for CHD presents several challenges. The general term “congenital heart defects” comprises a variety of malformations with varying prevalence, clinical features, natural history, management and hence anticipated benefit from screening. Furthermore, screening echocardiography when used in early life may reveal some structural heart malformations (e.g., ventricular septal defects [VSDs]) that are of no functional or clinical consequence and remain undiagnosed or resolve spontaneously. Clarity regarding the precise objectives of newborn screening is required if optimal screening strategies are to be selected and evaluated. We classified CHD into three groups according to the anticipated benefit from newborn screening (Box 1). There is increasing evidence to suggest that preoperative collapse is associated with higher postoperative mortality and morbidity and later neurological sequelae (Reference Bove, Bull and Stark7;Reference Brown, Ridout, Goldman, Hoskote and Penny10;Reference Clancy, McGaurn and Wernovsky13;Reference Knowles, Griebsch and Dezateux27), but data have not been related to mode of detection. We, therefore, selected timely diagnosis of “life-threatening” CHD (Box 1) as the primary outcome of the model, defined as a diagnosis made preoperatively before collapse or death occurs. This approach assumes definitive management is initiated at the time of diagnosis, and is effective in preventing pre-operative collapse. For instance, management might start with prostaglandin infusion and proceed with surgery. The secondary outcome was the diagnosis of “clinically significant” defects, along with the timely diagnosis of defects included in the primary outcome.
Box 1. Classification of congenital heart defects

No single clinical screening test will identify all defects—for instance, not all are associated with a murmur. Pulse oximetry will not detect noncyanotic defects. Although nonspecialist ultrasonographers located near obstetric units might recognize that the heart they were examining was not normal, they could rarely provide a confident diagnosis. For diagnostic echocardiography and any subsequent treatment, the baby must travel to a specialist center. As well as referrals that are clearly appropriate, screening echocardiographers might send babies with malformations that are of no functional consequence or that a specialist could be confident would resolve spontaneously.
The Decision Problem
We identified three potential screening strategies: (i) clinical examination alone, (ii) pulse oximetry in addition to clinical examination, and (iii) screening echocardiography in addition to clinical examination. With clinical examination alone, the test is positive if the infant has visible cyanosis, a cardiac murmur, or diminished femoral pulses. Pulse oximetry screening involves measurement of post-ductal oxygen saturation as an adjunct to clinical examination; a positive result is defined by arterial saturation of less than 95 percent on two consecutive occasions (Reference Richmond, Reay and Abu34), with or without positive findings on clinical examination. Screening echocardiography involves an echocardiogram performed by a nonspecialist in addition to clinical examination; a positive screening result is defined by an abnormal appearance on four chamber, outlet, or arch views with or without positive findings on clinical examination. A “no screening” strategy was not considered to be a practical or ethical option. We assumed newborn screening would take place at 24 hours of age.
Development of Decision-Analytic Model
We developed a decision model to simulate the sequence of events experienced by 100,000 live-born infants up to the point of diagnosis. Figure 1 presents a simplified version of this model. Infants with undiagnosed life-threatening CHDs are assumed to be at risk of cardiovascular collapse and death anytime before diagnostic echocardiography by a specialist is performed. An affected infant might collapse after either a positive or a false-negative screening test, if they were not screened. As data were not available for infants who missed screening, we assumed they had a similar risk of cardiovascular collapse to infants with false-negative screening results. Infants with life-threatening CHDs who screen positive and do not collapse before diagnostic echocardiography are considered to have received a “timely diagnosis” (primary outcome). We assumed all pathway probabilities varied according to specific CHD but were constant across screening strategies, with the exception of the detection and false-positive rates, which varied by both these factors.

Figure 1. Schematic representation of screening and management pathways. Pathways for all screening strategies are the same.
Data Used in the Model
Data for prevalence at screen and birth, test performance, and risk of cardiovascular collapse were derived from a systematic review (Medline [1966 onward], Embase [1980 onward], Cinahl [1982 onward]) and from a population-based register of CHDs in the Northern Region of England (Reference Wren, Richmond and Donaldson43). No randomized trials comparing the different screening strategies were found. Hence, published and unpublished observational data were used in the model. Where data were not available from these sources, input parameters were obtained as subjective probabilities (Reference Hunink, Glasziou and Siegel26) from two or three pediatric cardiologists who provided individual estimates that were negotiated until consensus was reached (Reference Clemen and Winkler17). Resulting probability ranges were translated into full probability distributions (Reference Spiegelhalter, Harris, Bull and Franklin38).
