Introduction
The conversion of seaweed beds to sea urchin barrens has been reported in temperate rocky reefs in both the northern and southern hemispheres (Steneck et al., Reference Steneck, Graham, Bourque, Corbett, Erlandson, Estes and Tegner2002; Filbee-Dexter & Scheibling, Reference Filbee-Dexter and Scheibling2014). In some places, such regime shifts have been accompanied by a hysteresis effect of approximately one order of magnitude in urchin biomass, which has maintained barrens between the critical threshold of overgrazing and recovery (Ling et al., Reference Ling, Scheibling, Rassweiler, Johnson, Shears, Connell, Salomon, Norderhaug, Pérez–Matus, Hernández, Clemente, Blamey, Hereu, Ballesteros, Sala, Garrabou, Cebrian, Zabala, Fujita and Johnson2015). Sea urchin barrens can persist in the absence of seaweeds because surviving urchins can shift their food source to less nutritious microalga or invertebrates growing on the open rocky substrates (Ling & Johnson, Reference Ling and Johnson2009), or acquire floating algae, algae fragments and detritus via their spines and tube feet (Campbell et al., Reference Campbell, Dart, Head and Ormond1973; Russo, Reference Russo1980; Filbee-Dexter et al., Reference Filbee-Dexter, Fredriksen, Norderhaug, Rinde, Kristiansen, Albretsen and Wernberg2020). Previous research has shown that predation pressure by sea urchins with high population densities continues to exceed seagrass growth and prevent recovery of kelp forest (Sivertsen Reference Sivertsen1997; Filbee-Dexter & Scheibling, Reference Filbee-Dexter and Scheibling2014), and that the absence of sea urchin predators by fisheries prevents seaweed recovery (Johnson et al., Reference Johnson, Ling, Ross, Shepherd and Miller2005). On the other hand, abiotic factors that control sea urchin populations and promote seaweed beds recovery remain poorly understood.
In tropical waters, it is known that Echinostrephus aciculatus and Echinometra mathaei bore pits on coral reef (Russo, Reference Russo1980) and Stomopneustes variolaris inhabit pits. The pits of S. variolaris are utilized by small organisms such as snails and crabs (Chanket & Wangkulangkul, Reference Chanket and Wangkulangkul2019) as refugia. In the warm temperate coasts of southern Japan, persistent sea urchin barrens have been observed in intertidal rocky reefs, where sea urchins inhabit pits bored on the rocky substrate. In pits, mainly three species of sea urchins dwell: Echinostrephus molaris, Anthocidaris crassispina and Echinometra sp. B. Echinostrephus molaris has been considered to be borers of these pits, because the shapes of the urchin tightly fit the pit (Yamamori & Kato, Reference Yamamori and Kato2017), and as mentioned above E. aciculatus is also known to bore pits on coral reef (Russo, Reference Russo1980). Because nearly all of these excavated pits are occupied by these urchins, seaweeds (e.g. Sargassum hemiphyllum and Sargassum thunbergii) around the pits are subject to continuous and severe overgrazing by these urchins, which also consume floating seaweed fragments (Yamamori & Kato, Reference Yamamori and Kato2017). In contrast with the subtidal barrens that form in the kelp forest ecosystems of cold temperate coasts and macroalgal beds in temperate coasts (Hernández et al., Reference Hernández, Clemente, Sangil and Brito2008), some barrens in West Pacific warm temperate coasts with soft bedrock are characterized by the coexistence of pit-inhabiting sea urchins and a high diversity of symbiotic biota within pits, particularly the commensal limpet-like trochid snail, Broderipia iridescens (Yamamori & Kato, Reference Yamamori and Kato2017). In these intertidal barrens, there are no sea urchins outside pits, and sea urchin density is determined by the number of pits. It is known that pit-inhabiting urchins seldom move out from their pits even if the conspecifics living outside pits move around. The pit-inhabiting urchins develop heavier gonads than the ones outside pits, which is considered to be caused by immobility and saving resources utilized for spine growth inside narrow pits (Yusa & Yamamoto, Reference Yusa and Yamamoto1994). In that study, it is also considered that pits are protected areas which reduce predation risks. Thus, sea urchins may be most successful by foraging inside or in the close vicinity of their pits.
Juvenile sea urchins are typically absent from intertidal barrens. Sea urchin larvae are likely to settle and grow in subtidal areas with pebbly substrates; adults may later migrate to the intertidal zone, as has been observed in Mediterranean urchins (Fernandez et al., Reference Fernandez, Caltagirone and Johnson2001). Accordingly, sea urchin populations in barrens could be limited by the number of pits, the mortality rate of pit occupants, and recruitment from the subtidal zone. Urchin mortality can be influenced by harsh environmental conditions, such as unusually low temperature (Tokioka, Reference Tokioka1963; Barnes et al., Reference Barnes, Crook, O'Mahoney, Steele and Maguire2001, Reference Barnes, Verling, Crook, Davidson and O'Mahoney2002) and possibly ocean acidification (Brothers et al., Reference Brothers, Harianto, McClintock and Byrne2016), whereas recruitment is limited by reproduction and the growth of juveniles.
To investigate the population among three sympatric sea urchin species in an intertidal barren, we monitored pit occupancy in 2017–2019 at a warm temperate rocky reef in Shirahama, Japan.
An unusually cold event in February 2018 led to mass sea urchin mortality. Nearly half of the pits were vacant after the cold event, although they were almost entirely occupied originally. Thus, this event provided an opportunity to test whether the extent of pit occupancy affects the population and behaviour of urchins, by comparing their migration and recruit rate.
