The Constitution of the Slovak Republic guarantees all its inhabitants universal and free access to a wide package of basic health care covered by the public health insurance system. All residents are insured and they are obliged to pay a contribution to the public health insurance fund, which is operated by several health insurance companies. In Slovakia, a pluralistic system of health insurance companies is in place, and in 2016, three health insurance companies operated in the Slovak market. According to the Health Care Surveillance Authority (1), the state-owned Všeobecná zdravotná poisťovňa (general health insurance company) covered 63.31 percent of the insured population in 2015, while the privately owned Dôvera and Union health insurance companies covered 27.92 percent and 8.77 percent, respectively.
Szalay et al. (Reference Szalay, Pažitný and Szalayová2) maintain that the Slovak healthcare system is based on universal coverage, compulsory health insurance, a basic benefit package, and a competitive insurance model with selective contracting and flexible pricing.
According to Barnieh et al. (Reference Barnieh, Manns and Harris3), the Slovak Republic met the highest standard of the three criteria deemed important in the process of reimbursement for drugs, including consideration of clinical and cost evidence, full transparency, and the presence of a formal appeal mechanism.
METHODS
We performed a comprehensive analysis of the Slovak legislation on health technology assessment (HTA) of medicines. Furthermore, we focused on the reimbursement process and its local specifications. Additionally, we aimed at evaluating the current practices of the Working Group for Pharmacoeconomics, Clinical Outcomes, and HTA of the Slovak Ministry of Health.
RESULTS AND DISCUSSION
Pricing and Reimbursement of Medicinal Products
In Slovakia, a single application is filed for both the pricing and the reimbursement of medicinal products. However, in the following section the two processes are described separately. The required dossiers for the assessments provide the registration holders of the new medicine. These include basic drug information (name, manufacturer, authorization holder, pharmaceutical form, package size, and strength), evidence on its effectiveness, the standard therapeutic dose and the number of standard therapeutic doses per package. Applications also contain the proposed reimbursement rate, medical indication and restriction of prescription, and/or medical indication, if applicable.
Slovakia has implemented a reference pricing system for medicines. Based on this system, a maximum price is set for a standard daily dose (SDD) in each specific reference group of medicines. All medicines included in such a reference group (identical five-digit Anatomical Therapeutic Chemical [ATC] classification system code) contain the same active substance per dose and are administered in the same form. Additionally, internal reference pricing applies for some therapeutic groups, which form internal reference groups of medicines that have the same molecular structure (identical four-digit ATC code). Likewise, a maximum price for an SDD of medicines belonging to the same internal reference group is defined. As a result, prices of medicines with different active substances are linked to the least expensive alternative within an internal reference group. Changes in price for a particular medicine, thus, may influence the reimbursement of other medicines in the same external as well as internal reference group.
After a medicine receives market authorization, the Ministry of Health of the Slovak Republic determines its “maximum retail price” (ex-factory price) applying external reference pricing methodology. The ex-factory price of each medicine available on the Slovak reimbursement list may not exceed the average of the three lowest prices of the same medicine available on pharmaceutical markets across the European Union (EU).
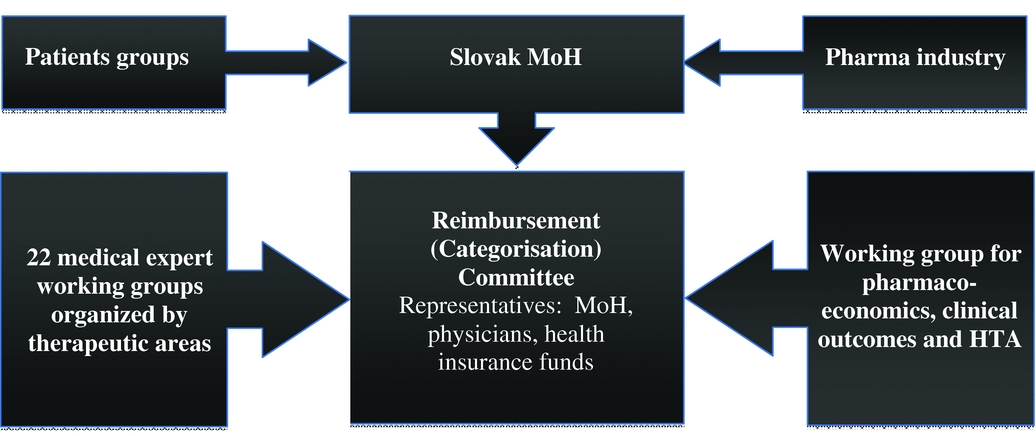
The Slovak Ministry of Health established the Reimbursement (or Categorization) Committee to act as its advisory body in regards to reimbursement processes. The Categorization Committee consists of three representatives from the Ministry of Health, three representatives from the Slovak Medical Chamber, and five representatives from health insurance companies. The Categorization Committee is supported by different advisory working groups, a medical board (assessing the effectiveness, safety and importance of the medicine), and the Working Group for Pharmacoeconomics, Clinical Outcomes, and HTA of the Ministry of Health as presented in Figure 1.

