Introduction
Seed dispersal is considered an important process for determining the spatial structure and dynamics of plant populations (Nathan and Muller-Landau, Reference Nathan and Muller-Landau2000; Terborgh et al., Reference Terborgh, Pitman, Silman, Schichter, Nunez, Levey, Silva and Galetti2002). Through seed dispersal, seeds are able to reach new sites for colonization, which influences the demography, genetics, spatial distribution and future vegetation structure (Dennis and Westcott, Reference Dennis and Westcott2006; Levey et al., Reference Levey, Tewksbury and Bolker2008).
Successful seed dispersal typically encompasses the removal of seeds from a source tree to deposition in micro-sites where seeds can germinate and seedlings can establish (Janzen, Reference Janzen1970; Schupp, Reference Schupp1993; Jordano et al., Reference Jordano, Garcia, Godoy and Garcia-Castano2007; McConkey and Chivers, Reference McConkey and Chivers2007). Therefore, seed germination and seedling establishment depend on factors that occur at the deposition sites such as intra- and interspecific competition, post-dispersal seed predation and environmental variation (Nathan and Casagrandi, Reference Nathan and Casagrandi2004; Nathan, Reference Nathan2006). Environmental variation can occur at small scales through changes in biotic or edaphic conditions and at large scales through these factors and different climatic conditions (Joshi et al., Reference Joshi, Schmid, Caldeira, Dimitrakopoulos, Good, Harris, Hector, Huss-Danell, Jumpponen, Minns, Mulder, Pereira, Prinz, Scherer-Lorenzen, Siamantziouras, Terry, Troumbis and Lawton2001).
In addition, it has been shown that plants are often adapted to their local soil and climate (Grondahl and Ehlers, Reference Grondahl and Ehlers2008), and because of restrictive logistic limitations, only very few studies simultaneously document the effect of seed origin and deposition sites on seed germination and seedling survival (Chapman, Reference Chapman1989; Zeiter and Stampfli, Reference Zeiter and Stampfli2008). The interaction between seed deposition and micro-environment variation is strongly scale dependent because the variance in important environmental variables increases with distance (Bell and Lechowicz, Reference Bell and Lechowicz1991). Therefore, the spatial patterns of plant recruitment will depend on the distance dispersed (Nathan and Casagrandi, Reference Nathan and Casagrandi2004), on the scales of heterogeneity in the landscape (Joshi et al., Reference Joshi, Schmid, Caldeira, Dimitrakopoulos, Good, Harris, Hector, Huss-Danell, Jumpponen, Minns, Mulder, Pereira, Prinz, Scherer-Lorenzen, Siamantziouras, Terry, Troumbis and Lawton2001) and on the interaction between them.
Generally, studies of seed dispersal support the idea that ‘farther is better’ (see Jansen et al., Reference Jansen, Bongers and van der Meer2008 for a detailed list); however, there is still some debate about the overall effects of dispersal distance for seed germination and seedling survival as stated by the Janzen–Connell model (Hyatt et al., Reference Hyatt, Rosenberg, Howard, Bole, Fang, Anastasia, Brown, Grella, Hinman, Kurdziel and Gurevitch2003). For example, it is important to consider that, in general, as the spatial scale of dispersal increases, environmental conditions are expected to diverge from conditions at the origin, which may reduce individual fitness and disfavour germination success. Given this ambiguity in the benefits of short- versus long-distance dispersal, it seems necessary to determine site origin (where seeds are produced) and site deposition effects for seeds dispersed by vertebrates, to understand the spatial patterns of survival of the tree species they disperse (Nathan and Muller-Landau, Reference Nathan and Muller-Landau2000). This poorly understood issue is of particular importance when evaluating the impacts of future scenarios of climate change on seed dispersal and recruitment success (i.e. due to changes in soil moisture due to higher precipitation or more frequent droughts).
Manilkara zapota is a dominant tree species in the Yucatan Peninsula. It is economically important for the extraction of latex (Pennington, Reference Pennington1990) and more recently zapote trees have been used for timber and charcoal, which has increased its economic value. Due to the large seed size of M. zapota (from 16 to 23 mm long; Morton, Reference Morton and Morton1987), its dispersal likely depends on large animals, as seeds are too heavy to be dispersed by wind or ingested by small animals. In the Neotropics, tapirs (Tapirus bairdii) are the last remaining large terrestrial mammals and recent reports indicate that they are capable of dispersing large-seeded plants (Fragoso et al., Reference Fragoso, Silvius and Correa2003), such as M. zapota (O'Farrill et al., Reference O'Farrill, Calmé and Gonzalez2006).