We also monitored the population of B. iridescens. This snail is an obligate commensal of the two pit-borrowing species assessed here, and has been observed migrating with their urchin hosts in aquaria (Yamamori & Kato, Reference Yamamori and Kato2018). To date, the population dynamics of sea urchin symbionts have only been assessed with regard to a symbiotic idoteid isopod (Stebbins, Reference Stebbins1989). Therefore, we assessed the role of sea urchin pits in intertidal barren ecosystems and the implications of regime shifts from seaweed beds to barrens by monitoring the three urchin species Echinostrephus molaris, Anthocidaris crassispina, Echinometra sp. B and the snail Broderipia iridescens.
Materials and methods
Study location
The study location was the rocky intertidal zone around the Seto Marine Biological Laboratory of Kyoto University in Shirahama, Wakayama Prefecture, Japan (Figure 1B, 33°69′51″N 135°33′58″E). The area is influenced by the warm Kuroshio Current, and the seawater temperature ranged between 11.5 and 27.8 °C throughout the study period, as measured at a nearby Kyoto University Shirahama aquarium 400 m away from the study site. For calibration, the seawater temperature at the study site on the survey day each month and the temperature measured at the aquarium on the same day are shown in Table S1. The study site was located at the mouth of Tanabe Bay, where variation in the spring tide is ~200 cm. The shore bedrock is comprised of conglomerate and brittle sandstone (Mii, Reference Mii1962) (Figure S1) and the rocky tide pools are heavily pitted by round-bottomed holes that are inhabited by sea urchins (Figure 1C–D). We selected two tide pools (P1 and P2) in the intertidal rock bed for sea urchin population monitoring. Both pools were 40 cm above the tide line and oval in shape. They were 1.79 and 2.00 m2 in size, had water depths at low tide of 42 and 68 cm, and had 295 and 217 pits, respectively.

Fig. 1. (A) Schema of interactions between organisms in warm temperate rock reefs. (B) Landscape view of the study site showing P1 and P2, (C) sea urchins in P1, (D) Echinostrephus molaris in a pit, (E) Broderipia iridescens in a sea urchin pit, (F) dead sea urchins at the bottom of P1 immediately after the cold event, and (G) E. molaris thrusting their bodies outward from the pit and wandering Echinometra sp. B in tide pool at night (indicated by the arrowhead). Scale bars: (B) 1 m; (C) 10 cm; (D) 3 cm; (E) 1 cm; (F) 10 cm; (G) 5 cm.
Monitoring of pit occupancy and sea urchin behaviour
We focused on one boring sea urchin (Echinostrephus molaris) and two non-boring, i.e. pit-borrowing, urchins (Anthocidaris crassispina and Echinometra sp. B). We performed census surveys of both pools between May 2017 and May 2019 (9 months before the cold event and 16 months after that). At the beginning of the study period, all pits were photographed and numbered and their water depth was measured. Every month, each of the species of pit-occupying sea urchins were recorded to estimate the migration rate. If the species of urchins differ in two consecutive months, movement of urchins took place, however, it is important to note that if there were the same species in the pit, we could not distinguish the individual and then the migration rate would be underestimated. Figure 2 presents a two-dimensional map of pits on the pool walls. In addition to pool monitoring, we surveyed urchins in the subtidal zone by skin diving once a month from June to October in 2017 and 2018. Finally, to describe the behaviour of urchins at night, we performed a behavioural observation survey in the field at low tide during the night on 17 December 2017.
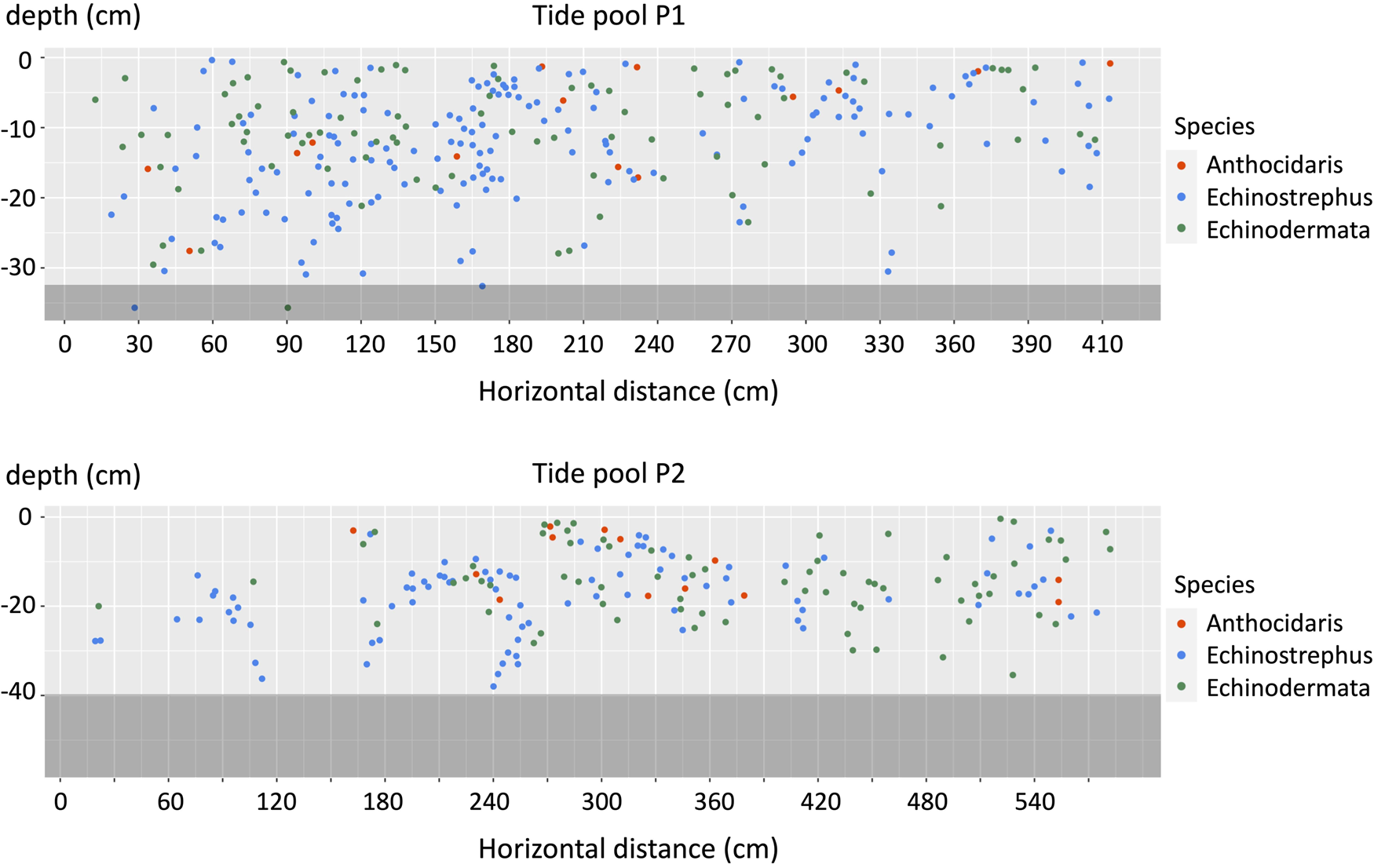
Fig. 2. Two-dimensional map of sea urchin pits on the walls of P1 and P2 at the beginning of the study period. The horizontal distance begins at the southmost points of the tidepools, and the shading (dark grey part) represents areas with a gentle slope.
