Introduction
Autism is characterized by abnormalities in communication, social interaction, and repetitive and stereotyped behaviors, which are typically observed before 3 years of age (American Psychiatric Association, 2000). Individuals diagnosed with an autism spectrum disorder (ASD) may have difficulties with language (delayed or stereotyped), initiating and maintaining conversations, processing facial expressions and emotions, recognizing social cues and displaying appropriate social behavior, and initiating and maintaining friendships. They may also have atypically intense interests and/or display repetitive behaviors. These symptoms may manifest in a multitude of combinations, range of severities, and in conjunction with comorbid diagnoses. The precise etiology of ASD remains unknown; however, it is postulated that a combination of genetic, developmental, and environmental factors may contribute to the presence of the disorder (Muhle, Trentacoste, & Rapin, Reference Muhle, Trentacoste and Rapin2004).
Recent research suggests that behavioral and/or pharmaceutical interventions may be effective (to varying degrees) in remediating ASD-related difficulties (for review, see Myers & Johnson, Reference Myers and Johnson2007). For example, Beversdorf, Carpenter, Miller, Cios, and Hillier (Reference Beversdorf, Carpenter, Miller, Cios and Hillier2008) recently reported improved verbal problem solving performance in individuals with ASD following administration of propranolol, a beta-adrenergic antagonist. In the present study, we sought to further this line of research and examine the possible effects of propranolol on other aspects of executive function, an area of cognitive ability that is particularly affected in individuals with ASD (for review, see Hill, Reference Hill2004).
Executive Function, Prefrontal Cortex, and ASD
Executive function refers to a set of higher-order cognitive processes that allow for the flexible modification of thought and behavior in response to changing cognitive or environmental contexts (Stuss & Benson, Reference Stuss and Benson1986). The ability to plan actions, inhibit prepotent responses and ignore distracting information, maintain and manipulate information in working memory, and focus and shift attention are all integral parts of directing and maintaining behavior. These processes are considered “executive” in that they guide and sustain goal-directed behavior.
From a neuroanatomical standpoint, the prefrontal cortex (PFC) of the brain appears to play a particularly critical role in supporting executive function (Stuss & Benson, Reference Stuss and Benson1986). Consistent with this, injury to the PFC and/or its network of connections is associated with problems with executive function processes, such as working memory and inhibitory control (e.g., Milner & Petrides, Reference Milner and Petrides1984; Shallice, Reference Shallice1982). Neuroimaging studies have also confirmed the PFC is consistently activated in neurologically intact individuals during performance of executive function tasks (e.g., Buchsbaum, Greer, Chang, & Berman, Reference Buchsbaum, Greer, Chang and Berman2005). In addition, childhood improvements in executive function performance appear to mirror the maturational trajectory of the PFC (e.g., Adleman et al., Reference Adleman, Menon, Blasey, White, Warsofsky, Glover and Reiss2002).
Building upon observations that individuals with acquired PFC damage and individuals with ASD appear to share some common symptomatology (e.g., repetitive behaviors, difficulty switching from one task to another), researchers (e.g., Damasio & Maurer, Reference Damasio and Maurer1978; Russell, Reference Russell1997) have proposed that disruptions of the PFC may contribute to the cognitive and social difficulties experienced by individuals with ASD. Additional support for this assertion comes from more recent studies documenting structural, functional, and metabolic abnormalities in PFC of individuals with ASD (Chugani et al., Reference Chugani, Muzik, Rothermel, Behen, Chakraborty, Mangner and Chugani1997; Levitt et al., Reference Levitt, Blanton, Smalley, Thompson, Guthrie, McCracken and Toga2003; Ohnishi et al., Reference Ohnishi, Matsuda, Hashimoto, Kunihiro, Nishikawa, Uema and Sasaki2000; Salmond, de Haan, Friston, Gadian, & Vargha-Khadem, Reference Salmond, de Haan, Friston, Gadian and Vargha-Khadem2003). A growing number of studies (for reviews, see Hill, Reference Hill2004; Kenworthy, Yerys, Anthony, & Wallace, Reference Kenworthy, Yerys, Anthony and Wallace2008) have also reported ASD-related impairments in PFC-mediated executive abilities such as cognitive flexibility (e.g., Ozonoff, Strayer, McMahon, & Fillouz, Reference Ozonoff, Strayer, McMahon and Fillouz1994; Pennington & Ozonoff, Reference Pennington and Ozonoff1996) and inhibitory control (e.g., Christ, Holt, White, & Green, Reference Christ, Holt, White and Green2007; Christ, Kester, Bodner, & Miles, Reference Christ, Kester, Bodner and Miles2011).
