Introduction
The management of newborns with extreme tetralogy of Fallot or pulmonary atresia with ventricular septal defect (with or without confluent branch and major aortopulmonary collateral artery) is multiple. Some centres suggest for these patients a biventricular repair in one time (unifocalisation),Reference Carotti1,Reference Asija, Koth and Velasquez2 while other centres suggest a repair in several stages (rehabilitation of pulmonary arteries).Reference Liava’a, Brizard and Konstantinov3,Reference Rome, Mayer, Castaneda and Lock4 Historically, for first-stage palliation, the main surgical option was the Blalock–Taussig shunt. But, the Blalock–Taussig shunt has two main complications: acute thrombosis and significant mortality.Reference Sasikumar, Hermuzi and Fan5 Thus, de Laval et al Reference de Leval, McKay, Jones, Stark and Macartney6 has been modified Blalock–Taussig shunt (modified Blalock–Taussig shunt) by interposing a polytetrafluoroethylene tube. The size of the tube is chosen according to the weight of the child. Modified Blalock–Taussig shunt allows a growth of the arterial tree.Reference Brandt, Camacho, Mahoney and Heintz7–Reference Amark, Karamlou and O’Carroll11
Other options have been proposed in alternative to modified Blalock–Taussig shunt: right ventricle to pulmonary artery connection,Reference Amark, Karamlou and O’Carroll11–Reference Gerelli, van Steenberghe and Murtuza17 central systemic-pulmonary shunts,Reference Kim, Sung, Choi, Lee, Ban and Chang18 and recently the stenting of the right ventricular outflow tractReference Quandt, Ramchandani and Stickley19 and the stent in the patent ductus arteriosus.Reference Boucek, Qureshi, Goldstein, Petit and Glatz20 Several studiesReference Amark, Karamlou and O’Carroll11,Reference Dragulescu, Kammache and Fouilloux15,Reference Gerelli, van Steenberghe and Murtuza17,Reference Lenoir, Pontailler and Gaudin21 show that the right ventricle to pulmonary artery connection allows a growth of the arterial tree with a decrease in the risk of thrombosis and an excellent ratio of biventricular repair. The ideal palliation is still debated especially for newborns. Thus, Choi et al Reference Choi, Sung and Kim22 reports their initial experience of the right ventricle to pulmonary artery connection. They warn about the complication of this technique especially the right ventricular outflow tract psendoaneurysm. One studyReference Zheng, Yang, Li and Li23 compares the right ventricle to pulmonary artery connection to modified Blalock–Taussig shunt only in older children. There is no study comparing the two procedures (modified Blalock–Taussig shunt versus right ventricle to pulmonary artery connection) in newborns.
Several studiesReference Ohye, Sleeper and Mahony24-Reference Pruetz, Badran, Dorey, Starnes and Lewis26 have suggested improved pulmonary arterial growth after Norwood procedure in hypoplastic left heart syndrome using Sano (ventricle to pulmonary artery connection) compared with a modified Blalock–Taussig shunt. The purpose of this report is to compare the short- and mid-term outcomes between the transannular path augmentation and the modified Blalock–Taussig shunt in the infants with tetralogy of Fallot or pulmonary atresia with ventricular septal defect.
Material and methods
Patients
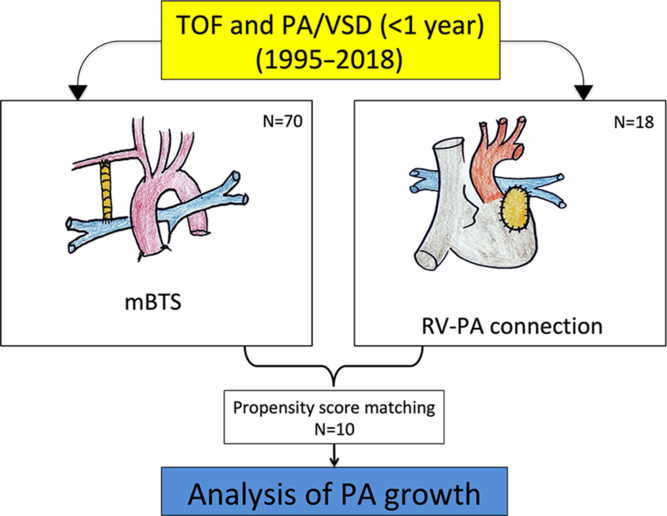
In this retrospective study, we considered infant (< 6 month) patients undergoing first palliation for critical tetralogy of Fallot or pulmonary atresia with ventricular septal defect between January 1995 and November 2018 (Fig 1). A total of 88 consecutive patients were included. Among those, 41 had a tetralogy of Fallot, and 47 had a pulmonary atresia with ventricular septal defect. There were 18 transannular path augmentations and 70 modified Blalock–Taussig shunt. During the same period, we did not perform a biventricular repair for these patients (ducto-dependence with hypoplasia pulmonary arteries).