In May 2017, February 2018 and July 2018, we carefully examined 20 pits occupied by each of the three sea urchin species (60 pits in total) by carefully removing the occupant and counting the number of B. iridescens in each pit, to determine the symbiosis rate. We also measured the shell length of each snail. Unfortunately, Echinometra sp. B was absent from both pools following the cold event in February 2018. Thus, we only included pits of the remaining two species in analyses.
Population structure of pit-inhabiting sea urchins
To assess patterns in the distribution of pits, we performed a spatial pattern analysis using a linear regression method (Iwao & Kuno, Reference Iwao and Kuno1968). We further quantified the relationship between the mean crowding index (m*) and mean density (m) using the equation below.
α: index of basic contagion, β: density-contagiousness coefficient.
Changes in pit occupancy can be assessed using a transition matrix representing the probability of moving from pit i to pit j in a one-month period (Pi,j,), where the monthly status of pits was recorded in categories representing occupancy by three species of sea urchins, A. crassispina, Echinometra sp. B and E. molaris, or vacant. We estimated two transition matrices for two distinct time periods in this study: before and after the cold event of February 2018. The retention rates of the pits’ occupants were calculated by dividing the number of months the pits were occupied by the observation period (24 months), and the turnover rates were calculated by dividing the number of months in which the transition of occupants occurred by the observation period. Generalized linear mixed model (GLMM) with tide pool (P1/P2) as a random effect was used to identify whether the turnover rate was different before and after the cold event.
Results
Distribution of sea urchin pits
Sea urchin pits were found on the side walls, but not the bottoms, of both P1 and P2. The mean water depth of pits were −16.5 cm (SD = ± 9.40) and −11.7 cm (SD = ± 7.91) in P1 and P2. The majority of pits occurred at depths <30 cm, and the deepest pits were observed at depths of 36 cm in P1 (total depth of 42 cm) and 56 cm in P2 (total depth of 68 cm). No pits were found above the ebb tide line, but some pits were found just below it. Total pit density, i.e. within both pools, was 164.7 m−2. The spatial pattern analysis indicted that pits had an aggregated distribution. In the spatial pattern analysis proposed by Iwao & Kuno (Reference Iwao and Kuno1968), the slope (β) indicates aggregated/random distribution (1 < β: aggregated, β = 1: random). As a result of the analysis, the equation m* = 1.50 m–0.875 was derived, which indicates aggregated distribution.
Sea urchin behaviour at low tide
Sea urchins retreated to the bottom of the pits during the day (Figure 1D). At night, they thrust their bodies slightly outward from the pit and actively moved their spines and tube feet to gather drifting seaweed (Figure 1G). We also observed a number of urchins wandering in tide pools at night (Figure 1G). At low tide, since there were no large fishes in the tide pool, the predation pressure is likely to be low. In contrast, at high tide, when fish are able to access the pits, sea urchins tended to remain in the innermost part of their pits.
Population of pit-inhabiting sea urchins
At the beginning of the study period, 294 and 212 sea urchins were observed in the 295 pits in P1 and 217 pits in P2, respectively. All occupied pits were inhabited by only one urchin, and few urchins were observed outside of pits at night. All urchins were >2 cm in diameter measurable from outside the pit, and we never detected juveniles in tide pools. The community was dominated by E. molaris, followed by Echinometra sp. B and A. crassispina. Species composition was similar between seasons until January 2018 (Figure 3C). Chi-square test indicates that there was no relationship between the change in occupancy and the change in month (χ2 = 16.448, df = 16, P = 0.4222). Therefore, it is suggested that there is no significant change in population size over the months.