Working memory is another aspect of executive function that relies heavily on the PFC (Owen, McMillan, Laird, & Bullmore, Reference Owen, McMillan, Laird and Bullmore2005) and is potentially affected in individuals with ASD. Previous behavioral findings on working memory and ASD have been mixed with some studies reporting comparable working memory performance in individuals with and without ASD (e.g., Koshino et al., Reference Koshino, Carpenter, Minshew, Cherkassky, Keller and Just2005, Reference Koshino, Kana, Keller, Cherkassky, Minshew and Just2008), and other studies reporting evidence of ASD-related impairment on tests of working memory (e.g., Joseph, McGrath, & Tager-Flusberg, Reference Joseph, McGrath and Tager-Flusberg2005; Steele, Minshew, Luna, & Sweeney, Reference Steele, Minshew, Luna and Sweeney2007). While the full nature of these discrepant findings remains unclear, there does appear to be a general trend toward ASD-related impairments in working memory being most evident on complex tasks that place a relatively greater demand on working memory (Williams, Goldstein, & Minshew, Reference Williams, Goldstein and Minshew2006; Williams, Goldstein, Carpenter, & Minshew, Reference Williams, Goldstein, Carpenter and Minshew2005). In addition, findings from functional neuroimaging studies suggest that the pattern of working memory-related neural activation and functional brain connectivity observed for individuals with and without ASD may differ even in the absence of differences in behavioral performance (e.g., Koshino et al., Reference Koshino, Carpenter, Minshew, Cherkassky, Keller and Just2005, Reference Koshino, Kana, Keller, Cherkassky, Minshew and Just2008).
Norepinephrine and PFC Functioning
The PFC is also sensitive to the effects of neurochemical substances such as norepinephrine (NE; for review, see Arnsten, Reference Arnsten1998). In addition to its role as a hormone, NE serves as an important neurotransmitter within the central nervous system, with noradrenergic neurons projecting from the locus coeruleus to several cortical regions, most notably the PFC (Berridge & Waterhouse, Reference Berridge and Waterhouse2003). Changes in central NE levels are known to impact PFC and thereby executive function (Arnsten & Li, Reference Arnsten and Li2005).
Whereas relatively low-to-moderate levels of the neurotransmitter NE can help to enhance PFC functioning by focusing attention and improving executive function (Aston-Jones, Rajkowski, & Cohen, Reference Aston-Jones, Rajkowski and Cohen1999; Coull, Frith, Dolan, Frackowiak, & Grasby, Reference Coull, Frith, Dolan, Frackowiak and Grasby1997), hyper-activation of the NE system can negatively impact PFC function. For example, individuals believed to be experiencing abnormally high NE levels related to acute cocaine withdrawal have been found to demonstrate impaired performance on tasks of cognitive flexibility, verbal fluency, and verbal memory (Kelley, Yeager, Pepper, & Beversdorf, Reference Kelley, Yeager, Pepper and Beversdorf2005).
Psychopharmacological studies provide additional evidence of the role of NE in higher-order cognitive abilities. For example, Coull, Middleton, Robbins, and Sahakian (Reference Coull, Middleton, Robbins and Sahakian1995a) reported impairments in sustained attention in human subjects following administration of therapeutic levels of clonidine, an alpha-2 NE receptor agonist. Clonidine has also been shown to have dose-dependent effects on other aspects of cognition such as working memory and visuospatial learning (Coull, Middleton, Robbins, & Sahakian, Reference Coull, Middleton, Robbins and Sahakian1995b; Jäkälä et al., Reference Jäkälä, Riekkinen, Sirviö, Koivisto, Kejonen, Vanhanen and Riekkinen1999).