Figure 1. Annual number of modified Blalock–Taussig shunt (n = 70) and right ventricle to pulmonary artery connection (n = 18) during the study period.
Baseline demographics, perioperative, and follow-up data were retrospectively collected from our local clinical database. The institutional review board approved the study, and a waiver of individual patient consent was obtained.
Study aim
We compared patients with transannular path augmentation (n = 18) and patients with modified Blalock–Taussig shunt (n = 70) (Fig 2).

Figure 2. Flowchart of 88 patients (pulmonary atresia with ventricular septal defect and tetralogy of Fallot) with first palliation.
Inclusion criteria
All patients with tetralogy of Fallot or pulmonary atresia with ventricular septal defect had a palliation (modified Blalock–Taussig shunt or transannular path augmentation) before 6 months.
Exclusion criteria were pulmonary atresia with ventricular septal defect type IV of the Castaneda classification (without pulmonary artery) and any patients whose initial palliative procedure was not a modified Blalock–Taussig shunt transannular path augmentation (e.g., central systemic-pulmonary shunts or stented arterial ducts). A univentricular heart or an atrioventricular septal defect was excluded.
Surgical technique
Conventional general anaesthesia was used in all patients.
Modified Blalock–Taussig shunt
The modified Blalock–Taussig shunt can be performed through a right or left thoracotomy or a median sternotomy. The decision to use a cardiopulmonary bypass was made by the attending surgeon at the time of surgery based on experience, the degree of saturation, and the haemodynamic stability. All patients underwent modified Blalock–Taussig shunt using a polytetrafluoroethylene tube without heparin bonded.
Transannular path augmentation
This surgical technique (technical points, safeguards, and pitfalls) has been extensively described previously,Reference Fouilloux, Bonello, Kammache, Fraisse, Macé and Kreitmann12-Reference Dragulescu, Kammache and Fouilloux15 including by our group.
Patients were operated via a median sternotomy with the use of a normothermic cardiopulmonary bypass usually on a beating heart. After controlling the right and left pulmonary artery with occlusion by loops, the main pulmonary artery was incised longitudinally, and an oval patch of autologous pericardium (prepared with glutaraldehyde) was sutured to edges of arteriotomy. There was no valved conduit or tube for the right ventricle to pulmonary artery connection. The incision was than extended to the right ventricle infundibulum. Finally, the right ventricle infundibulum was opened, and the patch was completed.
We sedate and ventilate the child for the first 24 hours. Intraoperative transfusion, fluid administration, insulin requirement, and use of inotropes were carried out at the discretion of the surgical team according to institutional protocols.
Follow-up
We conducted a retrospective single-institution study. Follow-up after palliation involved patient records and correspondences. Updating of patient follow-up was made in March 2019. We described the outcomes at a mean follow-up duration of 6.8 years (range, 0 days–20.2 years).
Therefore, the final cohort consisted of 88 consecutive patients undergoing a modified Blalock–Taussig shunt (n = 70) or a transannular path augmentation (n = 18) (Fig 3).

Figure 3. Initial angiogram through anterograde right ventricular outflow tract with tetralogy of Fallot (A). Initial angiogram through an arterial duct in a 25-day patient with pulmonary atresia with ventricular septal defect showing hypoplasia of native pulmonary artery (B).
Primary outcome
Pulmonary artery rehabilitation
Our primary outcome was pulmonary artery rehabilitation. Pulmonary anatomy evaluation was based on angiographic measurements. If these data were not available, echocardiographic or CT scan data were analysed. Right and left pulmonary artery diameters were measured and the Nakata indexReference Nakata, Imai and Takanashi27 was calculated. This analysis was performed before any palliative procedure and before biventricular repair. Delta (Δ) Nakata was defined as the difference between Nakata index before biventricular repair and before palliation. We defined an index of growth, which is the ratio between Δ Nakata and time between the two measurements (index of growth = Δ Nakata index/Δ time).