Prevalence of Unrecognized Congenital Heart Defects at the Point of Screening
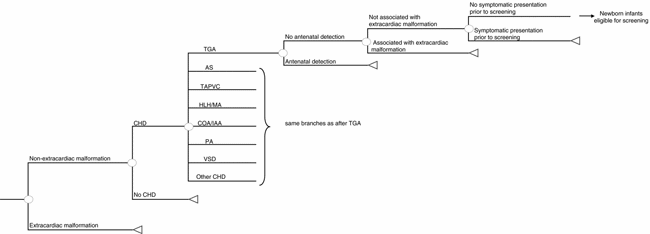
The Northern Region data set (Reference Wren, Richmond and Donaldson43) was the source of defect-specific prevalence estimates with a population-based denominator and follow-up of over 15 years, including postmortem diagnoses, to achieve complete case ascertainment with adjustment for the number of additional cases detected between 1 and 16 years (Reference Hoffman and Kaplan24;Reference Wren and O'Sullivan42). On the basis of Hoffman and Kaplan (Reference Hoffman and Kaplan24), we increased the baseline prevalence of ventricular septal defects (VSDs) by 95 percent to estimate those detected by screening echocardiography, and the prevalence of persistent ductus arteriosus, atrial septal defects, and pulmonary stenosis similarly by 61 percent. We assumed that, at the point of newborn screening, the prevalence of unrecognized CHDs would depend on the proportion detected by antenatal screening, or by the presence of readily recognizable extracardiac defects associated with CHDs, such as Down's syndrome, lethal trisomies (Reference Clancy, McGaurn and Wernovsky13 or Reference de Wahl, Mellander, Sunnegardh, Sandberg and Ostman-Smith18), gastroschisis, exomphalos or through symptomatic presentation before screening. Such infants require specialist cardiac assessment and were excluded from routine screening in the model. We used defect-specific antenatal detection and termination of pregnancy rates from the Northern Region (Reference Wren, Richmond and Donaldson43) in the base case and explored the effect of using average national UK antenatal detection rates (Reference Bull11) and of possible future improvements in fetal ultrasound screening in sensitivity analyses. Northern Region data were used to calculate the number of children with extracardiac defects, supplemented with data from a study of gastrointestinal defects (Reference Tulloh, Tansey and Parashar41), Eurocat surveillance data (19), and the UK Down's Syndrome Register (Reference Alberman3). We used Northern Region data to identify the number of infants with unrecognized CHDs at 24 and 48 hours of age (Table 1 and Supplemental Table 1 [http://www.journals.cambridge.org/jid_thc]). Figure 2 shows the decision model used to calculate the prevalence of CHDs at the point of screening.
Table 1. Source of Data for the Model

* http://www.journals.cambridge.org/jid_thc
CHDs, congenital heart defects.
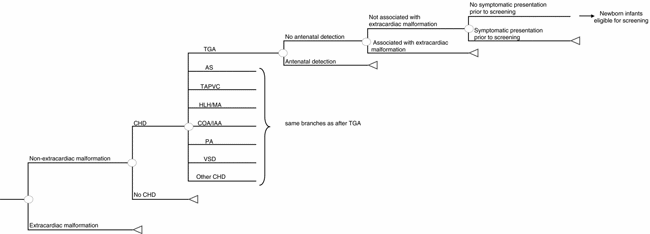
Figure 2. Decision tree to calculate the prevalence of congenital heart defects at screening. Infants with an extracardiac congenital malformation not associated with congenital heart defects (CHD), such as gastroschisis, require specialist examination and are, therefore, excluded from routine newborn screening. TGA, transposition of the great arteries; AS, aortic stenosis; TAPVC, total anomalous pulmonary venous connection; HLH/MA, hypoplastic left heart/mitral atresia; COA/IAA, coarctation of the aorta/interruption of the aortic arch; PA, pulmonary valve atresia; VSD, ventricular septal defect.