Figure 1. Categorization process of pharmaceuticals in Slovakia.
The Committee Prepares Recommendations for Reimbursement Levels, Patients’ Co-payments, and Conditions for Reimbursement
The decision about actual reimbursement levels of medicines eligible for reimbursement is based on the following criteria: therapeutic benefit of the medicine, cost-effectiveness, and the reimbursed levels of other medicines within the same reference group. The final reimbursement (or categorization) list also includes medicines with prescription or medical indication restrictions. Prescription restrictions limit the reimbursement of medicines only to prescription by certain specialists. Medical indication restrictions limit the reimbursement of medicines only to the prescription for specific medical indications, which might be narrower than specified by the Summary of Product Characteristics. In the case of certain medicines, the reimbursement can also be restricted to prescription solely in specialized hospitals.
Based on the recommendations from the Categorization Committee, the Ministry of Health issues final decisions. There is always at least one medicine available in determined therapeutic classes with no co-payment.
The final reimbursement decisions about new medicinal products are taken by the Slovak Ministry of Health within 180 days from submission of the reimbursement dossiers by manufacturers based on the Transparency Directive (Council Directive 89/105/EEC).
Kaló et al. (Reference Kaló, Docteur and Moïse4) maintained that several potentially not cost-effective pharmaceuticals had been reimbursed in Slovakia before 2008, especially because the strategic pricing of innovative medicinal products is not based on small markets with low purchasing power (such as the Slovak pharmaceutical market). The price level of new drugs is adjusted to meet the requirements of wealthier countries with greater willingness to pay for a quality-adjusted life-year (QALY).
Since 2011, new legislation regulating pricing and reimbursement of medicines, medical devices, and dietetic food has been in force in the Slovak Republic. Based on the evaluation of the medicine's effectiveness, safety, and importance as well as its economic benefits, the Categorization Committee determines the therapeutic and social value of the medicine as summarized in Table 1.
Table 1. Criteria Determining the Therapeutic and Social Value of Medicines (Reference Szalay, Pažitný and Szalayová2)

Concerning economic and legal dimensions, there is a basic threshold defined in the legislation, reflecting the benefits displaced elsewhere in the Slovak healthcare system when funds are allocated to new medicines.
Act No. 363/2011, coll. (5) provides that pharmacoeconomic reports are mandatory in the decision process on reimbursement of medicinal products. As stated by Decree No. 422/2011 of the Ministry of Health (6), the decision within the drug reimbursement process requires a pharmacoeconomic analysis (cost minimization, cost-utility, or cost-effectiveness analysis) and budget impact analysis. A special working group focuses on assessing the therapeutic value of the evaluated medicine, while a separate working group evaluates the pharmacoeconomic analysis. The latter working group assesses incremental costs incurred from public health insurance for one unit of health improvement due to the effects of the appraised medicine in comparison to the standard of care. Afterward, based on the thresholds (commonly described with the Greek letter “λ”) set forth by Act No. 363/2011 Coll. (5), the group decides about the reimbursement of the medicine. The thresholds are defined as follows: lower threshold (λ1): 24 times average monthly salary; upper threshold (λ2): 35 times average monthly salary.
The medicine is reimbursed from public health insurance (fully or partially) if the incremental costs are lower or equal to λ1 per one QALY. The medicine is conditionally reimbursed if the incremental costs lie within λ1 and λ2 per one QALY. Tesar (Reference Tesar7) elaborates that the submission for reimbursement in Slovakia has to include pharmacoeconomic analysis for all new molecules, new indications, as well as new galenic forms of medicines.
There is no doubt that economic evaluations of medicines should support and guide the decision-making process in terms of strengthening evidence-based decisions. The availability of professional, comprehensive, and well-structured information enables decision makers to issue informed decisions based on evidence, contributing to an efficient resource allocation.
The Slovak guidelines for pharmacoeconomic analysis (8) are published on the Web site of the Ministry of Health. These methodological guidelines guide the authorization holders and healthcare providers in preparing health economic evaluation to support submissions securing reimbursement for medicines covered by public health insurance.
The ultimate objective of the guidelines is to facilitate cost-effective use of scarce healthcare resources. The general principle is that the analysis should adopt the perspective of the audience targeted by the authors of the studies. For the reimbursement process from public insurance funds, the perspective of health insurance companies is mandatory. In the case of studies with a time horizon longer than 1 year, the principles of time preference as well as the opportunity costs of investments should be considered using discounting. In the basic case scenario, both future health gains and costs are discounted by 5 percent. When a more effective therapy has higher costs than an alternative intervention, the incremental cost-effectiveness ratio must be calculated.