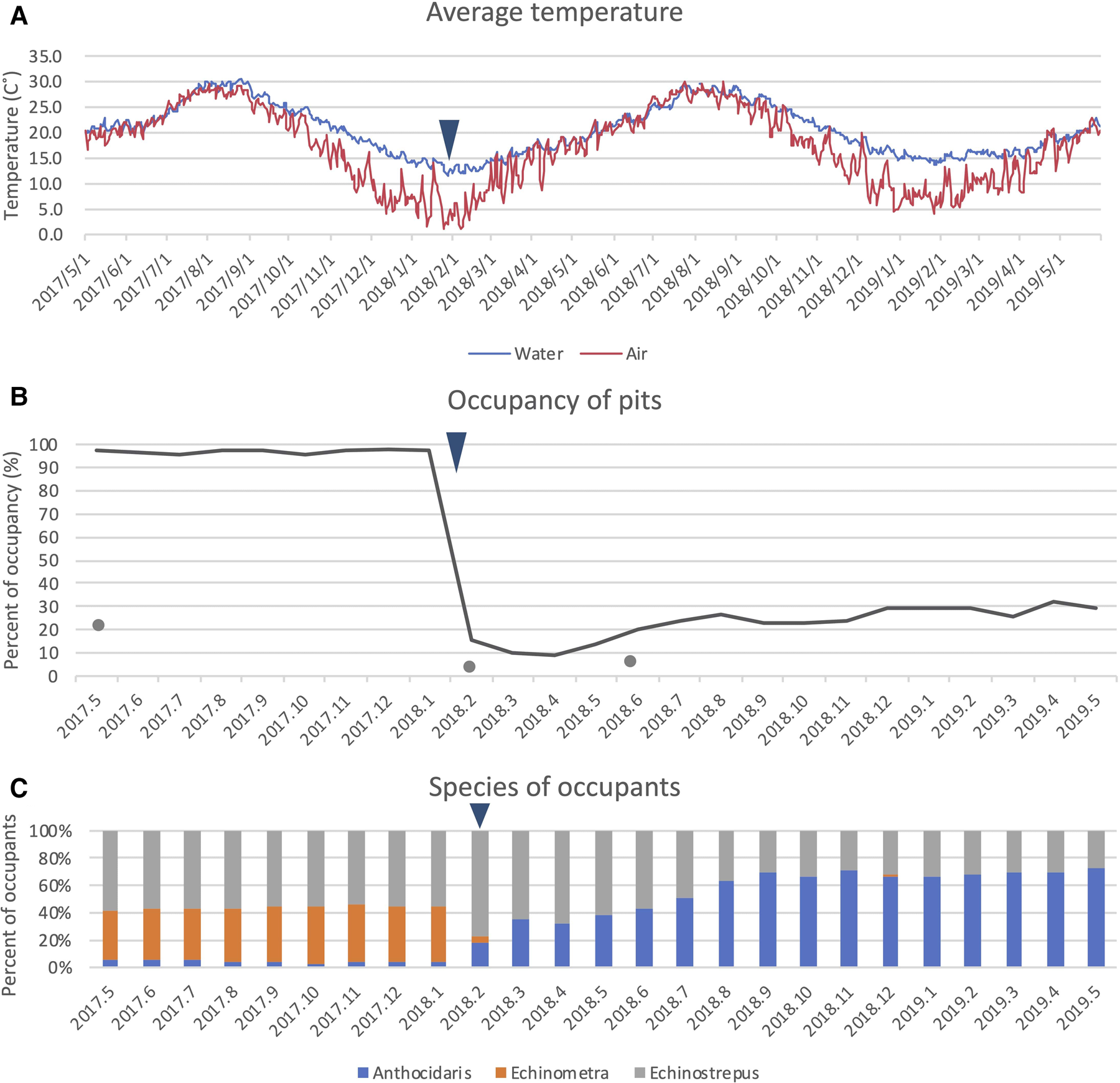
Fig. 3. (A) Daily average air and seawater temperatures between May 2017 and May 2019, obtained from an aquarium 400 m away from the study area; (B) per cent of pits occupied by sea urchins (lines) and Broderipia iridescens (dots); (C) composition of sea urchin species in pits. The cold event is denoted by an arrowhead.
The study area experienced an unusually cold event in February 2018, when the mean water temperature decreased to <16°C (Figure 3A). Immediately after the cold event, pit occupancy decreased from 97% to 16% (Figure 3B), and Echinometra sp. B was no longer found in P1 or P2 (Figure 3C). We observed dead or dying sea urchins drop from their pits and accumulate on the bottom of both tide pools (Figure 1F).
Over time, the populations of A. crassispina and E. molaris increased slowly; 14 months after the cold event, the total sea urchin population had reached 30% of the previous level when the survey started (Figure 3B). All newly recruited individuals were mature sized. We did not observe any recruitment of Echinometra sp. B in P1 or P2 15 months after the cold event. However, a diving survey in March 2019 revealed that this species was occupying pits in the subtidal zone. The reef areas around pits remained barren, i.e. essentially devoid of leafy seaweed, throughout the study period, even though on the wall without pits, a large amount of seaweed grew during spring and summer.