Palliation dysfunction (thrombosis and in-hospital mortality)
The diagnosis of acute palliation blockage was made on a combination of clinical and echocardiographic findings, supported by direct observation at surgery as appropriate. Hospital mortality defined as in-hospital and/or 30-day mortality.
Secondary outcomes
Morbidity
Our secondary outcomes assessed re-intervention for restrictive pulmonary blood flow after palliation (interstage) and before biventricular repair.
Biventricular repair
Based on clinical status, pulmonary angiography, anatomical pulmonary vascular tree, pulmonary vascular resistance, and Nakata index, a biventricular repair was performed. Ventricular septal defect was closed, and right ventricular outflow reconstruction was achieved either with a transannular pericardial patch or with a valved conduit.
Statistical analysis
Propensity score methodology was used to minimise bias between right ventricle to pulmonary artery connection patients and modified Blalock–Taussig shunt patients. Three variables considered relevant have been identified. Therefore, a propensity score was performed to match the tetralogy of Fallot group patients with pulmonary atresia with ventricular septal defect group patients (1:1) according to age at the palliation, sex, and weight. Patients were matched using the nearest neighbour method without replacement and a calliper with equal to (0.2). This process yielded 10 well-matched pairs from the 18 transannular path augmentation cases (55% matched).
Patients’ data were expressed as the mean ± standard deviation or as median ± range when data were skewed. Categorical data are summarised using frequencies and percentages; comparisons were made using the χ 2 test or Fisher exact test when the frequency was inferior 5 values. Statistical significance was established as p-value < 0.05. Actuarial survival and freedom from reintervention after palliation was analysed using the Kaplan–Meier method. The log rank test was used to explore the significance of the difference between two groups. Statistical analyses were performed using STATA statistics for Mac version 13 software (Stata Corporation, College Station, TX, USA).
Results
Pre-operative characteristics after matching
Mean age was 43 ± 51 days in the modified Blalock–Taussig shunt group and 63 ± 40 days in the transannular path augmentation group (p = 0.31). There were seven female in each group (70%). There was no difference between the two groups for the diagnosis of tetralogy of Fallot and pulmonary atresia with ventricular septal defect (p = 0.12), thus major aortopulmonary collateral artery was similar between two groups (p = 0.99). There were 1.3 (n = 13/10) major aortopulmonary collateral artery in the modified Blalock–Taussig shunt group and 2.1 (n = 21/10) major aortopulmonary collateral artery in the transannular path augmentation group. In addition, four patients had dual supply major aortopulmonary collateral artery in the modified Blalock–Taussig shunt group, and eight patients in the transannular path augmentation group (p = 0.06). Mean Nakata index was higher in the modified Blalock–Taussig shunt group (109 ± 31) than the transannular path augmentation group (52 ± 24) (p = 0.75). All other cardiac and non-cardiac co-morbidities were similar in the both groups (Table 1).
Table 1. Demographic data and morphological features before mBTS
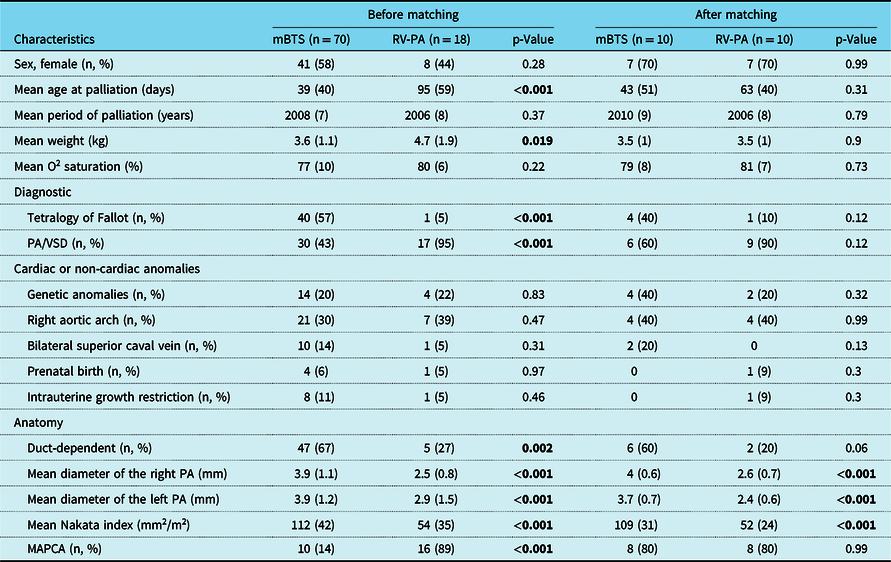
MAPCA = major aortopulmonary collaterals; mBTS = modified Blalock–Taussig shunt; PA = pulmonary artery; PA/VSD = pulmonary atresia with ventricular septal defect; RV-PA = right ventricular to pulmonary artery; TOF = tetralogy of Fallot.
