INTRODUCTION
Herbivorous predators may affect both the abundance and spatial distribution of seeds and seedlings (Augspurger & Kitajima Reference AUGSPURGER and KITAJIMA1992, Janzen Reference JANZEN1971, Nathan & Casagrandi Reference NATHAN and CASAGRANDI2004, Rey & Alcantara Reference REY and ALCANTARA2000). Seed predators can account for >90% of seed mortality in some species and may alter the relative regeneration success within a suite of tropical canopy trees (Augspurger & Kitajima Reference AUGSPURGER and KITAJIMA1992, Crawley Reference CRAWLEY and Fenner2001, Forget et al. Reference FORGET, MILLERON, FEER, Newbery, Prins and Brown1998, Hammond Reference HAMMOND1995, Hubbell Reference HUBBELL1979, Salvande et al. Reference SALVANDE, MULET and GONZALEZ2006, Sánchez-Cordero et al. Reference SÁNCHEZ-CORDERO, BRIONES-SALAS and SÁNCHEZ-ROJAS2006, Vallejo-Marin et al. Reference VALLEJO-MARIN, DOMINGUEZ and DIRZO2006). Yet, although the significant effect of seed predation on seedling recruitment has been well documented (Crawley Reference CRAWLEY, Davy, Hutchings and Watkinson1988, Curran & Webb Reference CURRAN and WEBB2000, Curran et al. Reference CURRAN, CANIAGO, PAOLI, ASTIANTI, KUSNETI, LEIGHTON, NIRARITA and HAERUMAN1999, DeMattia et al. Reference DEMATTIA, RATHCKE, CURRAN, AGUILAR and VARGAS2006, Grogan & Galvao Reference GROGAN and GALVAO2006, Janzen Reference JANZEN1971, Terborgh & Wright Reference TERBORGH and WRIGHT1994, Vallejo-Marin et al. Reference VALLEJO-MARIN, DOMINGUEZ and DIRZO2006), its influence on sympatric plant taxa is less well-understood (though see Worthy et al. Reference WORTHY, LAW and HULME2006).
A differential influence of seed predators on specific tree species may have important implications for entire forest communities. Herbivores that preferentially consume common species can alter the relative abundance of plants, and thus allow rare species to persist within a community (Connell et al. Reference CONNELL, TRACEY and WEBB1984, Hubbell Reference HUBBELL1979, McGuinness Reference MCGUINNESS1997, Wills et al. Reference WILLS, HARMS, CONDIT, KING, THOMPSON, HE, MULLER-LANDAU, ASHTON, LOSOS, COMITA, HUBBELL, LAFRANKIE, BUNYAVEJCHEWIN, DATTARAJA, DAVIES, ESUFALI, FOSTER, GUNATILLEKE, GUNATILLEKE, HALL, ITOH, JOHN, KIRATIPRAYOON, DE LAO, MASSA, NATH, NOOR, KASSIM, SUKUMAR, SURESH, SUN, TAN, YAMAKURA and ZIMMERMAN2006). Predators also can change the competitive interactions among their prey, and thus facilitate the growth of competitively inferior species (Paine Reference PAINE1966, Marquis Reference MARQUIS2004, Silman et al. Reference SILMAN, TERBORGH and KILTIE2003, Theimer Reference THEIMER2001, Worm & Duffy Reference WORM and DUFFY2003). However, little is known about the differential effects of seed predation on particular tree species and whether these differences influence adult recruitment and community structure. The role of mammalian seed predators may be particularly important in tropical rain forests, which contain more species of seed predators and large-seeded trees than any other forest formation (Flannery Reference FLANNERY1995, Richards Reference RICHARDS2004, Wright Reference WRIGHT2002).
The degree to which seed predation influences tree species recruitment ultimately depends on the life stages at which recruitment is most limited. Recruitment limitation may result as a function of: (1) low adult abundance and distribution; (2) low fecundity; (3) limited seed dispersal; and/or (4) poor establishment conditions due to mortality from both abiotic and biotic factors, such as pathogens and predators (Clark et al. Reference CLARK, MACKLIN and WOOD1998, Hubbell Reference HUBBELL2006). Seed predators only influence tree species recruitment when they reduce seedling densities below the level at which density-dependent seedling mortality occurs (Crawley Reference CRAWLEY, Davy, Hutchings and Watkinson1988, Hulme Reference HULME1996, Schupp Reference SCHUPP1990).