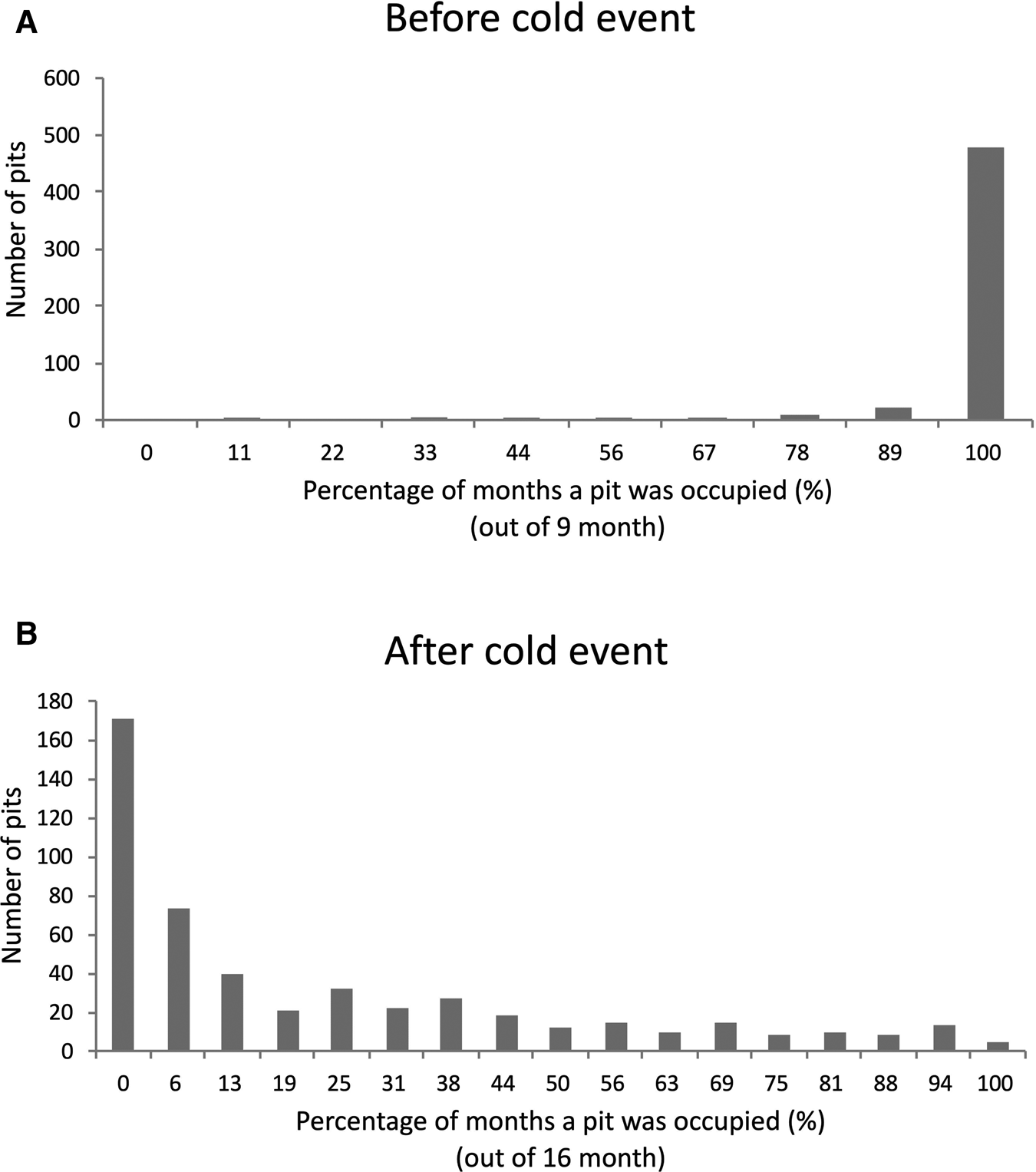
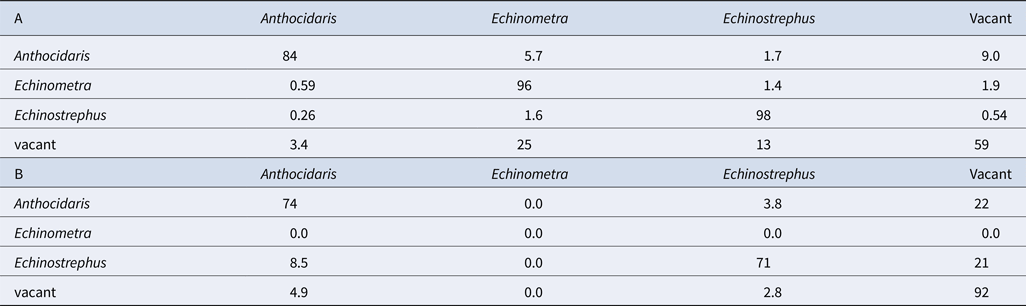
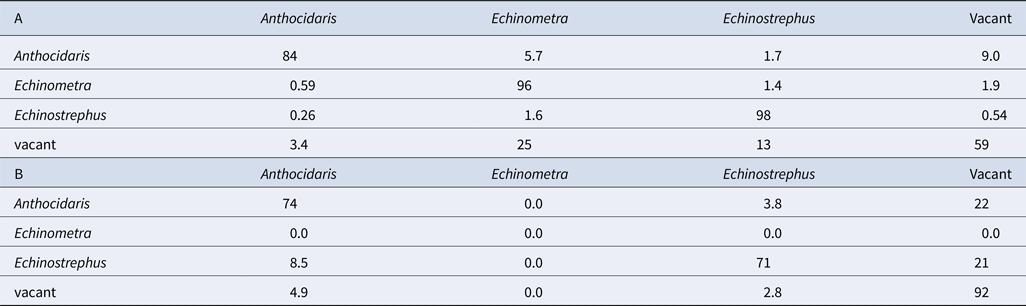
Although nearly all pits were continuously occupied prior to the cold event (Figure 4A), few pits were continuously occupied after the cold event (Figure 4B). Broadly, pit occupancy changed drastically following the cold event (Figure 5) and pits that were continuously occupied by sea urchins throughout the study period were distributed at intermediate depths, which were originally occupied by E. molaris. Due to the considerable population fluctuation over the study period, the transition matrices of pit occupants shifted between the time periods before and after the cold event (Table 1). Although the retention rate of pit occupants was > 83% for all three species prior to the cold event (83%, 96% and 98% for A. crassispina, Echinometra sp. B and E. molaris, respectively), retention rates decreased to <74% after the cold event (74% and 71% for A. crassispina and E. molaris). The retention rate of Echinometra sp. B could not calculated because all individuals died due to the cold event. The turnover rate of E. molaris was lower than 2.0% prior to the cold event, and increased to at most 28% after it (Figure 6). To identify whether the turnover rate was different before and after the cold event, we used generalized linear mixed model (GLMM) with tide pool (P1/P2) as a random effect. The turnover rate of A. crassispina was significantly higher after the cold event than before (Z = 0.4483, P < 0.05). The turnover rate of Echinometra sp. B and E. molaris was also significantly higher after the cold event than before (Z = 7.4661, P < 2 × 10−16; Z = 3.5077, P = 2 × 10−16).