More recent research, including a study by Alexander, Hillier, Smith, Tivarus, and Beversdorf (Reference Alexander, Hillier, Smith, Tivarus and Beversdorf2007), has shown that pharmacological-modulation of activity at beta-adrenergic receptor sites may also influence cognition. In the aforementioned study, researchers examined the effects of the beta-adrenergic antagonist propranolol (vs. placebo) on cognitive performance under conditions of high and low psychosocial stress. (Psychosocial stress was manipulated by having participants engage in a task involving public speaking and mental arithmetic.) Results of the study showed circumscribed, stress-induced impairments in performance on tasks (e.g., verbal anagram test), which place demands on cognitive flexibility and verbal problem solving. Importantly, administration of propranolol was found to attenuate the extent of the observed impairment. Subsequent research by Campbell, Tivarus, Hillier, and Beversdorf (Reference Campbell, Tivarus, Hillier and Beversdorf2008) further suggests that such effects of propranolol on cognition may also vary as a function of task difficulty. Lastly, administration of propranolol has also been shown to decrease the extent of cognitive impairment experienced by individuals undergoing acute withdrawal from cocaine (Kelley, Yeager, Pepper, Bornstein, & Beversdorf, Reference Kelley, Yeager, Pepper, Bornstein and Beversdorf2007).
Norepinephrine and ASD
The majority of evidence supporting a potential role of NE in ASD comes from psychopharmacological studies. Many such studies of ASD and NE have focused on the impact of alpha-2 adrenergic receptor agonists (e.g., clonidine and guanfacine) and beta-adrenergic antagonists (e.g., propranolol) on PFC and its function (Fankhauser, Karumanchi, German, Yates, & Karumanchi, Reference Fankhauser, Karumanchi, German, Yates and Karumanchi1992; Jaselskis, Cook, Fletcher, & Leventhal, Reference Jaselskis, Cook, Fletcher and Leventhal1992; Posey, Puntney, Sasher, Kem, & McDougle, Reference Posey, Puntney, Sasher, Kem and McDougle2004; Ratey et al., Reference Ratey, Bemporad, Sorgi, Bick, Polakoff, O'Driscoll and Mikkelsen1987). Findings from early research supported a potential beneficial effect of beta-adrenergic antagonists in reducing aggression, and improving socialization, in adults with ASD (Ratey et al., Reference Ratey, Bemporad, Sorgi, Bick, Polakoff, O'Driscoll and Mikkelsen1987). Subsequent research suggests that the benefits of pharmaceutical modulation of the NE system (via beta-adrenergic antagonists) in individuals with ASD may extend to cognitive function as well (Beversdorf et al., Reference Beversdorf, Carpenter, Miller, Cios and Hillier2008). Beversdorf and colleagues examined the effect of propranolol on performance during verbal problem solving tasks (easy anagrams) in individuals with and without ASD. ASD participants’ performance improved with the administration of propranolol, whereas non-ASD participants performed more poorly.
It is also worth noting that findings from non-pharmacological studies of NE have been much more equivocal. For example, consistent with a disruption in the noradrenergic system, early studies reported increased levels of NE in the plasma (Lake, Ziegler, & Murphy, Reference Lake, Ziegler and Murphy1977) and urine (Barthelemy et al., Reference Barthelemy, Bruneau, Cottet-Eymard, Domenech-Jouve, Garreau, Lelord and Pyrin1988) of individuals with ASD. It has been since argued, however, that these findings could have been due to a stress reaction in the participants with ASD to obtaining blood and urine samples rather than due to ASD itself (Minderaa, Anderson, Volkmar, Akkerhuis, & Cohen, Reference Minderaa, Anderson, Volkmar, Akkerhuis and Cohen1994). A neuroanatomical study of the locus coeruleus, the primary origin of noradrenergic projections to the cortex (Berridge & Waterhouse, Reference Berridge and Waterhouse2003), yielded no significant differences in the structure in individuals with ASD as compared to healthy non-ASD individuals (Martchek, Thevarkunnel, Bauman, Blatt, & Kemper, Reference Martchek, Thevarkunnel, Bauman, Blatt and Kemper2006).