Here we present the effects of mammalian seed predation on the seed survival and regeneration of five focal tree species in a mid-elevation Papua New Guinean rain forest. We examine species-specific predation and germination frequencies to assess whether there are differential effects of mammalian seed predation on regeneration. We then survey species-specific size distributions to determine the relative abundance of focal species in several size classes and identify potential limitations to sapling recruitment for each species. By comparing species-specific predation and germination frequencies to abundance measures at several life stage classes, we assess the potential for seed predation to influence the sapling recruitment of our focal tree species.
Our major questions addressed are: (1) how does mammalian seed predation affect the seed survival of the focal tree species? (2) Do seed predators impart consistent effects on germination across different species? (3) How do relative seed predation frequencies for each focal species compare to stage-specific size distribution? (4) Are species that display a high probability of mortality following seed damage potentially limited by seed recruitment? (5) For species that display a low probability of mortality following seed damage, are they limited at the seedling to sapling stage? These questions highlight several potential mechanisms by which seed predation can influence regeneration of rain-forest tree species and may help to reconcile the relative importance of seed predators within a suite of sympatric species.
METHODS
Study site
This study was conducted at the Crater Mountain Wildlife Management Area (CMWMA) in the Chimbu Province of Papua New Guinea. This site contains some of the world's largest expanses of intact rain forest (Mack Reference MACK1998a). Ninety-seven per cent of the reserve's 2700 km2 is primary rain forest and the remaining land contains patches of secondary forest regrowth from abandoned gardens and treefall gaps (Mack Reference MACK1998a). Approximately 2000 people live in CMWMA; the nearest village is 5 km from the study site. Local landholder agreements for the study area prohibit logging and hunting native animals, including rodents (Wright Reference WRIGHT1998).
Data were collected from 27 October 2004 to 23 March 2006 at a 40-ha study site within the CMWMA, located between 850 m and 1350 m asl (Wright et al. Reference WRIGHT, JESSEN, BURKE and DE SILVA GARZA1997). Temperatures range from 15 °C to 28 °C and the annual rainfall regime, which is ever-wet, averages 6.66 ±0.88 m (± SD) (Wright Reference WRIGHT1998, Reference WRIGHT, Dew and Boubli2005). We estimate that 36 terrestrial mammal species in the study area (29 murids) eat seeds (Flannery Reference FLANNERY1995). Other potential post-dispersal seed predators include the bandicoots (Microperoryctes longicauda Peters & Doria and Peroryctes raffrayana Milne-Edwards), pademelon (Thylogale browni Ramsay), ground cuscus (Phalanger gymnotis Peters & Doria), eight bird species (Megapodiidae and Columbidae), as well as insects and fungal pathogens (Beehler et al. Reference BEEHLER, PRATT and ZIMMERMAN1986, Flannery Reference FLANNERY1995).
Focal tree species
For this study, selected tree species met the following five criteria: (1) terrestrial mammals have been observed eating their seeds; (2) reproductive trees are sufficiently abundant for sample size; (3) seeds have been observed germinating immediately (no dormancy or delayed germination); (4) trees fruited at least annually; and (5) species represented a range of seed mass and volume.
The focal species were: Cerbera floribunda K. Schum. (Apocynaceae), Terminalia impediens Coode (Combretaceae), Pandanus penicillus Martelli (Pandanaceae), Microcos grandiflora Burret (Tiliaceae) and Endiandra latifolia Kostermans (Lauraceae) (Table 1). Average tree density (≥10 cm dbh) of these five species is ≤2 trees ha−1, which is typical of 69% of tree species recorded in the study area (Wright Reference WRIGHT1998, Reference WRIGHT, Dew and Boubli2005). Cerbera floribunda and P. penicillus produce fruit throughout most of the year, while E. latifolia, M. grandiflora and T. impediens fruit annually during the community-wide peak season (May–October: Wright Reference WRIGHT1998, Reference WRIGHT, Dew and Boubli2005; Table 1). Mean seed crop size of the focal species ranges from 62 to 356 seeds per tree and represents the low end of the tail of the broader-community fruiting distribution, which averages 336 seeds per tree (range: 2 to 10 385 seeds per tree; Table 1). This broader-community fruiting response is based on monthly phenological sampling of 113 species conducted by Wright from June 1990 to March 1993, with a sample of >7000 individual trees and lianas in 4.2 ha (Wright Reference WRIGHT1998, Reference WRIGHT, Dew and Boubli2005).
Table 1. Traits of focal species and 113 tree species studied in the Crater Mountain Wildlife Management Area of an Eastern Highlands tropical rain forest in Papua New Guinea (Wright Reference WRIGHT1998, Reference WRIGHT, Dew and Boubli2005).