Test Performance
The detection rate for specific CHDs and the false-positive rate were derived from the Northern Region data set and from a population-based study of newborn screening (Reference Glazener, Ramsay and Campbell21), respectively. Estimates of test performance for pulse oximetry were taken from published studies (Reference Richmond, Reay and Abu34), supplemented by expert opinion. As the only randomized trial identified was small and excluded many subjects before randomization (Reference Sands, Craig and Mulholland37), estimates of test performance for screening echocardiography were supplemented by expert opinion (Table 1 and Supplemental Table 2 [http://www.journals.cambridge.org/jid_thc]).
Table 2. Data Used to Calculate Unit Costs (2000/2001 Prices)

a Parameter for probability distributions: alpha, beta for Gamma distribution; minimum, maximum for Uniform distribution.
b SD = .56, n = 12.
c Uprated by 40% to take overheads into account.
d Low cost estimates assumed a high delivery rate (75th percentile of the national deliveries) and a low cost of equipment, whereas high cost estimates assumed a low delivery rate (25th percentile of the national deliveries) and high equipment cost. The midpoint was used in base case analysis.
Other Probabilities
The probability of being screened (coverage), was estimated as 93 percent for clinical examination (Reference Glazener, Ramsay and Campbell21), 93 percent for pulse oximetry (Reference Richmond, Reay and Abu34), and 91 percent for screening echocardiography (Reference Sands, Craig and Mulholland37). We derived probabilities of diagnosis without cardiovascular collapse in an affected infant given a negative screening result or no screening between 1 and 16 years from Wren and O'Sullivan (Reference Wren and O'Sullivan42) and Wren et al. (Reference Wren, Richmond and Donaldson43). Expert opinion was used for all remaining pathway probabilities (Table 1 and Supplemental Table 2 [http://www.journals.cambridge.org/jid_thc]]).
Costs
Costs were estimated from the UK health service perspective and included screening and diagnostic tests, management of collapsed infants, staff, equipment, consumables, and overhead (see Table 2). Costs were adjusted to 2000/2001 prices and were not discounted as the model considers only the first year of life. Costs were entered as pounds sterling, with £1 sterling equivalent to US $1.872 or €1.478 (as at 13 September 2006).
Evaluation of Cost-Effectiveness
The model was programmed and analyzed in Microsoft Excel 2000. The model was based on 100,000 live-born infants entering the screening pathway, and the “base case” analysis assumed that the antenatal detection rate for specific heart defects from the Northern Region applied, newborn screening was performed at 24 hours of age and the primary outcome was used. For each screening strategy, we calculated the overall detection rate, the number of infants with true-positive and false-positive screening results, the positive predictive value, and the false-positive rate for the primary and secondary outcomes (Reference Hunink, Glasziou and Siegel26). For each screening strategy, incremental cost-effectiveness ratios (ICERs) were presented as the additional cost per additional timely diagnosis compared with the next most effective strategy.
Assessing Uncertainty
Sensitivity analyses were undertaken to examine the robustness of the base case analysis to alternative assumptions. Within the model, we explored the implications of improved coverage for screening echocardiography, a detection rate of 100 percent for screening echocardiography, differing antenatal detection rates, immediate access to diagnostic testing after a presumptive positive screen, and varying age at screening (birth or 48 hours). To derive a distribution of expected costs and outcomes (Reference Briggs, Drummond and McGuire9), we assigned probability distributions to input parameters and conducted probabilistic sensitivity analyses (i.e., Monte Carlo, 10,000 iterations; see Tables 1 and 2 and Supplemental Table 1). The probability of a strategy being cost-effective using a range of different thresholds for cost-effectiveness was estimated using the net benefit approach (Reference Stinnett and Mullahy39) and displayed using cost-effectiveness acceptability curves (Reference Fenwick, Claxton and Sculpher20).