The Current Study
The current study builds upon the previously described psychopharmacologic research (Beversdorf et al., Reference Beversdorf, Carpenter, Miller, Cios and Hillier2008) and is designed to further examine the role of NE in executive function in individuals with ASD. Specifically, we investigated the performance of individuals with and without ASD on an AX continuous performance test (AX-CPT) following administration of the beta-adrenergic antagonist propranolol versus a placebo. The AX-CPT is a well-established task of continuous performance that examines working memory and inhibitory control (Rosvold, Mirsky, Sarason, Bransome, & Beck, Reference Rosvold, Mirsky, Sarason, Bransome and Beck1956), and has been used to reliably evaluate executive dysfunction in a variety of clinical populations, such as schizophrenia (Barch, Carter, MacDonald, Braver, & Cohen, Reference Barch, Carter, MacDonald, Braver and Cohen2003; Riccio, Reynolds, Lowe, & Moore, Reference Riccio, Reynolds, Lowe and Moore2002).
In a standard AX-CPT (including the one used in the present study), participants are shown a series of letters one at a time. They are instructed to press a “target” button when they see a cue letter X that immediately follows a probe letter A (i.e., an AX trial). For all other stimulus combinations, they must press a “non-target” button. AX trials comprise a large portion (70%) of the overall task thus creating a strong tendency toward responding with the target button. Participants’ ability to inhibit this prepotent tendency is particularly evident on the small portion (10%) of the remaining trials in which the cue letter A is followed by a probe letter other than X (i.e., an AY trial). On another portion (10%) of the non-target trials, a cue letter other than A is followed by the probe letter X (i.e., a BX trial). Performance on this trial type is hypothesized to assess working memory to the extent that failure to remember the cue letter's identity (i.e., not an A) may result in mistakenly pressing the target button when the probe letter X is presented. The remaining non-target trials (10%) comprise trials in which a cue letter other than A followed by a probe letter other than X (i.e., BY trial). Performance on BY trials, as well as on the AX target trials, reflects participants’ generally ability to maintain the task instruction set and attend to the stimuli throughout the task.
Based on past findings of executive function impairments in individuals with ASD (Hill, Reference Hill2004), we anticipate that the group of participants with ASD will demonstrate impaired AX-CPT performance as compared to non-ASD individuals. In addition, we hypothesize that down-regulation of the NE system (via administration of propranolol) will result in attenuation of any observed executive function impairments in individuals with ASD.
METHOD
Participants
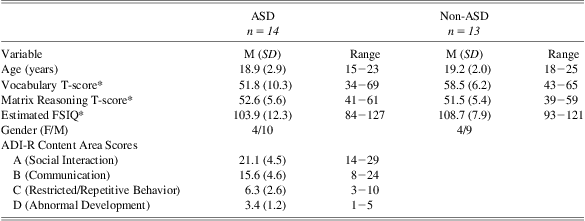
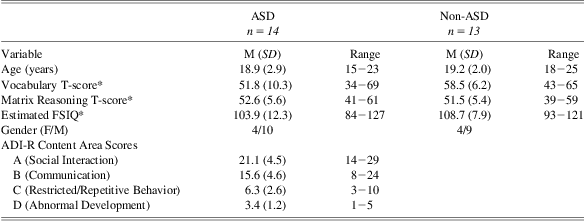
A sample of 14 participants with ASD (mean age = 18.9 years; 71% male) and a comparison group of 13 healthy participants without ASD (mean age = 19.2 years; 69% male) participated. Additional sample characteristics are included in Table 1.