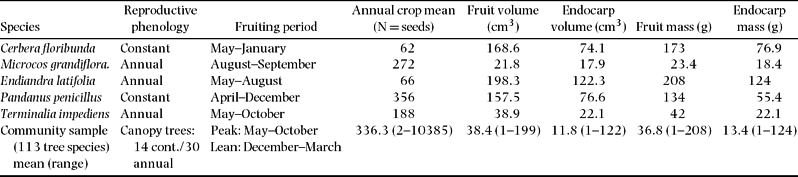
All five focal species are canopy trees, except M. grandiflora, which is a mid-storey tree (Wright Reference WRIGHT1998). Cerbera floribunda, Endiandra latifolia, Microcos grandiflora and Terminalia impediens have cylindrical boles with no spines; buttresses are only present in Terminalia impediens. Pandanus penicillus has thick, forking, spiny trunks; young trees have a single bole before forking.
These five species produce drupes, with the endocarp mass and volume ranging from 18.4 to 124.0 g and 17.9 cm3 to 122 cm3, respectively. This seed size range is representative of the broader community (N = 113), where endocarp mass ranges from 1 g to 124 g and endocarp volume ranges from 1 cm3 to 122 cm3 (Wright Reference WRIGHT1998, Reference WRIGHT, Dew and Boubli2005; Table 1). Pandanus penicillus produces a multiple-fruit head containing dozens of tightly clustered wedge-shaped drupes. Endiandra latifolia is the largest and heaviest seed of 113 species studied by Wright (Reference WRIGHT1998).
Seed predation and germination
Mammal exclosures and controls
Wire-mesh (1 cm2) exclosures and adjacent control plots measuring 1 m2 were constructed around 50 trees (10 individuals per focal species) to examine whether predation following seed-fall influences seed survival, regeneration and seedling composition. Exclosures were built in November and December 2004, and the exclosure tops were open to permit seed rain comparable to that of the control plot. Individuals of the focal species were identified and chosen to be a treatment tree if reproductive. The exclosures and control plots were located at 0 m, 5 m and 10 m from the base of each tree trunk, totalling three treatments per tree, 30 per species and 150 total.
Beginning 1 January 2005, after all exclosures were constructed, a baseline dataset was compiled for each exclosure and control by recording all pre-existing conspecific seeds, seedlings and saplings within the plot. The life stage class and condition (intact vs. predated) were recorded for each individual, where predation type was distinguished as mammal (tooth marks present), avian (seed broken and segment removed without teeth marks present), or insect (small hole or insects present). Changes in the seed and seedling composition of each plot were monitored on a fortnightly rotation, recording the presence and condition of both new and old seeds and seedlings. Individual seeds were marked and coded with dots of paint, and seedlings were labelled with tags to monitor the fate of individuals. Data collection spanned from 1 January 2005 to 23 March 2006.
Shade-house experiment
A shade house experiment was conducted to assess the germination potential of partially eaten and intact seeds in a controlled environment. For each focal species, 20 intact seeds and 20 seeds damaged by mammalian seed predators (approximately 50% embryo damage per seed), were planted in seed trays, and placed in an open shade house. All seeds were collected in the field and planted within 1 d. Seed germination was monitored fortnightly. Data collection extended from 10 November 2004 to 23 March 2006.
Life stage distributions
Transect counts
We conducted 150 transect counts to estimate the density of the five species and their relative abundance in different life stage classes. Each transect was 50 m × 2 m, thus representing a 100-m2 area. Stratified random sampling was employed to select transect location by delineating 30 points 50 m apart on each of five trails in the study area. From each point, a distance from which to start each transect was randomly assigned (0–49 m), and a compass bearing was randomized to determine the transect orientation.
Within each 100-m2 transect area, all individuals of the five species were recorded and species, location, life stage or size class (seedling, sapling (≥0.5 m height and ≤10 cm dbh), tree (>10 cm dbh)), sapling height and tree dbh were determined. Transect sampling was conducted from 22 February 2005 to 8 July 2005.
Radial counts
We delineated a 10-m-radius circle around each study tree and counted all conspecific individuals to estimate the relative abundance of conspecifics in each life stage class near parent trees. The following data were recorded: intact seeds, predated seeds, seedlings, saplings (≥0.5 m height and ≤10 cm dbh), and trees ≥10 cm dbh. Radial counts were conducted from 5 March to 30 July 2005 for 75 trees, equally distributed among the five focal species.