Bold values represents p < 0.05.
Operative characteristics
Mean modified Blalock–Taussig shunt polytetrafluoroethylene size was 4 ± 1 mm, and mean transannular path augmentation size was 7 ± 3.4 mm. Associated procedures with palliation were similar in the both groups. Operative details are listed in Table 2. Using cardiopulmonary bypass was higher in transannular path augmentation groups than modified Blalock–Taussig shunt (n = 10, 100% versus n = 5, 50% for the modified Blalock–Taussig shunt group, p = 0.01). Mean cardiopulmonary bypass time was similar in both the groups (82 ± 41 min in the modified Blalock–Taussig shunt versus 87 ± 53 min in the transannular path augmentation group, p = 0.63).
Table 2. Operative and post-operative characteristics after mBTS

CPB = cardiopulmonary bypass; HFO = high-frequency oscillations; LPA = left pulmonary artery; mBTS = modified Blalock–Taussig shunt; PA = pulmonary artery; PA/VSD = pulmonary atresia with ventricular septal defect; RPA = right pulmonary artery; RV-PA = right ventricular to pulmonary artery; TOF = tetralogy of Fallot.
Bold values represents p < 0.05.
Pulmonary arterial growth
The left and right pulmonary artery diameter before biventricular repair were similar between the two groups (p = 0.8 and p = 0.48, respectively). Mean Nakata index before biventricular repair and the Δ mean Nakata were significantly better in transannular path augmentation (p = 0.03 and p < 0.001, respectively). Thus, the mean index of growth was better growth in transannular path augmentation group (62 ± 27 mm2/m2/month) compared in the modified Blalock–Taussig shunt group (9.2 ± 10 mm2/m2/month) (p < 0.001). Data for pulmonary artery growth are shown in Table 3 and Figure 4.
Table 3. Pulmonary artery size characteristics before and after mBTS

mBTS = modified Blalock–Taussig shunt; PA = pulmonary artery; PA/VSD = pulmonary atresia with ventricular septal defect; RV = right ventricular; RV-PA = right ventricular to pulmonary artery.
Bold values represents p < 0.05.
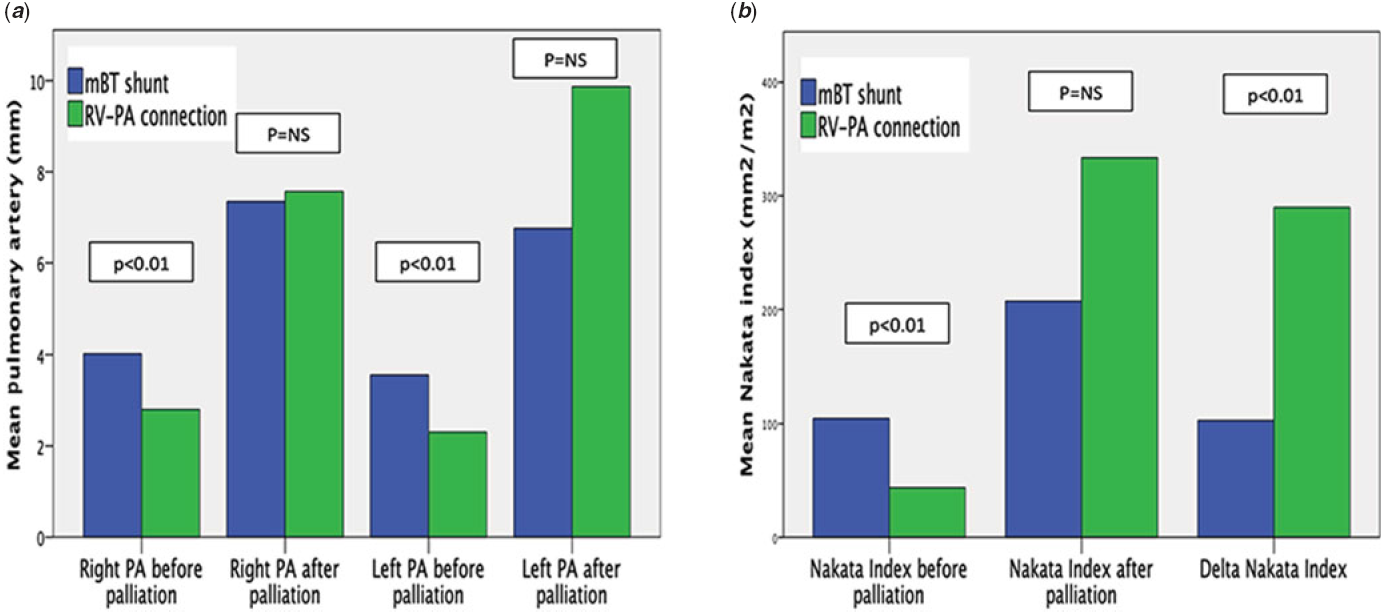
Figure 4. (A) Mean pulmonary artery diameter before and after palliation for two groups. (B) Mean Nakata index before and after palliation and Delta Nakata index for two groups. (modified Blalock–Taussig shunt, blue bar ; right ventricle to pulmonary artery connection, green bar).