The present study quantifies the effect of seed origin and deposition sites on the germination and recruitment probabilities of M. zapota in two environments within the habitat of the Baird's tapir. It was hypothesized that the spatial precipitation gradient and soil moisture conditions within the Greater Calakmul Region at origin and deposition sites would affect the germination probability and seedling survival when seeds are displaced over realistically large distances.
Methods
Study site
This research was conducted in the centre of the Yucatan Peninsula in southern Mexico near the Calakmul Biosphere Reserve in the Greater Calakmul Region (19°15′ to 17°50′N and 90°20′ to 89°00′W; Fig. 1). The climate of this region is classified as tropical with pronounced wet (June–October) and dry seasons; the annual precipitation is around 945 mm and the mean annual temperature is c. 22°C (Xuluc-Tolosa et al., Reference Xuluc-Tolosa, Vester, Ramirez-Marcial, Castellanos-Albores and Lawrence2003; Vester et al., Reference Vester, Lawrence, Eastman, Turner, Calmé, Dickson, Pozo and Sangermano2007). The Greater Calakmul Region is the largest expanse of mature, seasonal tropical forest in Mexico, and one of the last large remaining tropical forests in Mesoamerica (Vester et al., Reference Vester, Lawrence, Eastman, Turner, Calmé, Dickson, Pozo and Sangermano2007). This region is also considered a hot spot of tropical deforestation (Abizaid and Coomes, Reference Abizaid and Coomes2004). The Greater Calakmul Region hosts more than 1600 plant species, of which Lysiloma latisiliqua, Bursera simaruba, Cedrela odorata, Brosimum alicastrum and M. zapota represent some of the dominant plant species (Martínez and Galindo-Leal, Reference Martínez and Galindo-Leal2002).

Figure 1 Study site showing the location of deposition sites.
The Calakmul Region does not have rivers; the majority of the water runs underground and stores naturally in waterholes locally called aguadas (García-Gil et al., Reference García-Gil, Palacio and Ortiz2002). There is a west–east and north–south precipitation gradient across the Yucatan Peninsula (north to north-west Peninsula total ≤ 800 mm; east Peninsula total ≥ 1400 mm; White and Hood, Reference White and Hood2004). Therefore, there are wetter conditions to the east and south of the Peninsula than in the west and north (García, Reference García1965; White and Hood, Reference White and Hood2004). Recent work revealed that approximately 10% of the forest has been recently disturbed by human activities (Turner et al., Reference Turner, Villar, Foster, Geoghegan, Keys, Klepeis, Lawrence, Mendoza, Manson, Ogneva-Himmelberger, Plotkin, Salicrup, Chowdhury, Savitsky, Schneider, Schmook and Vance2001), that precipitation has decreased considerably over the past 40 years (Martínez and Galindo-Leal, Reference Martínez and Galindo-Leal2002) and that precipitation will continue to decrease based on global climate change models (McSweeney et al., Reference McSweeney, New and Lizcano2007). This decrease in precipitation directly influences the vegetation and the availability of water in this karst landscape, as much of the water percolates through the limestone soil with only a few waterholes maintained during the peak of the dry season.
In the Greater Calakmul Region, the Baird's tapir (T. bairdii) is the largest terrestrial mammal and a potential short- and long-distance seed disperser of large-seeded plants. In April 2006, we observed a maximum number of 42 M. zapota seeds in a single dung pile, with a mean number ( ± SD) of seeds per dung pile of 4.07 ± 5.36 seeds. In addition, previous studies suggest that tapirs are able to disperse viable zapote seeds to favourable places for germination (O'Farrill et al., Reference O'Farrill, Calmé and Gonzalez2006) and that germination probabilities do not differ when seeds are collected directly from zapote fruits or from tapir dung (O'Farrill et al., submitted). Individuals of lowland tapir (Tapirus terrestris) are known to move up to 13 km in a 24-h period in Peru (Tobler, Reference Tobler2008), indicating that tapirs can be effective long-distance dispersers of large seeds (Fragoso et al., Reference Fragoso, Silvius and Correa2003).