Data analysis
Germination and predation frequencies in the field were calculated by dividing the total number of seeds that geminated or were partially eaten during the monitoring period by the total number of intact seeds that occurred in a given treatment during that time period. Germination frequencies in the shade house were calculated by dividing the number of seeds that germinated by the number of seeds that were planted for each group. All germination and predation percentage values were arcsine-transformed to normalize their distribution and paired t-tests were employed on seed predation and germination data to determine the difference in predation frequencies between field and shade house treatments. Species-specific and life stage-specific differences in seed predation and germination frequencies were tested with ANOVA and pairwise comparisons.
Transect and radial count data were used to calculate individual densities for each species at each life stage class. Species-specific and stage-specific differences in distribution were tested with an ANOVA and pairwise comparisons.
RESULTS
Seed predation
Mammalian seed predation and seed removal frequencies in the control plots differed significantly from the exclosure treatments, which prevented small mammals from accessing seeds (Paired t-test: t = 4.76, df = 49, P < 0.001 and t = 5.21, df = 49, P < 0.001, respectively; Figure 1). Insect predation frequencies did not differ significantly between treatments (Paired t-test: t = 0.55, df = 49, P < 0.29; Figure 1). Post-dispersal seed predation by terrestrial birds was not observed.
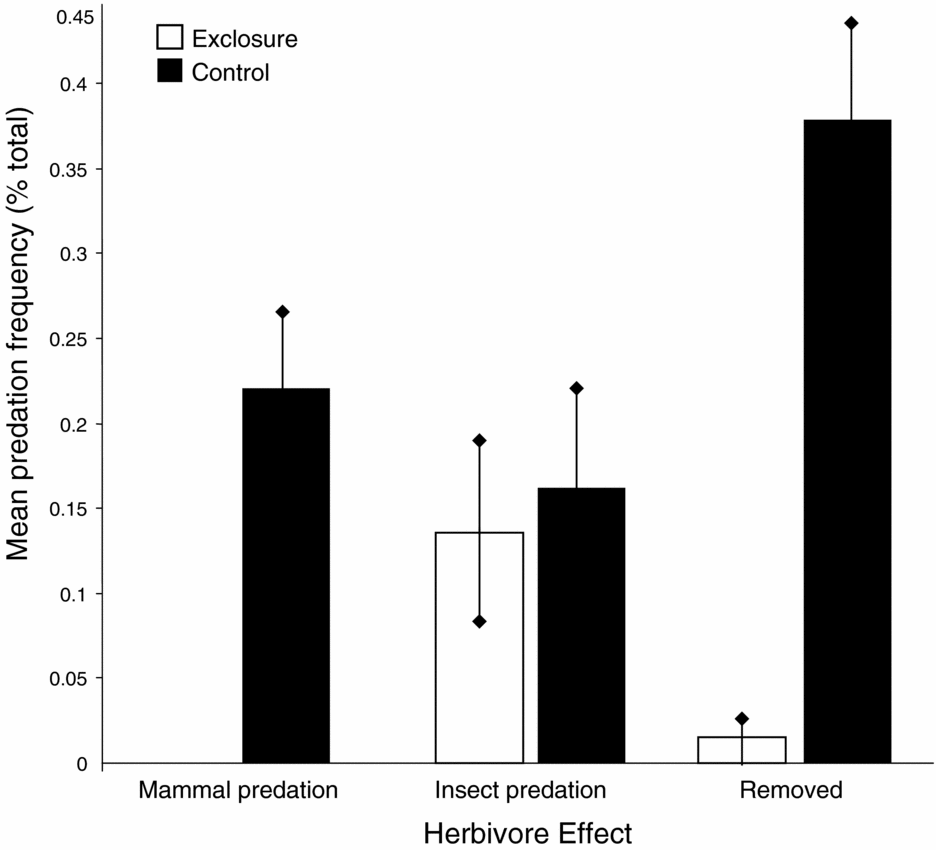
Figure 1. Post-dispersal seed predation patterns for focal seed species in the Crater Mountain Wildlife Management Area of an Eastern Highlands tropical rain forest in Papua New Guinea. Data acquired from 150 paired exclosure/control treatments. Mammal predation and seed removal frequencies differed significantly between control and exclosure treatments (Paired t-test: t = 4.76, df = 49, P < 0.001 and t = 5.21, df = 49, P < 0.001, df = 49, respectively). Insect predation frequencies did not differ significantly between treatments (Paired t-test: t = 0.55, df = 49, P < 0.29). Error bars represent ±1 SE.