There was 67% of right side modified Blalock–Taussig shunt (47/70). The side of the modified Blalock–Taussig shunt was not found a factor of pulmonary artery growth (Table 4).
Table 4. Effect of the side of mBTS

mBTS = modified Blalock–Taussig shunt; PA = pulmonary artery.
Hospital mortality
Before matching, among 88 patients, 4 patients died (5.6%), 3 in the modified Blalock–Taussig shunt group, and one in the transannular path augmentation group (Table 1). One patient had intraoperative death; it was not possible to wean the cardiopulmonary bypass for oxygenation reasons in transannular path augmentation. One patient died at 5 days after modified Blalock–Taussig shunt thrombosis. One patient had a cardiac arrest immediately after catheterising. One patient died for hypoxic cardiac arrest at 1 month of the modified Blalock–Taussig shunt. After matching, there were one in-hospital deaths in the transannular path augmentation group (10%) and no death in the modified Blalock–Taussig shunt group.
Survival
Before matching, a total of nine patients died at follow-up, four patients died in hospital mortality, and five patients in late death: 1 due to a modified Blalock–Taussig shunt thrombosis at 5.7 months, one due to a enteropathy at 7.5 months after transannular path augmentation, one due to cardiac failure in post-operative biventricular repair after transannular path augmentation, one due to hypoxia and supra-systemic right ventricular pressure in post-operative biventricular repair after modified Blalock–Taussig shunt, and one unknown causes. There were three late deaths in the transannular path augmentation group and two in the modified Blalock–Taussig shunt. There are trended for better survival in the modified Blalock–Taussig shunt group than in the transannular path augmentation group (survival: 92%, 92% at 1 and 10 years in modified Blalock–Taussig shunt group versus 88%, 75% at 1 and 10 years in the transannular path augmentation group; log rank = 0.06) (Fig 5).

Figure 5. Survival Kaplan Meyer between modified Blalock–Taussig shunt (Blue line) and right ventricle to pulmonary artery connection (red line) (log rank = 0.06).
Morbidity
Thrombosis
Before matching, there were six modified Blalock–Taussig shunt thrombosis and no transannular path augmentation thrombosis. Two patients in transannular path augmentation (11%) developed right ventricular outflow tract pseudoaneurysm without thrombosis (Fig 6).

Figure 6. 3D reconstruction showing infundibular pseudoaneurysm.