Study organism
M. zapota (zapote) is a dominant species in the Greater Calakmul Region and is among the co-dominant species in the upper storey of evergreen lowland forest and semi-evergreen forest (Pennington, Reference Pennington1990; Cruz-Rodriguez and Lopez-Mata, Reference Cruz-Rodriguez and Lopez-Mata2004; Weterings et al., Reference Weterings, Weterings-Schonck, Vester and Calmé2008). Its distribution encompasses the Pacific and gulf coasts of Mexico, the Yucatan Peninsula in particular, as well as Guatemala, northern Belize and the Atlantic coastal forest of Nicaragua (Morton, Reference Morton and Morton1987) and it appears to be native only in Mexico, Guatemala, Belize and the Atlantic coast forests of Nicaragua (Pennington, Reference Pennington1990). Zapote fruits have large seeds and therefore are likely to depend on large mammals for long-distance dispersal. In the Greater Calakmul Region, the fruits contain, on average, 2.00 ± 1.58 seeds per fruit and mean seed dimensions of 1.99 ± 0.26 cm length, 1.13 ± 0.26 cm width and 0.62 ± 0.17 cm thickness (n = 926). The combination of human exploitation of gum and fruits and the predation on fruits by animals seems to operate synergistically to inhibit the regeneration process of this species (Roldan and Simonetti, Reference Roldan and Simonetti2001). M. zapota is a plant species with a high economic and biological value, which has historically provided important ecosystem services to the people of the region (Fig. 2).

Figure 2 Manilkara zapota: tree (left), germinating seed (middle top), fruit (middle bottom), seedlings growing in tapir dung (top right) and experimental cages (bottom right). (A colour version of this figure can be found online at www.journals.cambridge.org/ssr).
Experimental design
In April 2008 a 12-month seed and seedling reciprocal transplant experiment was implemented within the tapir's habitat to evaluate M. zapota seed germination and seedling survival when deposited in two environments with measured differences in soil moisture levels. The collection and deposition site of each seed was recorded with a GPS (Garmin Ltd, Olathe, Kansas, USA), and distance between collection and deposition site was estimated with ESRI ArcMap 9.3 (ESRI Inc., Reston, Virginia, USA). A collection site corresponded to a small area within the zapote distribution area where zapote trees with mature fruits were found for the collection of seeds. The two deposition sites selected were found within the zapote distribution area located at 14 km from each other within the north-west to south-east rainfall gradient of the Calakmul Region. Within each site four sub-sites were selected, separated by 100 m for seed and seedling deposition (Fig. 1). Sub-sites had an average area of 421 ± 146 m2 (1SD). Both collection and deposition sites correspond to the mature forest type commonly visited by tapirs, while 14 km represents approximately the maximal travel distance reported (13 km) by tapirs in a day (Tobler, Reference Tobler2008). A recent study with captive lowland and Malayan tapirs showed mean fluid and particle retention times ranging from 25 to 81 h (Clauss et al., Reference Clauss, Lang-Deuerling, Müller, Kienzle, Steuer and Hummel2010). This suggests that within a day seeds ingested by tapirs can be deposited up to 13 km from their site of origin.
Climate data
Field observations from 2005 to 2008 indicated that the deposition site located in the north-east (‘wet site’ from here on) was characterized by higher humidity than the site located at the south-west (‘dry site’ from here on). To further quantify humidity, long-term precipitation and temperature data were obtained from the Comisión Nacional del Agua (National Water Commission of Mexico). These data showed a mean decrease in precipitation of 16% from 1950 to 2009 (Zoh Laguna weather station). To further monitor the differences in moisture availability between the sites, in April 2008 a Micro Hobo station (Onset computer corporation, Bourne, Massachusetts, USA), adapted with four data loggers, was placed in each deposition site to record variation in soil moisture (S-SMx-M005), air temperature (S-TMB-M022) and soil temperature (S-TMB-M022). The micro weather stations were placed in random locations within the sites and data were recorded every 10 min. One of the Hobo stations stopped functioning in February 2009 (after 10 months). Absolute differences between site means of soil moisture and air and soil temperatures were assessed to quantify ecological differences between sites. Air and soil temperature was similar between the wet and dry sites. Even though soil moisture was only measured in two sites without any replication, differences in mean soil water content were found between sites: dry site 0.04 ± 0.05 and wet site 0.17 ± 0.06 (m3/m3 ± 1SD).