Mammal and insect predation frequencies differed significantly across species (ANOVA: F = 4.64, df = 4, P < 0.003; F = 13.5, df = 4, P < 0.001, respectively; Figure 2) while removal frequencies did not (ANOVA: F = 1.02, df = 4, P < 0.410; Figure 2). The totalled post-dispersal predation and removal frequencies for M. grandiflora and C. floribunda in control plots were relatively high, at 50.5% (N = 47 of 93 total) and 44.2% (N = 34 of 77 total), respectively (Figure 2). Pandanus penicillus and T. impediens had lower total predation and removal frequencies, with 18.5% (N = 25 of 135 total) and 14.5% (N = 17 of 117 total), respectively (Figure 2). No post-dispersal predation was recorded for E. latifolia, but this species had a 3.2% removal frequency (N = 3 of 95 total; Figure 2). Predation frequency was consistent among individuals within each focal species and did not vary with distance up to 10 m from each parent tree. For all species, seed abundance peaked at 5 m from parent trees and terminated at approximately 15 m from parent trees, except for P. penicillus, where seeds occurred up to 25 m from the focal tree.
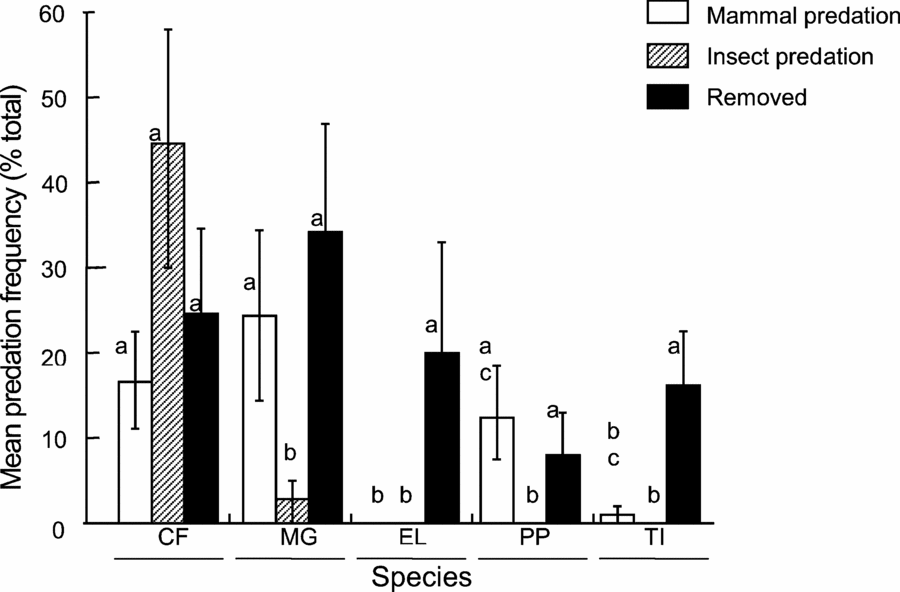
Figure 2. Predation frequencies for each focal species in the Crater Mountain Wildlife Management Area of an Eastern Highlands tropical rain forest in Papua New Guinea. Data acquired from 150 control treatments. Mammal and insect predation frequencies differed significantly across species (ANOVA: F = 4.64, df = 4, P < 0.003 and F = 13.5, df = 4, P < 0.001, respectively). Removal frequencies did not vary significantly across species (ANOVA: F = 1.02, df = 4, P < 0.410). Error bars represent ±1 SE. For each predation type, values with same letter do not differ significantly. Species names: CF = Cerbera floribunda, MG = Microcos grandiflora, EL = Endiandra latifolia, PP = Pandanus penicillus, TI = Terminalia impediens.
Germination
Germination frequency varied significantly across species (ANOVA: F = 7.01, df = 4, P < 0.0001) and between exclosure and control treatments (Paired t-test: t = 2.29, df = 49, P < 0.012). Cerbera floribunda had the lowest germination frequency in the absence of predation (3%, N = 2 of 65 total), while E. latifolia had the highest germination frequency (38%, N = 38 of 101 total). Microcos grandiflora and T. impediens both exhibited approximately three times the germination frequency in the exclosure treatments than in the control (12% (N = 13 of 109 total) vs. 4% (N = 4 of 99 total) and 10% (N = 13 of 134 total) vs. 3% (N = 4 of 117 total), respectively). Germination frequencies were consistent among individuals within each focal species.
Species-specific differences in the shade house were significant (ANOVA: F = 14.9, df = 4, P < 0.005; Figure 3), whereas treatment differences were not (Paired t-test: t = 0.56, df = 4, P < 0.295; Figure 3). Endiandra latifolia germinated more successfully than other species in the shade house, with 80% germination of both intact and damaged seeds. For Pandanus penicillus the germination frequency for damaged seeds was twice that of intact seeds (30% vs. 15%).