Participants with ASD were patients receiving clinical services at the University of Missouri Thompson Center for Autism and Neurodevelopmental Disorders, an interdisciplinary academic medical center specializing in diagnosis and treatment of ASD. Diagnostic interviews, caregiver questionnaires, and observation focusing on DSM-IV criteria (American Psychiatric Association, 2000) were used for the diagnosis of ASD in these individuals. The diagnosis of ASD was further confirmed using the Autism Diagnostic Interview-Revised (ADI-R; Lord, Rutter, & Le Couteur, Reference Lord, Rutter and Le Couteur1994). Of the 14 participants with ASD, 7 met criteria for Autistic disorder and 7 met criteria for Asperger's disorder. Individuals with a history of learning disorders or major medical disorders unrelated to ASD were excluded. Participants in the comparison group were recruited from the local Columbia, Missouri, community.
A total of 24 individuals with ASD were approached and screened for participation in the current study, and 14 individuals were willing and able to participate (58%). A similar participation rate was found in the comparison group with 21 individuals approached and screened for participation, and 13 were willing and able to participate (62%).
The two groups were found to differ slightly in terms of educational attainment (mean number of years of education completed: ASD = 11.4 years; Control = 12.9 years), t(24) = 2.37, p = .03. Participants completed the Wechsler Abbreviated Scale of Intelligence (WASI; Psychological Corporation, 1999) to estimate full scale IQ (FSIQ). All participants had an estimated FSIQ > 80, and no significant differences were observed in FSIQ between the ASD and comparison groups (mean FSIQ = 103.9 and 108.7, respectively), t(25) = 1.19, p = .25. Neither educational level nor FSIQ correlated with task performance in either pharmaceutical condition (p > .05 in all instances). Consequently, these variables were not considered further.
Procedure
The present study was approved by the University of Missouri-Columbia Internal Review Board Study and completed in accordance with the Helsinki Declaration. Informed consent was obtained for all participants in the present study, and an assent was obtained for all participants under 18 years in addition to obtaining a parental consent. Participants attended two testing sessions over the course of a week. Each participant received 40 mg of propranolol upon arrival at one testing session, and a placebo (sugar) pill at the other session. The medication/placebo was administered to participants in a double blinded manner, with the order of drug administration counterbalanced within each group. Following a 60-min delay (to allow for complete absorption and maximization of plasma levels), participants completed the AX-CPT.
AX-CPT
The task apparatus and procedures were similar to what has been described previously (Barch et al., Reference Barch, Carter, MacDonald, Braver and Cohen2003). In brief, participants were seated in front of a computer monitor in a well-lit, sound-attenuated room. The sequence of trial events is shown in Figure 1. Each trial began with the presentation of a cue letter for 500 ms. Following a 5000-ms delay (blank screen), a probe letter was then presented for 500 ms. Letter stimuli subtended approximately 1.8 vertically and 1.3 horizontally.

Fig. 1 An example of the sequence of events on two consecutive trials of the AX-CPT. Trial 1 displays the target pair condition (AX) and Trial 2 displays the working memory condition (BX).
Participants were instructed to respond as quickly as possible by pressing a button with their right middle finger when the probe letter X followed the cue letter A. For all other cue-probe pairs (e.g., a letter A followed by an S; a letter F followed by an X), participants responded by pressing a button with their right index finger. Response time and accuracy were recorded. After an inter-trial interval of 3000 ms, a new trial was presented.
Following explanation of the task instructions, participants were administered a block of 15 practice task trials. Performance during the practice trials was closely monitored by the examiner and additional prompting was given (as needed) so as to ensure full understanding of the task before initiation of the experimental trials. After successful completion of the practice trials, participants completed 150 experimental trials. AX cue-probe stimuli were presented on 70% of the task trials. The remaining 30% of trials were split evenly across 3 additional conditions: (1) the AY condition in which the cue letter A was followed by a letter other than X, (2) the BX condition in which the cue was a letter other than A but the probe was X, and (3) the BY condition in which neither the cue nor probe were A or X. The conditions were mixed randomly. At intervals of 30 trials, participants were offered a break.