Seed reciprocal transplant experiment
In April 2008 seeds were collected from mature zapote fruits found on the ground, close to seven zapote trees distributed to the north, south and close (called ‘middle’) to each deposition site. In the wet area, these collection sites were located at an average distance of 6.2 ± 3.05 km (1SD) from the wet deposition site, and collection sites of the dry area were at an average distance of 6.1 ± 5.37 km (1SD) from the dry deposition site. The mean number of seeds collected per tree ranged between 12 and 72 (average 48.1 ± 21.3, 1SD) in the wet area and between 13 and 149 in the dry area (average 45 ± 46.7, 1SD). Before planting, seeds from all trees at the same collection site were randomized to minimize bias due to maternal effects. In this transplant experiment, the tapir's long-distance seed dispersal was mimicked by moving and planting seeds at two sites observed to be visited by tapirs and separated by 14 km. Seeds were not planted at intermediate distances for logistic constraints and because the aim of this study was to compare seed and seedling recruitment at the maximum dispersal distance reported for tapirs. Seeds were collected directly from fruits rather than from tapir dung to be able to record the origin of seeds. The use of seeds extracted from fruits rather than from tapir dung did not influence the results since we have found that the two seed types have very similar germination probabilities (O'Farrill et al., submitted).
In each deposition site (dry and wet site), germination stations were placed in pairs, with each pair always corresponding to a collection site (north, middle or south) and an origin site (dry or wet) combination (i.e. north–dry site and north–wet site). The locations of the germination stations were selected randomly with 1–5 m between them. Depending on the availability of seeds per collection site, 3–5 seeds were planted in each germination station, which corresponded to the average number of zapote seeds found in tapir dung in the Calakmul Region (O'Farrill, pers. obs.). Seeds were planted directly in the soil at a depth of 1 cm and with at least 2 cm between them. The area where the germination stations were placed was checked for other M. zapota seeds (from the seed bank) which, given their size, were easily observed and removed. The germination stations were covered with a cylindrical wire cage of 20 × 15 cm with a wire mesh of 1 cm to avoid predation by small predators, such as mice (Peromyscus yucatanicus), doubled with a mosquito net covering the first lower 15 cm of the cage. Although the mesh size allows ants and beetles to enter, it is not large enough to permit the removal of seeds. Even though dung beetles and ants might move or bury the seeds within the cages, influencing their germination success, by keeping the seeds within the cages their germination success could be recorded. In total, 625 seeds were planted across the two sites; 313 seeds were placed in 82 cages in the four sub-sites of the wet deposition site and 312 seeds in 83 cages in the four sub-plots of the dry deposition site, with 20 cages on average per sub-site (see Table 1 for the distribution of seeds per collection and origin site).
Table 1 Distribution of seeds planted per origin site

Seedling reciprocal transplant experiment
In April 2008, 401 zapote seedlings were collected around six trees in each deposition site, with an average of 33.3 ± 6.5 seedlings (1SD) per tree in the wet site and 33.3 ± 7.5 seedlings (1SD) per tree in the dry site. Seedlings with more than two leaves were selected to facilitate survival after transplantation. Those collected in the wet site had an average height of 5.5 ± 1.5 cm (1SD) and average number of 4.5 ± 1.0 leaves (1SD), while seedlings collected in the dry site had an average height of 6.6 ± 1.6 cm (1SD), and average number of 4.8 ± 2.9 leaves (1SD). Seedlings were removed from the soil with a small shovel and with extreme care not to cut the roots. If the roots were damaged during the process other seedlings were chosen and planted. Soil attached to the roots was kept until transplantation. Seedlings were kept humid until planted with a delay that varied between 40 and 48 h.