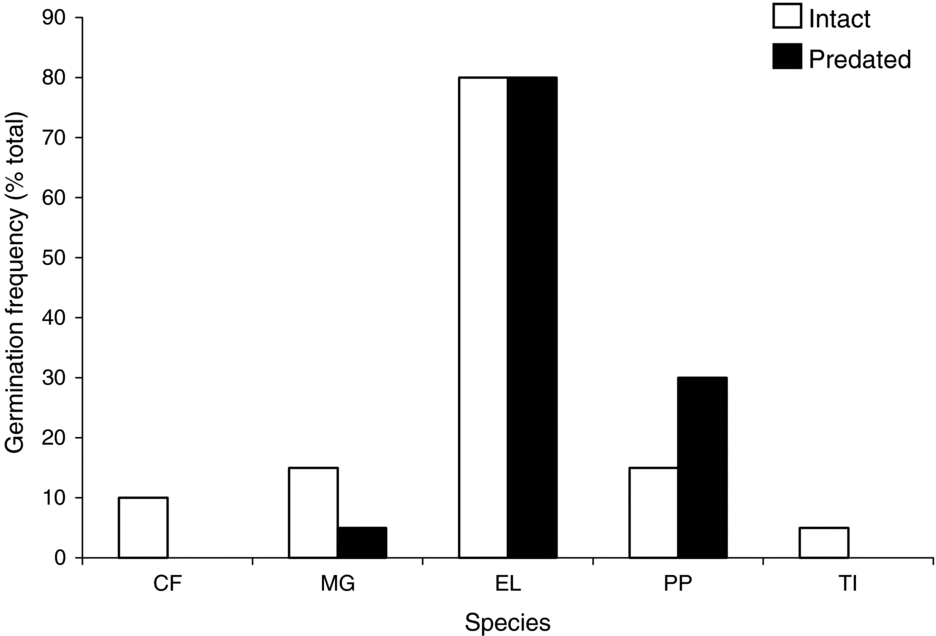
Figure 3. Germination frequency for each focal species in the Crater Mountain Wildlife Management Area of an Eastern Highlands tropical rain forest in Papua New Guinea. Per cent values were arcsine-transformed and used in a paired t-test and ANOVA. Differences among species in the shade house were significant (ANOVA: F = 14.9, df = 4, P < 0.005), whereas treatment differences were not (paired t-test: t = 0.56, df = 4, P < 0.295). Species names: CF = Cerbera floribunda, MG = Microcos grandiflora, EL = Endiandra latifolia, PP = Pandanus penicillus, TI = Terminalia impediens.
Life stage distributions
Tree species differed significantly in the distribution of abundances in each life stage class for the 19 712 m2 sampled (ANOVA: F = 3.48, df = 4, P < 0.026 and F = 10.4, df = 4, P < 0.0001 respectively; Figure 4). Two distinct species groups emerged and are distinguished by seedling abundance. Microcos grandiflora and C. floribunda each had < 20 seedlings ha−1, while E. latifolia, P. penicillus and T. impediens display ![]() = 380 seedlings ha−1 (Figure 4). Sapling densities were more uniform across the five species, with a maximum abundance of 163 ±15.7 individuals ha−1 (± SE) for E. latifolia. Tree (≥10 cm dbh) densities did not differ significantly across species (ANOVA: F = 1.13, df = 4, P < 0.371; Figure 4).
= 380 seedlings ha−1 (Figure 4). Sapling densities were more uniform across the five species, with a maximum abundance of 163 ±15.7 individuals ha−1 (± SE) for E. latifolia. Tree (≥10 cm dbh) densities did not differ significantly across species (ANOVA: F = 1.13, df = 4, P < 0.371; Figure 4).