Value of Information Analysis
Because a preference for a particular screening strategy is subject to various uncertainties in the input parameters of the model, we used value of information analysis (Reference Chilcott, Brennan, Booth, Karnon and Tappenden12;Reference Claxton, Cohen and Neumann16) to assess the value of perfect information (EVPI) and the partial expected value of information (EVPPI) for groups of model input parameters. This analysis assumes the maximum value placed on research to eliminate uncertainty is equivalent to the opportunity cost of making the wrong decision under current uncertainty and can be used to identify those parameters for which further research would be most valuable. The population EVPI (i.e., EVPI for the whole current and future population of interest; in this case, all infants undergoing newborn screening) was calculated by multiplying the “per person” EVPI (in this model per 100,000 persons) by the number of newborns (I) in each time period (t) discounted at rate r over the assumed lifetime of the screening technology (T):
An effective lifetime of the screening technology of 5 years was assumed, and the number of newborns (549,566) per year over this 5-year period was based on the number of hospital deliveries in England during the year 2000/2001. A discount rate of 6 percent per annum was applied.
RESULTS
Overall Screening Test Performance
Table 3 details the clinical results of the model analysis. Based on the base case decision model for 100,000 infants, 167 are predicted to have life-threatening CHDs, of whom 121 will remain unrecognized at 24 hours of age (see Table 2). A further 543 infants are predicted to have clinically significant CHDs, of whom 425 will remain unrecognized at 24 hours. The percentage of live-born infants with positive screening results is highest for screening echocardiography (5.4 percent; 4,940) and lowest for clinical examination alone (.5 percent; 499).
Table 3. Estimated Performance per 100,000 Live Birthsa of Alternative Screening Strategies to Detect Congenital Heart Defects (CHD): Base Case Analysis

a Rounded to the nearest whole number, unless otherwise stated.
b Anatomically defined cardiac malformations of no functional clinical significance, for example, tiny ventricular septal defects.
c Affected infants diagnosed before collapse or death occurs.
Clinical examination alone results in 34.0 timely diagnoses per 100,000 live births, compared with 70.6 for pulse oximetry and 71.3 for screening echocardiography. Screening echocardiography and pulse oximetry detect a similar proportion of infants with life-threatening CHDs (69 percent and 68 percent, respectively), twice the proportion detected by clinical examination (32 percent). Using the secondary outcome (all clinically significant and life-threatening CHDs combined), the detection rates for pulse oximetry and screening echocardiography are 62 percent and 50 percent, respectively, compared with 32 percent for clinical examination.
Screening echocardiography is associated with the highest false-positive rate (5.4 percent; 4857), which includes 3,644 infants with clinically nonsignificant CHDs. This number is substantially lower for clinical examination (.5 percent, 460 infants) and pulse oximetry (1.3 percent, 1,168 infants). The predictive value of a positive screening test for life-threatening CHDs is 7.8 percent for clinical examination, 6.6 percent for pulse oximetry, and 1.7 percent for screening echocardiography.
Cost-Effectiveness
Total costs are lowest for clinical examination (£296,891 per 100,000 live births) and highest for screening echocardiography (Table 4). The cost per additional timely diagnosis is £4,894 for pulse oximetry compared with clinical examination, and £4,496,666 for screening echocardiography compared with pulse oximetry. Using the secondary outcome, the cost per additional diagnosis falls to £1,489 for pulse oximetry and £36,013 for screening echocardiography.
Table 4. Results of the Economic Analyses per 100,000 Live Births (2000/2001 Prices)

Sensitivity Analyses
These results are sensitive to detection rates for screening echocardiography and age at screening, but robust to assumptions about antenatal detection rates using published ranges, availability of diagnostic echocardiography and coverage of screening echocardiography. If the detection rate for screening echocardiography is assumed to be 100 percent, the cost per timely diagnosis falls to £126,606 and £22,291 for the primary and secondary outcomes, respectively. Similarly, if newborn screening were to be performed immediately after birth, the cost per timely diagnosis for pulse oximetry falls to £3,406 and to £1,176 for the secondary outcome, whereas screening echocardiography becomes more costly and less effective than pulse oximetry. Conversely, if screening were to be undertaken at 48 hours, the cost per timely diagnosis rises to £8,195 and £1,928,151 for pulse oximetry and screening echocardiography, respectively, for screening at 48 hours of age, and for the secondary outcome to £2,183 and £46,010, respectively. If antenatal detection rates rise due to future improvements in fetal ultrasound, then the cost of detecting additional cases with newborn screening increases but only begins to rise steeply once antenatal detection rates of 85–90 percent are achieved (data not shown).