In the dry and wet sites germination stations were placed in pairs, with seedlings from each origin site (dry and wet sites) kept together. Extra soil from the origin site was removed and two seedlings were planted per cage directly in the soil from the deposition site. Supplemental watering was provided once seedlings were planted, to minimize transplant shock. In total, 160 seedlings from each origin site were planted in each deposition site, randomized in four sub-sites. At the end of the experiment all the seedlings were measured to evaluate differences in growth based on the deposition site. Seedlings grew less than 2 cm.
Data collection
All the sites were visited 1 and 3 months after planting (May and July 2008) and after a year, in April 2009, to monitor the status of the seeds, seedlings and cages. During each visit emergence from planted seeds and planted seedling survival were recorded. After a year, seeds were recorded as germinated if a seedling developed, even if it died some time after germination. A seedling was reported as ‘survived’, if it had not died 1 year after planting. The height of all the seedlings was measured. All the cages were removed, but seedlings were left in the field for a long-term survival study.
Data analysis
All statistical tests were conducted using R 2.70 (R Development Core Team, 2005). The effects of origin site (wet and dry), deposition site (wet and dry) and their interaction on the germination of seeds were analysed, and the effects of origin and deposition site on seedling survival, with a hierarchical mixed model. In all cases a generalized mixed model function lmer (lme4 package) was used. Collection site (north, middle and south), sub-site and cage were included as random effects. Where applicable, collection site, sub-site and cage were nested hierarchically. Sub-site, cage, collection, origin and deposition site were treated as categorical variables. The following were tested: whether plant germination and survival decreased when seeds or seedlings were planted in a new location that showed differences in soil moisture; and whether seed germination varied when seeds were collected from different collection sites (north, middle, south) and planted in different sites that show similar or different soil moisture conditions. The response variable was binomial: germinated or not germinated and survived or not survived. The proportions of seeds germinated and seedlings survived were compared with a two-sample binomial test (prop. test in R).
Results
Reciprocal seed transplant experiment
After 1 year 61% of the seeds germinated (381 seeds out of 625 seeds). A significant effect of deposition site on germination (z = − 2.63, P = 0.008) was observed; seeds planted in the dry deposition site germinated less (56.7%) than seeds planted in the wet site (65.2%) (χ2 = 4.33, df = 1, P = 0.037). Seeds collected in the wet conditions did not germinate with equal probability in the wet and dry sites (χ2 = 6.87, df = 1, P = 0.008), exhibiting higher germination probabilities when planted in a site with similar soil moisture conditions (68.5%) to their site of origin than when planted in a dryer site (53.7%). Seeds collected in the dry area did not show a significant difference in germination when planted in sites with wetter or similarly dry moisture conditions (Table 2, Fig. 3). Cage nested in sub-site and collection site explained 49% of the variation of the random effects, while sub-site nested in collection site explained 51%.
Table 2 Germination and survival probabilities of seeds and seedlings by origin and deposition site±SE (SE refers to probabilities of individual seeds/seedlings)
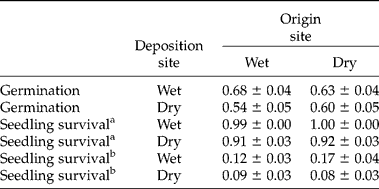
a Seedling survival: results from seed transplant experiment.
b Seedling survival: results from seedling transplant experiment.

Figure 3 Mean germination and seedling survival ± SE by origin and deposition sites.
In the wet deposition site, no significant effect of seed origin on germination (z = − 0.402, P = 0.687) was found, suggesting that seed germination did not differ when seeds originated from the dry or wet environment. The nested term ‘sub-site’ in collection site explained 100% of the variation of the random effects. Seeds collected from the three collection sites in the wet site (north, middle, south) showed significant germination differences (χ2 = 15.71, df = 2, P = 0.0003) when planted in the wet deposition site (north>middle>south) and higher overall probabilities of germination, but no differences were observed for germination of seeds planted in the dry site (Table 3). In particular, seeds collected in the most northerly locality of the wet site germinated significantly more when planted in the wet environment (93.7%) than in the dry environment (59.4%). Seeds collected in the south locality of the wet site germinated less than seeds from the north and middle localities when planted in the dry (south 44% < north 59% < 61% middle) or wet sites (south 53.7% < middle 70.6% < north 93.7%).