Results
One participant with ASD was unable to perform the task. Mean response time (RT) and error rate data for the remaining 26 participants are shown in Figures 2 and 3, respectively. Trials on which an error occurred were excluded from the RT analysis. Data were analyzed separately for each condition (AX, AY, BX, and BY) using a repeated measures analyses of variance (ANOVA) with medication (placebo and propranolol) serving as a within subjects factor and group (ASD and non-ASD comparison) serving as a between subjects factor.

Fig. 2 Mean error rates (%), shown separately for drug (placebo and propranolol), task condition (AX, BX, AY, and BY), and group (ASD and non-ASD). Error bars represent standard error of the mean.

Fig. 3 Mean response time (%), shown separately for drug (placebo and propranolol), task condition (AX, BX, AY, and BY), and group (ASD and non-ASD). Error bars represent standard error of the mean.
Working Memory (BX condition)
Consistent with the presence of ASD-related working memory difficulties, the ASD group made more errors in the BX condition as compared to the non-ASD group, F(1,24) = 11.2, p < .05, ηP 2 = .31 (mean error rate = 18.5% and 3.1%, respectively). A main effect of medication and an interaction between medication and group were also seen, Fs(1,24)>5.5, ps < .05, ηP 2 > .18 in both instances. Additional analysis confirmed that these effects were driven primarily by the fact that propranolol treatment was associated with significant improvements in working memory (i.e., decreased number of BX errors) for the ASD group (mean error rate for propranolol and placebo conditions = 7.2% and 18.5%, respectively), t(12) = 2.58, p < .05, Cohen's d = .72. A similar benefit, however, was not observed for the non-ASD group (mean error rate for propranolol and placebo conditions = 3.1% and 3.1%, respectively), t(12) < 1, p > .05. Analysis of the RT data for the BX condition did not reveal any significant main effects or interaction, Fs(1,24) < 1, ps > .6, ηP 2 < .01, in all instances.
Inhibitory Control (AY Condition)
Performance of individuals with ASD did not differ significantly from that of the comparison group in the AY condition, F(1,24) = 1.6, p = .21, ηP 2 = .06. The interaction between medication and group was also not significant, F(1,24) = 2.7, p = .11, ηP 2 = .10. The administration of propranolol did not appear to improve inhibitory performance for either group (mean AY error rate for propranolol and placebo conditions: ASD group, mean error rate = 14.9% and 15.4%, respectively; non-ASD group = 9.7% and 4.6%, respectively). Similarly, RT analysis for the AY condition did not reveal any significant main effects or interaction, Fs(1,24) < 1, ps > .5, ηP 2 < .02, in all instances.
General Sustained Attention Ability (AX & BY Conditions)
In the target condition (AX), there was a non-significant trend toward a higher error rate among individuals with ASD as compared to the non-ASD group (mean error rate = 6.2% and 3.4%, respectively), F(1,24) = 3.8, p = .06, ηP 2 = .14. The propranolol treatment did not significantly influence AX error rate for either group (mean error rate for propranolol and placebo conditions: ASD group = 5.9% and 6.2%, respectively; non-ASD group = 2.6% and 3.4%, respectively), as indicated by a lack of main effect of medication and interaction between medication and group, Fs(1,24) < 1, ps > .6, ηP 2 < .01 in both instances.
In the BY condition, individuals with ASD again made more errors than the non-ASD comparison group, F(1,24) = 8.9, p < .05, ηP 2 = .27 (mean error rate = 4.1% and 0.5%, respectively). However, neither a main effect of medication nor interaction between medication and group was observed (mean error rate for propranolol and placebo conditions: ASD group 2.6% and 4.1%; non-ASD group 0.0% and 0.5%), Fs(1,24) < .1, ps > .3, ηP 2 < .04 in both instances. Analysis of RT in the AX and BY conditions did not reveal any significant main effects or interactions, Fs(1,24) < 1, ps > .5, ηP 2 < .02, in all instances.
Discussion
A previous study reported improvements in cognitive flexibility during a verbal problem solving task in individuals with ASD following administration of propranolol, a beta-adrenergic antagonist (Beversdorf et al., Reference Beversdorf, Carpenter, Miller, Cios and Hillier2008). In the current study, we used an AX continuous performance test to explore whether this effect would generalize to other executive function processes such as working memory and inhibitory control, which are critical for proper day-to-day functioning.