Probabilistic Sensitivity Analysis
Uncertainty around these estimates is represented by cost-effectiveness acceptability curves, which show the probability that one of the three screening combinations/options is cost-effective compared with a maximum willingness to pay that decision makers might have for these health outcomes (Figures 3 and 4 for the primary and secondary outcome, respectively). For example, the probability that pulse oximetry is cost-effective is .53 if the willingness to pay per timely diagnosis of life-threatening CHDs is £5,000. This probability increases to over .90 if decision makers are willing to pay between £10,000 and £100,000 for a timely diagnosis (Figure 3). The cost-effectiveness acceptability for the secondary outcome is shown in Figure 4, indicating that the probability of screening echocardiography is over .50 at a willingness to pay of around £50,000 per timely diagnosis of clinically significant CHDs.

Figure 3. Cost-effectiveness acceptability curves for base case analysis and primary outcome. Curve shows the probability that a screening modality is cost-effective for a range of decision-makers' maximum willingness to pay per timely diagnosis of life-threatening heart defects.

Figure 4. Cost-effectiveness acceptability curves for base case analysis and secondary outcome. Curve shows the probability that a screening modality is cost-effective for a range of decision-makers' maximum willingness to pay per timely diagnosis of life-threatening heart defects and diagnosis of clinically significant heart defects.
Value of Information Analysis
The maximum monetary value of further research for an arbitrary cost-effectiveness threshold of £50,000 per timely diagnosis is £744,000 and £14,450,000 for the primary and secondary outcomes, respectively. At this threshold, key determinants of cost-effectiveness with a maximum value of future research for primary and secondary outcome, respectively, are detection rates of pulse oximetry (£557,000; £11,320,000), screening echocardiography (£0; £4,958,000), and screening test (£275,000; £5,285,000) costs.
DISCUSSION
Our findings suggest that the addition of pulse oximetry to clinical examination is likely to detect twice as many life-threatening CHDs in affected infants before death or collapse occurs. This strategy appears cost-effective but further research is required before this change should be recommended as policy.
Although encouraging, this study alone is not sufficient to permit recommendation of adding pulse oximetry to a newborn screening checklist immediately. First, our value of information analysis supports targeting future research at reducing uncertainty around detection and false-positive rates for pulse oximetry, reflecting the fact that the few observational studies reporting experience with this test have been based on small samples (Reference Arlettaz, Archer and Wilkinson4;Reference Bakr and Habib5;Reference de Wahl, Mellander, Sunnegardh, Sandberg and Ostman-Smith18;Reference Hoke, Donohue and Bawa25;Reference Koppel, Druschel and Carter28;Reference Reich, Miller and Brogdon33;Reference Richmond, Reay and Abu34). Second, protocols to specify the measurement of oxygen saturation (including operator, algorithm, and number and timing of measurements) and the proper investigation of positive screening results, considering the benefit of identifying respiratory and neurological abnormalities also, are essential before this screening strategy could be adopted. Existing studies of the feasibility and performance of pulse oximetry in the detection of cyanotic CHD have reported occasions when cyanotic life-threatening defects have been missed (Reference Richmond, Reay and Abu34). Larger studies are needed to estimate the detection rate of pulse oximetry more precisely. Similarly, surveillance of screen-negative populations is needed to define the negative predictive value of pulse oximetry, that is, to estimate the risk of falsely reassuring parents that their child does not have a CHD when the defect is acyanotic.
Although screening echocardiography and pulse oximetry may lead to a similar proportion of timely diagnoses of life-threatening CHDs, screening echocardiography is more costly in financial terms and in consequences for families of infants with false-positive diagnoses, which include a high number of those with clinically nonsignificant CHDs. This remains true for the secondary outcome, although in this case, screening echocardiography and pulse oximetry are more differentiated and screening echocardiography appears more attractive in terms of cost-effectiveness. In practice, it would be difficult to disregard information about clinically nonsignificant CHDs, although their detection is of arguable benefit to the child. Because newborn screening for CHD should be considered in conjunction with the total antenatal and newborn experience of mothers and infants, the additive effects of false-positive diagnoses becomes highly relevant (Reference Kwon29).