Reintervention
Reinterventions for restrictive pulmonary blood flow were similar between two groups (p = 0.63). Four patients (40%) underwent reoperations in transannular path augmentation (one extracorporeal membrane oxygenation, 2 days after palliation, one angioplasty of left pulmonary artery stenosis, one enlargement of right ventricular-pulmonary artery connection, and one unifocalisation) and three patients (30%) in modified Blalock–Taussig shunt group (two modified Blalock–Taussig shunt revision for acute modified Blalock–Taussig shunt thrombosis and one unifocalisation). Before matching, freedom for reintervention was similar between both groups (88% at 1 year in modified Blalock–Taussig shunt group versus 69% at 1 year in the transannular path augmentation group; log rank = 0.18) (Fig 7).

Figure 7. Kaplan Meyer curve freedom from reintervention (before matching) between modified Blalock–Taussig shunt (Blue line) and right ventricle to pulmonary artery connection (red line) (log rank = 0.18).
No significant differences were seen among the two groups in respect of delayed sternal closure (p = 0.11), and the mean mechanical ventilation support duration was similar in both groups in modified Blalock–Taussig shunt group (4 ± 4 days) and transannular path augmentation group (9 ± 15 days), p = 0.21. Data are shown in Table 2.
Biventricular repair
All patients in modified Blalock–Taussig shunt group have undergone biventricular repair, and eight patients (80%) in transannular path augmentation. Median time to biventricular repair was similar in the modified Blalock–Taussig shunt (8.9 [5.7–89] months) compared with transannular path augmentation (11.4 [7.8–39] months) p = 0.46). Data are shown in Table 2.
Discussion
This is the first comparison with propensity score matching of early outcomes of rehabilitation of the native pulmonary arteries between modified Blalock–Taussig shunt and transannular path augmentation for critical infants with tetralogy of Fallot or pulmonary atresia with ventricular septal defect. The present study set out to assess pulmonary arterial growth in a cohort of patients undergoing biventricular repair of tetralogy of Fallot or pulmonary atresia with ventricular septal defect having required initial palliation by either modified Blalock–Taussig shunt or transannular path augmentation over a 23-year period. The institutional experience with transannular path augmentation has been published previously.Reference Fouilloux, Bonello, Kammache, Fraisse, Macé and Kreitmann12–Reference Schouvey, Dragulescu and Ghez14,Reference Metras, Chetaille, Kreitmann, Fraisse, Ghez and Riberi16
Principal funding
Despite a smaller pulmonary artery, transannular path augmentation is equivalent to modified Blalock–Taussig shunt for rate of biventricular repair, time to biventricular repair, and morbidity (re-intervention for restrictive pulmonary blood flow). The transannular path augmentation is better for the rehabilitation of the extreme smaller native pulmonary artery.
Pulmonary artery growth
The initial palliation allows growth of native pulmonary arteries by increasing blood flow through the pulmonary artery.Reference Rome, Mayer, Castaneda and Lock4,Reference Chetaille, Fraisse and Ghez13,Reference Schouvey, Dragulescu and Ghez14,Reference Metras, Chetaille, Kreitmann, Fraisse, Ghez and Riberi16,Reference Amark, Karamlou and O’Carroll28 This study shows that right ventricle to pulmonary artery connection promotes better pulmonary arterial growth than modified Blalock–Taussig shunt palliation. In right ventricle to pulmonary artery connection group, the mean delta Nakata is similar to other series.Reference Amark, Karamlou and O’Carroll11,Reference Zheng, Yang, Li and Li23,Reference Zhang, Hua and Yang29 Thus, Amark et al Reference Amark, Karamlou and O’Carroll11 in 2006 found a delta Nakata of 74 mm2/m2 for the modified Blalock–Taussig shunt and 102 mm2/m2 for right ventricle to pulmonary artery connection, concluding that the right ventricle to pulmonary artery connection seemed to promote better growth of the pulmonary arteries relative to modified Blalock–Taussig shunt.
Tamisier et al Reference Tamisier, Vouhé, Vernant, Lecá, Massot and Neveux30 found pulmonary artery stenosis in 19% of the patients after modified Blalock–Taussig shunt in infants less than 3 months of age. Thus, Odim et al Reference Odim, Portzky and Zurakowski31 report an incidence of 16% of this complication; particularly, they found that the side of the modified Blalock–Taussig shunt was a significant predictor of pulmonary artery stenosis. However, in our study, the side of the modified Blalock–Taussig shunt was not a significant predictor of the pulmonary artery growth.
The growth rate of the pulmonary arteries was higher in transannular path augmentation than modified Blalock–Taussig shunt.