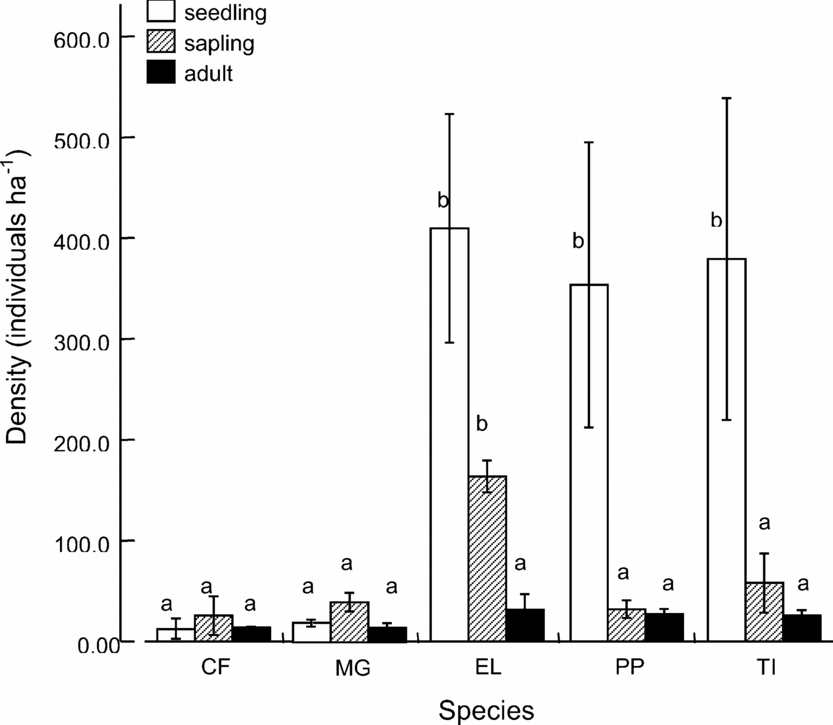
Figure 4. Density (individuals ha−1) of seedling, sapling and adult (≥10 cm dbh) life stage classes for each focal species in the Crater Mountain Wildlife Management Area of an Eastern Highlands tropical rain forest in Papua New Guinea Data averaged from transect and radial counts, where total area sampled = 19 712 m2. Species-specific densities differ significantly for seedlings (ANOVA: F = 3.48, df = 4, P < 0.026) and saplings (ANOVA: F = 10.4, df = 4, P < 0.0001) and non-significantly for adults (ANOVA: F = 1.13, df = 4, P < 0.371). Error bars represent ±1 SE. Stage-specific values with same letter do not differ significantly. Species names: CF = Cerbera floribunda, MG = Microcos grandiflora, EL = Endiandra latifolia, PP = Pandanus penicillus, TI = Terminalia impediens.
DISCUSSION
Seed predation
Excluding mammals from seeds significantly reduced both predation and removal frequencies for the five focal species, indicating that mammalian seed predators affect seed survival. Although comprehensive species-specific larder-hoarding and scatter-hoarding behaviour has not been documented among New Guinea murids, our field observations showed that mammals typically eat the focal species after removal, therefore it is likely that high removal frequencies signify high seed predation frequencies for these species. Insect predation frequencies did not differ significantly between the two treatments, which was expected because the treatments did not exclude insects from the 1-m2 treatment area.
Species-specific predation frequencies differed greatly, indicating strong mammalian preference for seed resources. Total predation and removal of M. grandiflora and C. floribunda seeds was approximately three-fold greater than that of P. penicillus and T. impediens and 17-fold greater than E. latifolia. However, E. latifolia germinates immediately, therefore some predated seeds may not have been detected.
Germination
The five focal species differed in their ability to germinate after seed predator attacks. For each species, germination frequency in the field corresponded well to results generated from the shade house, indicating strong species-specific traits. Endiandra latifolia and P. penicillus germinated following seed damage, as shown in both the shade house and exclosure treatments. Endiandra latifolia seeds were the most robust of the focal species; in the shade house, they germinated at an almost eight-fold greater frequency than the average of the other species, and, in the field, they germinated at a four-fold greater frequency than the other species. Endiandra latifolia also germinated in a diversity of microsites, which indicates that its seeds contain ample resource reserves to withstand unfavourable establishment conditions (Harms & Dalling Reference HARMS and DALLING1997, Mack Reference MACK1998b). Cerbera floribunda, M. grandiflora and T. impediens failed to germinate following seed damage, but germinated in the absence of seed predator attacks in both the shade house and field experiment.
Life stage distributions
The focal species exhibited two distinctive life stage distributions: a significant loss of individuals at the seed stage for M. grandiflora and C. floribunda, and a significant loss of individuals at the seedling stage for E. latifolia, P. penicillus and T. impediens. Microcos grandiflora and C. floribunda had a relatively even distribution of individuals at the seedling, sapling and adult stage classes, indicating a potentially high probability of survival once individuals become seedlings. High seed mortality suggests that surviving the seed-to-seedling transition may limit recruitment for these species (Clark et al. Reference CLARK, BECKAGE, CAMILL, CLEVELAND, HILLE RIS LAMBERS, LITCHTER, MCLACHLAN, MOHAN and WYCKOFF1999, Connell & Green Reference CONNELL and GREEN2000, Lichstein et al. Reference LICHSTEIN, GRAU and ARAGÓN2004).