Table 3 Germination probability of seeds by origin and deposition site±SE

In the dry deposition site, no significant effect of the origin site on germination was found (dry or wet; z = 1.19, P = 0.233). Cage nested in sub-site and collection site explained most of the variation of the random effects (69%). Seeds obtained from the three collection sites of the dry area did not show significant differences in germination when planted in the dry (χ2 = 0.186, df = 2, P = 0.911) or wet sites (χ2 = 4.229, df = 2, P = 0.121; Table 3); overall, seeds germinated only 3% more when planted in the wet site than in the dry site (Table 2). Seeds collected to the south of the dry site germinated less than seeds from the middle and north origin sites irrespective of their deposition site (south 51% < north 63% < middle 88% in wet deposition site and south 59% < middle 62% < north 63% in dry deposition site). Seeds collected in the middle of the dry site germinated 26% more when planted in the wet deposition site (17 km away from their collection site) than in the dry deposition site (4 km away from their collection site); showing statistically significant differences (χ2 = 5.258, df = 1, P = 0.02).
After a year, 93.7% of the seedlings that emerged survived, which corresponds to 57.1% of the total number of seeds planted. There was a significant effect of deposition site, with higher seedling survival when planted in the wet site than in the dry site (z = − 3.085, P = 0.002), irrespective of their origin (Table 2, Fig. 3). Sub-site nested in collection site explained 55% of the variation of the random effects while sub-site explained 45% of the variation. The length of seedlings after 1 year showed a significant effect of deposition site (z = − 2.64, P = 0.008), with sub-site explaining 100% of the variation in the random effects.
Reciprocal seedling transplant experiment
One year after sowing, only 11.4% of the transplanted seedlings survived (46 out of 401) suggesting a transplantation shock. There were no significant effects of origin (wet or dry; z = 0.151, P = 0.880) or deposition site (wet or dry; z = − 0.342, P = 0.732) on seedling survival (Table 2). The mean survival of seedlings planted in the wet site was higher than seedlings planted in the dry site, irrespective of their origin site; however, these differences were not significant (origin: wet site, χ2 = 0.692, df = 1, P = 0.405; origin: dry site χ2 = 02.925, df = 1, P = 0.08).
Discussion
In this study, the origin of seeds (wet, dry, or north, middle, south) showed a strong influence on germination probabilities. For example, seeds collected in the north of the wet area germinated 40% more than seeds collected at the south of the wet area when both were planted in the same wet deposition site. This might indicate maternal or origin differences across local scales; however, the experimental design does not allow discrimination between these differences.
In addition, deposition sites had a strong influence on seed germination and seedling survival. For example, more seedlings from the seed and seedling transplant experiments survived in the wet than in the dry site. The effect of deposition sites on germination suggests that even within relatively small regions (sites found within 14 km from each other) the displacement of seeds far from parent trees does not ensure their survival (Hyatt et al., Reference Hyatt, Rosenberg, Howard, Bole, Fang, Anastasia, Brown, Grella, Hinman, Kurdziel and Gurevitch2003) and that seeds need to be deposited in places with favourable environmental conditions for germination (Schupp, Reference Schupp1993; Jordano and Schupp, Reference Jordano and Schupp2000; Hansen et al., Reference Hansen, Kaiser and Müller2008). The vast majority of seed dispersal studies assume biotic factors are the major determinants of seedling viability; this study suggests that in some cases this is not true and that the trade-off between the importance of biotic and abiotic factors is dependent on spatial scale.
Local adaptation and seed dispersal
The present results show that germination probabilities had different patterns depending upon the origin and deposition sites. If seeds were moved from a wet to a dry site there was an expected decrease in germination, while seeds moved from a dry to a wet site did not differ; this was an unexpected result. In addition, a degree of local adaptation of the wet-site-originated seeds (within the same soil moisture conditions) was found, because the probability of seed germination was greatest at the wet than at the dry site (Byars et al., Reference Byars, Papst and Hoffmann2007; Zeiter and Stampfli, Reference Zeiter and Stampfli2008). Although these results should be interpreted cautiously, given the lack of replication on the soil moisture measurements, this study suggests that the moisture gradient affects the success of establishment, and that the fitness costs of moving across this gradient are direction dependent. The effects of environmental heterogeneity on the distribution and fitness of animal-mediated seed species are poorly understood but merit further work.