First, it is speculated that the right ventricle to pulmonary artery connection increases pulsatile forward flow of systemic venous blood to the pulmonary artery promotes better pulmonary arterial growth.
Secondary, the blood in the pulmonary arteries is more oxygenated by the modified Blalock–Taussig shunt than by the right ventricle to pulmonary artery connection. We know that hyperoxia leads to vasoconstriction in human pulmonary arteries.Reference Ariyaratnam, Loubani and Bennett32 The slow growth of the pulmonary arteries in the modified Blalock–Taussig shunt group can be explained by the vasoconstriction of the pulmonary arteries secondary to the hyperoxia of modified Blalock–Taussig Shunt.
Another major benefit, in cardiac catheterisation lab, it is easier to through the right ventricular pulmonary artery connection to dilate pulmonary arteries or embolisation the major aortopulmonary collateral arteries than through modified Blalock–Taussig shunt.
This finding is similar with most of the latest literature on pulmonary artery growth after Sano or modified Blalock–Taussig shunt of the Norwood palliation for patients with hypoplastic left heart syndrome,Reference Ohye, Sleeper and Mahony24-Reference Pruetz, Badran, Dorey, Starnes and Lewis26 or stenting of the right ventricular outflow tract compared modified Blalock–Taussig shunt in tetralogy of Fallot palliation.Reference Quandt, Ramchandani and Stickley19
This excellent growth of the pulmonary arteries allowed for the good rate of biventricular repair achieved in our series. The rate of biventricular repair is comparable with other studies.Reference Lenoir, Pontailler and Gaudin21,Reference Zheng, Yang, Li and Li23
Complications
Mortality-thrombosis
In our study, the hospital mortality was low compared to those ever published.Reference Amark, Karamlou and O’Carroll11,Reference Do, Hill and Wallace33 In the right ventricle to pulmonary artery connection group, there was no thrombosis against 8.6% of acute thrombosis in modified Blalock–Taussig shunt group. In contrast, in the right ventricle to pulmonary artery connection group, we had infundibular pseudoaneurysm. Choi et al Reference Choi, Sung and Kim22 reported that infundibular pseudoaneurysm that is sometimes deadly. Lenoir et al Reference Lenoir, Pontailler and Gaudin21 found the same complication. We think perhaps that preventing infundibular pseudoaneurysm formation by adding several pledgetted sutures and regular echocardiographic control of this patch could reduce interstage mortality.
The interstage morbidity is our key concern. Unfortunately, morbidity remains high in our series. Reoperations for restrictive pulmonary blood flow were frequent in the both groups.
Study limitations
This study has several limitations. This is a single-centre retrospective observational study comparing transannular path augmentation and modified Blalock–Taussig shunt, which may limit its generalisation to other surgical teams. Patients were propensity-matched to try to minimise differences in baseline pre-operative characteristics. This resulted in a relatively small sample size, which may influence the power of some analyses and increase the risk of a type II statistical error. Moreover, despite propensity-matching, we were not able to capture all disease-related, surgeon-specific, and procedure-specific details that may modify in the perioperative care and influence clinical outcomes. Thus, the propensity-matched was using only three parameters. After matching, there was significant difference for pulmonary size. The patients with transannular path augmentation were smaller pulmonary artery than modified Blalock–Taussig shunt, and the Delta Nakata could be more easily increased.
Conclusion
Despite smaller pulmonary arteries, transannular path augmentation is equivalent to modified Blalock–Taussig shunt for: biventricular repair rate and time to biventricular repair. The right ventricle to pulmonary artery connection is better for the rehabilitation of the native pulmonary artery.
The modified Blalock–Taussig shunt option remains acceptable and allows excellent rate of biventricular repair, when the right ventricle to pulmonary artery connection is not possible (i.e., coronary anomaly).
Acknowledgements
Acknowledgment to Christine Ferrer and Suzanne Jolly for the help you have given us for the development of the database.
Financial support
This research received no specific grant from any funding agency, commercial or not-for-profit sectors.
Conflict of interest
None.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guidelines on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008, and has been approved by the institutional committees (Assistance Publique Hopitaux de Marseille Protection des Données N° :2018-87). The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national guides on the care and has been approved by the institutional committee (Assistance Publique Hopitaux de Marseille Protection des Données N° :2018-87).