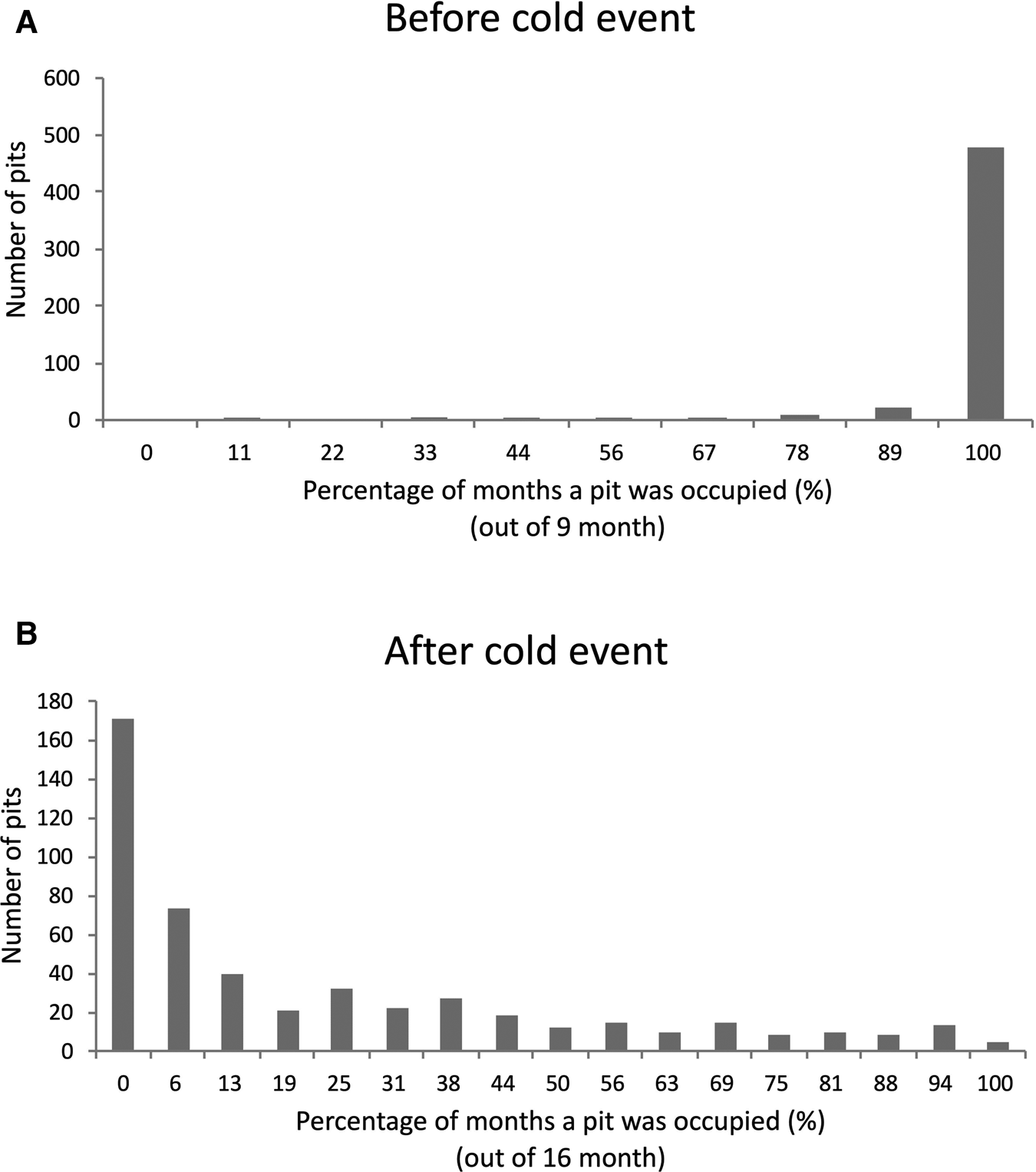
Fig. 4. The number of pits indicating the per cent of study period (months) in which a pit was occupied by a sea urchin (A) before the cold event and (B) after the cold event.