In a recent study, Hansen et al. (Reference Hansen, Kaiser and Müller2008) found strong negative effects of proximity to maternal trees for seedling growth and survival in the critically endangered endemic Mauritian tree Syzygium mamillatum. Their results reinforce our understanding of the benefits of short-distance dispersal, but the present results suggest that conclusions from these types of experiments cannot be extrapolated to long-distance events (Muller-Landau et al., Reference Muller-Landau, Levin and Keymer2003; Bullock and Nathan, Reference Bullock and Nathan2008). Short-distance dispersal is advantageous because it might reduce the intraspecific competition and pathogens associated with conditions close to the parent tree (Janzen, Reference Janzen1970; Hyatt et al., Reference Hyatt, Rosenberg, Howard, Bole, Fang, Anastasia, Brown, Grella, Hinman, Kurdziel and Gurevitch2003; Jansen et al., Reference Jansen, Bongers and van der Meer2008). However, long-distance dispersal might also result in the deposition of seeds close to adult trees of the same species, resulting in intraspecific competition and predation. Although adult trees were present at the deposition sites in the present study, the cage exclosures ensured that seedling survival was not affected by predation as predicted by the Janzen–Connell Model (Janzen, Reference Janzen1970; Connell, Reference Connell, Den Boer and Gradwell1971).
At the individual level, the fitness advantages of dispersal are expected to decline after a short distance from the parent's crown. Under long-distance dispersal seed survival probability is affected by increasing environmental variance at larger spatial scales (Bustamante and Simonetti, Reference Bustamante and Simonetti2000; Muller-Landau et al., Reference Muller-Landau, Levin and Keymer2003), which will, in general, decrease seed fitness (Bell and Lechowicz, Reference Bell and Lechowicz1991). The distribution of fitness costs associated with seed dispersal is likely to be complicated for plant species exhibiting a broad range of seed dispersal distances. Most studies on distance effects have only focused on short-distance movements ( < 300 m) (Hyatt et al., Reference Hyatt, Rosenberg, Howard, Bole, Fang, Anastasia, Brown, Grella, Hinman, Kurdziel and Gurevitch2003). We suggest that seed dispersal studies, especially on trees whose dispersal is mediated by large mammals, should assess the fitness of seeds deposited across a range of spatial scales (i.e. at the maximum extent of animal home-range movements). The effect of dispersal distance per se should also be evaluated in conjunction with the abiotic effects of origin and deposition sites. For example, a comparison between frugivore species with different dispersal distances but with similar microhabitat preferences for deposition sites would allow the study of dispersal distance in combination with the effects of origin and deposition sites on germination and plant recruitment (Jordano et al., Reference Jordano, Garcia, Godoy and Garcia-Castano2007; Bradford and Westcott, Reference Bradford and Westcott2010). Transplant experiments like the one presented here are necessary to control some of the many potentially confounding factors that can complicate interpretation of comparative studies.
Conclusion
This study focused on the effects of seed origin and deposition sites on seed germination and seedling survival. It revealed that differences between origin and deposition sites of seeds, which may be due to soil moisture differences, affected seed germination and seedling survival in the field. In addition, this study underscores the value of reciprocal transplant field experiments. It should be emphasized that these experiments should be conducted over variable spatial and temporal scales that are relevant to different dispersers and the tree species in question. In the case of the Calakmul Region, changes in animal movements in response to trends in precipitation and water availability, may influence dispersal of M. zapota seeds, and the overall recruitment of zapote populations at the regional scale.
Acknowledgements
This study was carried out with the financial support of Idea Wild, National Science and Engineering Research Council from Canada (NSERC). The Consejo Nacional de Ciencia y Tecnología-México (CONACyT) supported G.O. through a doctoral fellowship. A.G. acknowledges support from the NSERC Discovery grant and the Canada Research Chair Program. We are grateful to the people of Nuevo Becal and Zoh Laguna. Nicolas Arias, Selvin Hernandez, Victor Bacab, Ana Chi and Kim Gauthier Schampaert provided invaluable assistance during fieldwork. Sophie Calmé provided helpful suggestions for improving the experiment. We thank Sophie Calmé and Martin Lechowicz for their suggestions on a previous version of this manuscript. Kim Gauthier Schampaert drew Fig. 1.