The limitations of our study reflect limitations in primary data sources as well as methodological limitations in this field. There was a paucity of studies comparing longer-term outcomes between screened and unscreened populations and model parameters were based on evidence from observational studies and, extensively, on expert opinion. We could not quantify the disbenefits associated with false-positive screening results and the application of generic measures that potentially could capture these disbenefits, for example quality-adjusted life-years, was limited by the lack of outcome data (Reference Knowles, Griebsch and Dezateux27) and the problems of using existing generic measures for health-related quality of life in newborn infants and young children (Reference Griebsch, Coast and Brown22;Reference Petrou32;Reference Tilford40). Sensitivity analyses demonstrated that relative cost-effectiveness is determined mainly by the choice of outcome measure (life-threatening or all CHD diagnoses) and the value society attaches to these outcomes. By including value of information analysis, we were able to identify parameters for which more precise estimates would reduce the uncertainty surrounding the choice of screening strategy and indicate where further research would be of most value. The value of information approach has been highlighted recently as a potential aid for setting research priorities in the context of health technology assessment (Reference Chilcott, Brennan, Booth, Karnon and Tappenden12;Reference Claxton14–Reference Claxton, Cohen and Neumann16).
A further limitation in the model is the lacking information on the dependence of age at screening and test performance. When different ages at screening were investigated in the model, we assumed that the mean detection rate and its associated uncertainty remained the same. Further research is needed to determine whether such a dependency exists in clinical practice and, if so, then the optimal age at screening needs to be determined.
Clearly, strategies for the early detection of CHDs require an integrated approach across antenatal and newborn screening programs and postnatal referral protocols. Antenatal diagnosis allows delivery in specialist centers with prompt intervention available. Our model would accommodate future updating with population-based antenatal screening data. Although the timely management of infants who “screen positive” does not differ between screening strategies, it is a crucial practical consideration. Telemedicine transmission of scanned images for specialist evaluation might bring forward effective treatment for some conditions, but others need urgent surgical intervention.
POLICY IMPLICATIONS
The policy implications resulting from our analysis are clear. This analysis suggests that there is scope to improve the effectiveness of newborn screening policies for CHDs by implementing a combined strategy of pulse oximetry and clinical examination. Before implementing this strategy in clinical practice, the development of protocols to specify the measurement of oxygen saturation and the proper investigation of positive screening results, considering the benefit of identifying respiratory and neurological abnormalities also, are essential.
Further research is about to be commissioned by the National Health Service Research and Development Health Technology Assesssment Programme as a result of our study and will be aimed at reducing uncertainties surrounding the use of pulse oximetry as a population screening strategy (see www.ncchta.org for more information). This research is important in view of the significant contribution of CHD to infant mortality in industrialized countries (Reference Rosano, Botto, Botting and Mastroiacovo36).
CONTACT INFORMATION
Ingolf Griebsch, MSc, MPH (Ingolf.Griebsch@merck.de), Research Associate, MRC Centre of Epidemiology for Child Health, Department of Social Medicine, University of Bristol, Whiteladies Road, Bristol BS8 2PR, UK
Rachel L. Knowles, MBChB, FFPH (r.knowles@ich.ucl.ac.uk), MRC Research Fellow, MRC Centre of Epidemiology for Child Health, UCL Institute of Child Health, 30 Guilford Street, London WC1N 1EH, UK
Jacqueline Brown, PhD (jackie.brown@lilly.com), MRC Senior Scientist, MRC Health Services Research Collaboration, Department of Social Medicine, University of Bristol, Whiteladies Road, Bristol BS8 2PR, UK
Catherine Bull, FRCP (BullC@gosh.nhs.uk), Medical Advisor, Department of Family Policy, Great Ormond Street Hospital for Children, Great Ormond Street, London WC1N 3JH, UK
Christopher Wren, FRCP (Christopher.wren@nuth.nhs.uk), Consultant Pediatric Cardiologist, Department of Pediatric Cardiology, Freemann Hospital, High Heaton, Newcastle upon Tyne NE7 7DN, UK
Carol A. Dezateux, FRCP (c.dezateux@ich.ucl.ac.uk), Professor of Pediatric Epidemiology, Director, MRC Centre of Epidemiology for Child Health, UCL Institute of Child Health, 30 Guilford Street, London WC1N 1EH, UK