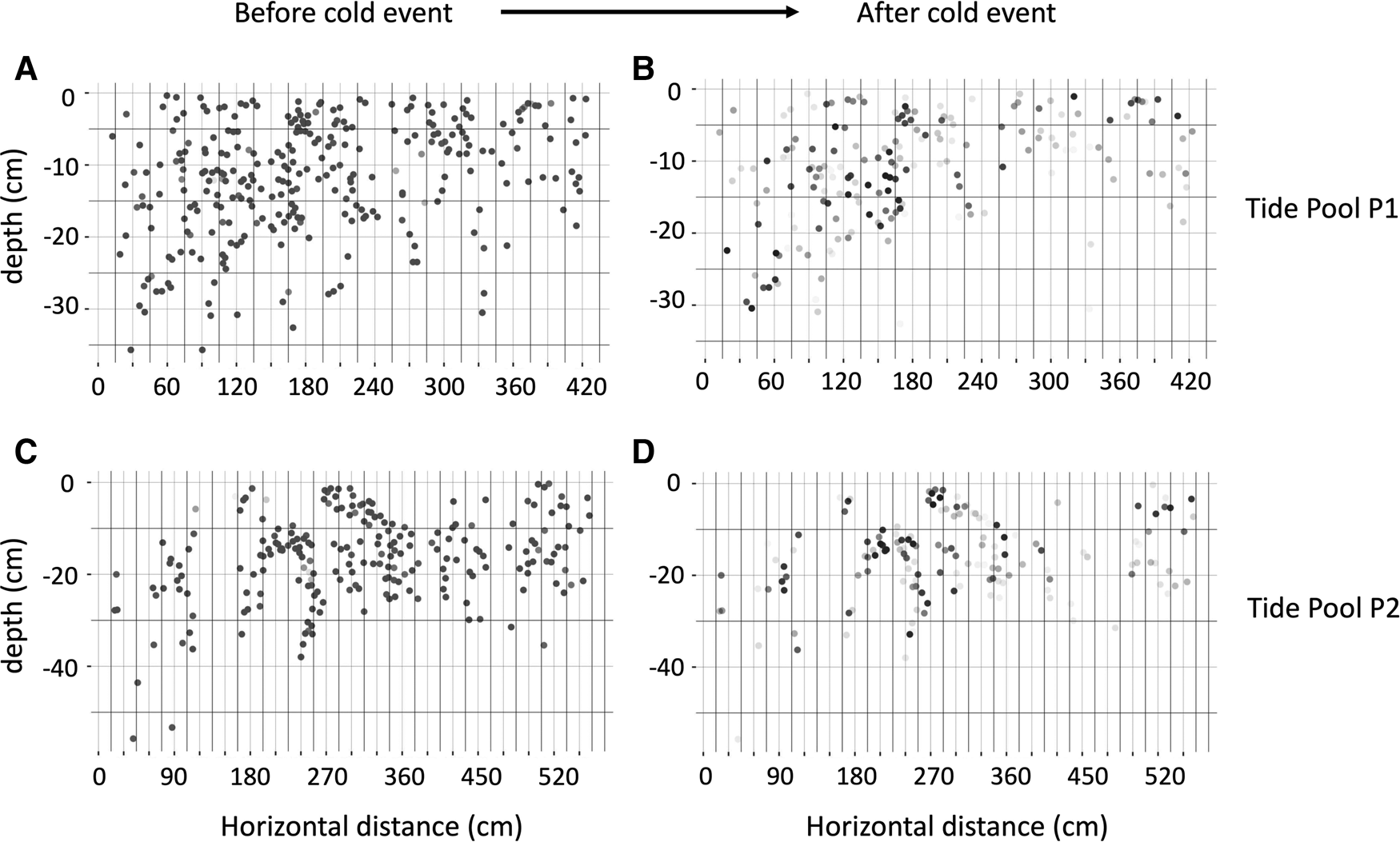
Fig. 5. Maps of sea urchin pits in (A, B) P1 and (C, D) P2, (A, C) before the cold event and (B, D) after the cold event. Each dot indicates a pit. Colour intensity reflects pit occupancy, wherein white and black dots correspond to unoccupied and always occupied pits, respectively, and grey tones indicate intermediate levels of occupancy.