These life stage distributions differed remarkably from those of E. latifolia, P. penicillus and T. impediens species, which each have an order of magnitude more seedlings per hectare than either M. grandiflora or C. floribunda. Additionally, E. latifolia, P. penicillus and T. impediens exhibited a significant decrease of individuals at the seedling-to-sapling transition. These distributions demonstrate that microsites, pathogens, or other factors may increase mortality at the seedling-to-sapling transition (De Steven Reference DE STEVEN1994, Lichstein et al. Reference LICHSTEIN, GRAU and ARAGÓN2004, Rey & Alcantara Reference REY and ALCANTARA2000).
Species-specific patterns and implications for sapling recruitment
Seed predation and germination frequencies displayed by E. latifolia and P. penicillus differed from those of M. grandiflora and C. floribunda in several aspects. Endiandra latifolia and P. penicillus germinated after partial seed damage, experienced fewer predator attacks, had high seedling abundance, and exhibited widespread seedling mortality. These traits suggest that mammalian predation had relatively little influence on the fate of the seed population, and the stage-specific distribution data support this prediction. Higher seed survivorship may enhance seedling density, but may not increase seedling survival or the abundance of individuals at later life stage classes.
Conversely, M. grandiflora and C. floribunda both experienced high levels of seed predation and low germination success after mammalian seed predator attacks. Furthermore, the distribution of M. grandiflora and C. floribunda seedlings, saplings and adults was relatively even when compared to that of E. latifolia, P. penicillus and T. impediens. This combination of traits suggests that surviving mammalian seed predation may influence the recruitment of M. grandiflora and C. floribunda seedlings. However, an increase in seed survival may not alter M. grandiflora and C. floribunda sapling and sub-adult abundance if mammals kill individuals that would die at later life stage classes. Understanding the relative competitive ability of M. grandiflora and C. floribunda seedlings would further elucidate the relative role of seed predation in their recruitment.
Terminalia impediens displayed traits similar to all other focal species. Its size distribution resembled that of E. latifolia and P. penicillus, where seedling density was relatively high. Unlike E. latifolia and P. penicillus, and similar to M. grandiflora and C. floribunda, T. impediens seeds failed to germinate after mammalian seed predator attacks, which suggests that seed predation may influence sapling recruitment. Although damaged T. impediens seeds failed to germinate, its relatively low predation frequency permitted seed survival and facilitated high seedling densities. Increased predation on T. impediens seeds may reduce seedling abundance, but would minimally influence recruitment if seedling mortality were relatively high.
Although many tropical trees have seed predators, the influence of seed damage on seed survival and seedling regeneration varies significantly. Of the five focal species, mammalian seed predation potentially influences the regeneration of only two: Cerbera floribunda and Microcos grandiflora. These species demonstrate high mortality from seed predation by mammals and display a potential reduction in recruitment at the seed-to-seedling stage transition. These predation and size distribution patterns differ significantly from those of Endiandra latifolia, Pandanus penicillus and Terminalia impedians, where mortality from seed predation is low and the seedling-to-sapling transition indicates a reduction in recruitment. These findings suggest that the influence of seed predation varies among five New Guinean tree species and that these differences may relate to the distribution of abundances in the three life stages. By influencing seed survival and seedling composition, seed predation also may play a key role in the recruitment of individuals to the sapling stage, which may thereby influence adult community structure (Connell & Green Reference CONNELL and GREEN2000). Future research is needed to assess how the differential effects of seed predators on seed survival and seedling growth influence the adult recruitment and community composition.
This study also highlights the importance of examining the relative effects of seed predators on both populations and communities across multiple seasons and for various food webs. The relative abundance of mammals, especially murid rodents, and their seed resources fluctuate seasonally and may represent variable effects of vertebrates averaged over many years (DeMattia et al. Reference DEMATTIA, CURRAN and RATHCKE2004, Wright Reference WRIGHT1998, Reference WRIGHT, Dew and Boubli2005).
ACKNOWLEDGEMENTS
We thank A. Arihafa and the entire team at the Wildlife Conservation Society-Papua New Guinea Program whose dedication and fieldwork made this study possible. M. Smith and field assistants from the Haia, Toyari and Herowana villages provided logistical and field assistance. O J. Schmitz and D. K. Skelly provided support and guidance throughout this study.