Participants with ASD were found to have performed more poorly than a non-ASD comparison group on the BX condition of the AX-CPT. Poor performance in this condition can arise from problems with maintenance and/or recall of information from working memory. Specifically, failure to maintain the cue identity (i.e., a letter other than A) in working memory during the cue-probe delay can result in a participant responding to the probe letter X incorrectly (i.e., he/she might respond as if it had been an AX trial). The finding of ASD-related impairment in this condition is consistent with previous studies reporting working memory difficulties in individuals with ASD (Hill, Reference Hill2004).
Individuals with ASD also performed more poorly than their non-ASD counterparts in the BY condition of the task. In addition, there was a trend (p = .06) toward poorer performance by the ASD group in the target (AX) condition as well. These findings may point toward a general ASD-related difficulty in the ability to sustain attention to the task.
Of interest, performance of ASD group did not differ significantly from that of the non-ASD group in the AY condition, which assesses inhibitory control. [The inhibitory demands associated with this condition arise from the fact that 7 of 8 times (88%) when the cue letter is the letter A, it is followed by the probe letter X. As such, when the cue letter A is followed by a probe letter other than X (i.e., the AY condition), participants must inhibit the prepotent tendency to respond as if it were an AX trial.] The present study follows several previous studies that have also failed to find evidence of ASD-related impairments in prepotent response inhibition (e.g., Christ et al., Reference Christ, Kester, Bodner and Miles2011).
Effect of Propranolol Administration
Most importantly, administration of propranolol resulted in a marked improvement in working memory performance (i.e., an increase in accuracy from 82% to 93% on BX trials) for individuals with ASD. This benefit was observed for only the ASD group. Propranolol had no apparent effect on BX trial performance for the non-ASD group; it also did not appear to affect performance in any other task condition (AX, AY, BY) for either group. The present finding of improved working memory performance following propranolol administration is in line with previous studies (Beversdorf et al., Reference Beversdorf, Carpenter, Miller, Cios and Hillier2008; Ratey et al., Reference Ratey, Bemporad, Sorgi, Bick, Polakoff, O'Driscoll and Mikkelsen1987) documenting cognitive and behavioral improvements following propranolol treatment in this population.
Propranolol is as a noradrenergic antagonist, blocking action on both β1- and β2-adrenergic receptors, thus resulting in post-synaptic down regulation of the noradrenergic system. Taken together with past reports of higher-than-normal NE levels in individuals with ASD (Barthelemy et al., Reference Barthelemy, Bruneau, Cottet-Eymard, Domenech-Jouve, Garreau, Lelord and Pyrin1988; Lake et al., Reference Lake, Ziegler and Murphy1977), the present finding of improved working memory performance following administration of propranolol is consistent with the hypothesis that ASD is associated with abnormalities of the noradrenergic system. Indeed, cognitive improvements following administration of propranolol have been documented in other clinical populations associated with increased noradrenergic activation (e.g., Kelley et al., Reference Kelley, Yeager, Pepper, Bornstein and Beversdorf2007).
Although the precise mechanism underlying the observed improvements in working memory performance remains unclear, one possibility is that propranolol may affect brain function by increasing the relative signal-to-noise ratio (SNR) of neural activity between cortical regions (Hasselmo, Linster, Patil, Ma, & Cekic, Reference Hasselmo, Linster, Patil, Ma and Cekic1997). Consistent with this notion, a recent pilot neuroimaging study by Narayanan et al. (Reference Narayanan, White, Saklayen, Scaduto, Carpenter, Abduljalil and Beversdorf2010) reported that propranolol treatment was associated with increased functional brain connectivity in individuals with ASD during performance of a phonological task. Of particular relevance to the current study, past neuroimaging studies (e.g., Just, Cherkassky, Keller, Kana, & Minshew, Reference Just, Cherkassky, Keller, Kana and Minshew2007) have reported decreased frontal-parietal functional connectivity in individuals with ASD during performance of a working memory task. Building on these past studies, we soon plan to investigate the potential link between propranolol-related improvements in working memory and potential increases in functional brain connectivity in individuals with ASD.