Medicines whose additional costs per QALY were below the lower threshold (λ1), which is €20,592 per QALY in 2016, could be included in the reimbursement list. Medicines whose additional cost per QALY were between the lower and upper threshold (λ1 and λ2), which is between €20,592 and €30,030 per QALY in 2016, could be included in the reimbursement list with condition(s). Medicines whose additional costs per QALY exceeded the upper threshold (λ2), which is €30,030 per QALY in 2016, could not be included in the reimbursement list.
Van Wilder et al. (Reference Van Wilder, Mabilia, Kuipers Cavaco and McGuinn9), based on specific patient access schemes available in the Slovak Republic, concluded that thresholds for additional costs per QALY are a tool for assessing cost-effectiveness of medicines and not a rule for excluding medicines from public reimbursement.
This principle is included in the Slovak legislation. Some highly innovative medicines whose additional costs exceed λ2, but for which there are no currently available (effective) alternative medicines on the pharmaceutical market, can be still reimbursed from public health insurance funds. Exceptions can be issued based on specific patient access schemes for certain innovative medicines. Such schemes are negotiated directly between the authorization holders of the innovative medicines, which are not included in the reimbursement list, and the health insurance companies. Usually, price discounts or price limits are offered in exchange for public reimbursement. Furthermore, these thresholds are not applicable for orphan drugs indicated in the therapy of rare diseases with prevalence in Slovakia lower than 1:100,000.
HTA
HTA is still a relatively recent concept in Slovakia and the country is in the early stage of its implementation. In 2012, the Working Group for Pharmacoeconomics, Clinical Outcomes and Health Technology Assessment of the Slovak Ministry of Health was established.
The Working Group represented the Slovak Republic in the European Network for Health Technology Assessment Joint Action 2 (EUnetHTA JA 2). Furthermore, one member of the Working Group operated also within the European HTA Network, defined by Directive 2011/EU/24. The participation of Slovakia in the EUnetHTA JA 3 and the European HTA Network has a potential to facilitate the dissemination of HTA activities. Projects of the EUnetHTA JA 2 with the participation of Slovak Ministry of Health are presented in Table 2. Efficient use of HTA is starting to appear in the decision-making processes in Slovakia.
Table 2. EUnetHTA JA 2 Projects with the Participation of Slovak Ministry of Health

Based on the Slovak experience in the European collaboration, we can conclude that Rapid Relative Effectiveness assessments prepared at the EU level are very useful for Slovakia's healthcare system. There is no doubt that several dimensions of the HTA Core Model® are context specific. Cost and economic evaluation of the HTA Core Model® are especially sensitive issues for Slovakia. However, the current status concerning organization of HTA in Slovakia can be described as intermediate. Further legislative activities in this field are required and expected as a result of the approved strategy for EU Cooperation on HTA. Reuse of Joint Work in the field of HTA will be an important factor for Slovak activities. The term “Joint Work” means activities in which countries and/or organizations work together to prepare shared products or agreed outcomes. These may include, for example, literature reviews, structured information for rapid or full HTAs, early dialogues or scientific advice on research and development planning and study design.
We have identified five main domains of practical barriers to implementation of HTA in the Slovak Republic, which are summarized and briefly elaborated in Table 3. We have found three key factors of success of implementation of HTA in the Slovak Republic: results, quality of reimbursement processes, and acceptance. In terms of results, it can be seen that participation within EUnetHTA JA 2 and EUnetHTA JA 3 projects has significantly increased the quality of HTA processes in Slovakia.
Table 3. Domains of Practical Barriers of HTA Implementation in the Slovak Republic

The majority of decision makers in Slovakia accept and support the idea of increased use, quality, and efficiency of HTA production. In Slovakia, several tools developed at the EU level are used for production of selected HTA, including the HTA Core Model®, Methodological Standards for HTA, training material, and Information and Communication Technology tools (including the existing Planned and Ongoing Projects [POP] database and EVIDENT Database).
CONCLUSIONS
Results of HTAs are widely accepted by Slovak decision makers. The most recent Slovak legislation, introduced in 2011, implemented the requirement of providing an HTA as a mandatory part of applications for public health insurance reimbursement. Further legislative activities in this field are required in Slovakia as a result of the approved strategy for EU Cooperation on Health Technology Assessments. Such action is crucial to overcoming several practical barriers to progress in the field of HTAs within the Slovak Republic.
CONFLICTS OF INTEREST
The authors declare that they have no conflict of interest.