Whereas the presence of a NE disruption in individuals with ASD is the most parsimonious explanation for the current findings, other possibilities exist. It may be that noradrenergic dysregulation is not the primary cause of the neurocognitive impairment observed for individuals with ASD, but rather manipulation of the noradrenergic system via pharmacological intervention (as was done in the present study) could be compensating for unrelated disruptions in other neurotransmitter systems (e.g., gamma-aminobutyric acid/GABA: Dhossche et al., Reference Dhossche, Applegate, Abraham, Maertens, Bland, Bencsath and Martinez2002; glutamate: Purcell, Jeon, Zimmerman, Blue, & Pevsner, Reference Purcell, Jeon, Zimmerman, Blue and Pevsner2001; serotonin: Rapin & Katzman, Reference Rapin and Katzman1998) and/or neurophysiologic irregularities in regions that do not receive an abundance of NE projections (e.g., basal ganglia; Nickl-Jockschat et al., Reference Nickl-Jockschat, Habel, Maria Michel, Manning, Laird, Fox and Eickhoff2011). For example, down-regulation of the NE system via projections to working memory-related brain regions (e.g., prefrontal cortex, hippocampus, amygdala) may be compensating in part for the functional impact of a principal abnormality in other neurotransmitter systems (e.g., GABA). Future research is clearly necessary to better delineate the role of the NE system and propranolol in the etiology and potential treatment of ASD.
Additional Limitations
The present sample size provided sufficient statistical power to detect ASD-related impairment in working memory and the positive impact of propranolol administration on performance in individuals with ASD. An additional concern with smaller sample sizes, however, is the ability to generalize findings to the broader population. The current study focused on higher functioning adults on the autism spectrum (FSIQ range: 84–127). Future research using larger, better characterized samples of individuals with ASD will be critical in determining to what extent factors such as age, overall levels of cognitive and adaptive functioning, and socioeconomic status may moderate the effect of beta-adrenergic modulation on working memory and other neurocognitive processes.
As detailed above, propranolol administration had no apparent effect on task performance for the non-ASD group. It is worth noting, however, that the group's generally high level of performance on the task (e.g., mean error rate for BX condition = 3.1%) may have precluded our ability to detect any propranolol-related effects. Additional research, possibly using a more challenging task, is necessary to fully evaluate whether working memory performance in healthy typically developing individuals may benefit from propranolol administration as well.
Conclusions
In summary, the present findings suggest that individuals with ASD experience difficulties in working memory. Furthermore, the magnitude of these difficulties appeared to lessen with the administration of propranolol, a beta-adrenergic antagonist. This finding is consistent with past studies reporting cognitive (Beversdorf et al., Reference Beversdorf, Carpenter, Miller, Cios and Hillier2008) and behavioral (Ratey et al., Reference Ratey, Bemporad, Sorgi, Bick, Polakoff, O'Driscoll and Mikkelsen1987) improvements in individuals with ASD following administration of beta-adrenergic antagonists. Of note, the observed benefit did not appear to extend to either (1) participants without ASD or (2) non-impaired aspects of functioning (e.g., inhibitory control). Future cognitive and neurophysiological research is necessary to better understand the nature of the present findings and their potential clinical relevance. Regardless, the current study, along with the handful of related studies on adrenergic modulation in ASD, provides a strong foundation for these efforts.
Acknowledgments
This research was supported in part by a Research Scholar grant from the Thompson Center for Autism and Neurodevelopmental Disorders (D.Q.B., S.E.C.) and the Department of Radiology Research Investment Fund at the University of Missouri (D.Q.B.). The authors would also like to thank Stephen Kanne and Steve Hackley for their helpful comments on earlier drafts of this manuscript. No authors had any potential conflicts of interest. This manuscript is dedicated to the memories of Patricia Reiser and Joshua Morgano (K.E.B.).