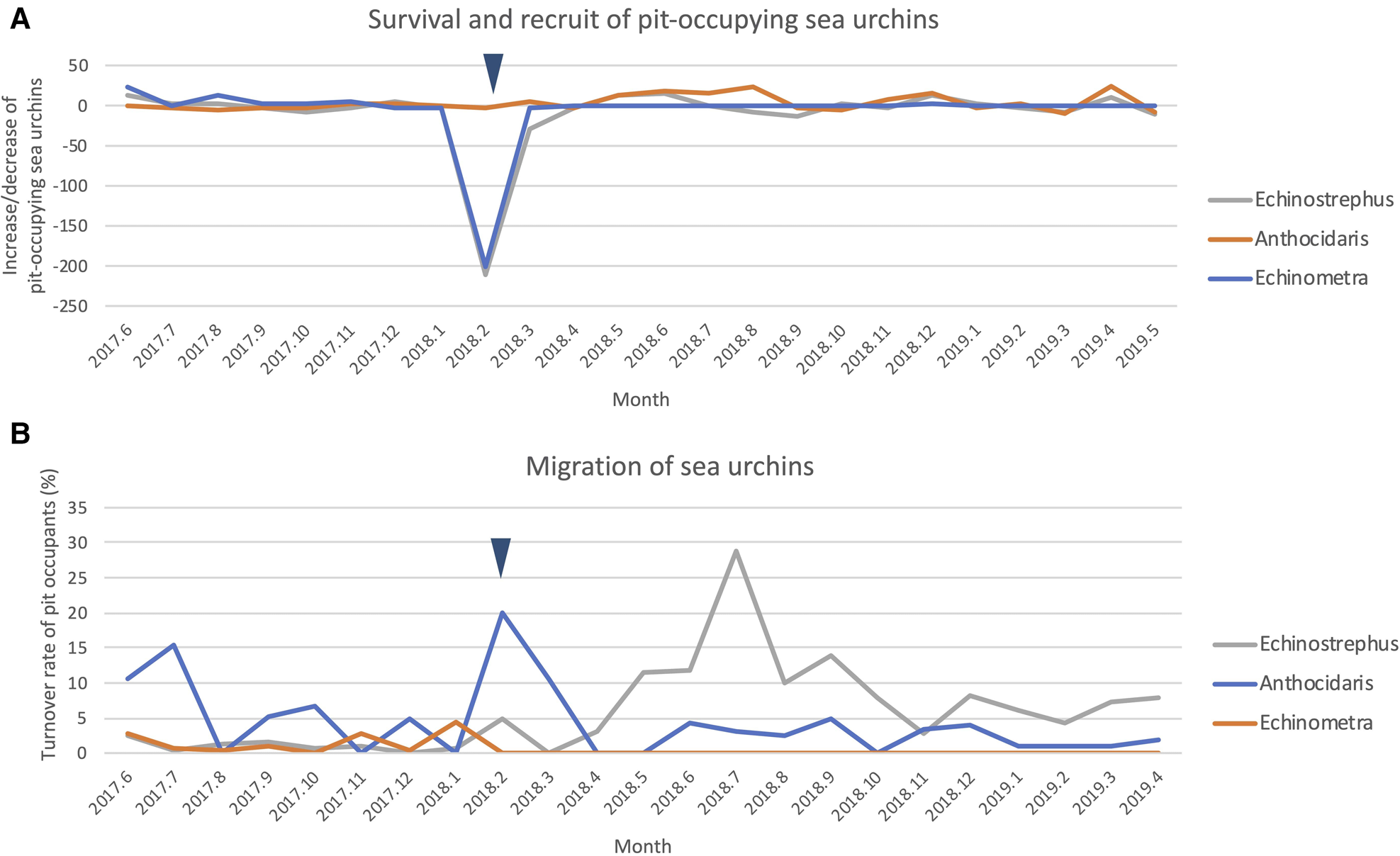
Fig. 6. (A) Monthly increases and decreases in each sea urchin population, and (B) turnover rates in pit occupants. The cold event is denoted by an arrowhead.
Table 1. Transition matrices (Pi, J) of sea urchin species occupying pits (A) before the cold event and (B) after the cold event (%)
Broderipia iridescens populations
Pits that were occupied by the two pit-borrowing sea urchin species were often shared with B. iridescens prior to the cold event, but the occupancy rate declined dramatically following the cold event, in response to the decrease in sea urchin hosts (Figure 3B). Before the cold event (April 2017), the average numbers and shell lengths of the snails were 1.58 ± 0.865/pit and 3.94 ± 0.311 mm in pits of A. crassispina, and 1.50 ± 0.887/pit and 5.59 ± 0.703 mm in pits of Echinometra sp. After the cold event, the numbers and shell lengths of the snails found in the pits of A. crassispina were 1.08 ± 0.557/pit and 6.21 ± 0.600 mm in February 2018, and 1.36 ± 0.767/pit and 5.28 ± 0.801 mm in July 2019. The limpet-like trochid snail was never found in pits that were not occupied by urchins or outside of pits. However, the symbiosis rate, which means the number of urchins sharing a pit with B. iridescens, was relatively constant throughout the study period, between 50–55% (55% in April 2017, 50% in February 2018 and 55% in July 2018).
Discussion
Distribution of pit-inhabiting sea urchins on temperate rocky shores
We found large numbers of excavated pits on the sidewalls of tide pools at Shirahama, 98% of these were occupied by sea urchins and there were no urchins outside pits. It is likely that no pits were excavated at the bottom of the pools due to the high risk of sedimentation. Pit density in our study site (total number of pits divided by the total area of the tidal pool) was remarkably high, 164 m–2, relative to the densities reported for sea urchin populations with outbreak status in other parts of the world (Ling et al., Reference Ling, Scheibling, Rassweiler, Johnson, Shears, Connell, Salomon, Norderhaug, Pérez–Matus, Hernández, Clemente, Blamey, Hereu, Ballesteros, Sala, Garrabou, Cebrian, Zabala, Fujita and Johnson2015). Thus, the near complete absence of seaweeds in the tide pools is likely due to high grazing pressure by sea urchins. Pits tended to have an aggregated distribution in the two tide pools, which may be indicative of an effect of substrate heterogeneity, i.e. the areas where conglomerate is distributed in abundance in the sandstone are difficult to bore, in contrast, the areas where conglomerate is scarce or walls with conglomerate runoff are easy to bore.
Sea urchin behaviour and community prior to the cold event
Sea urchin behaviour differed between day and night. We found that inter-pit migration was fairly rare, which may be an adapted response to reduce predation risk from fish at high tide or from birds and crabs during daytime low tides, as well as the risk of usurpation by other sea urchins. The overall absence of inter-pit migration was supported by high scores on the diagonal elements in the transition matrices (Table 1) for all three species and by the low turnover rate of pit occupants. If the urchins wander about the tide pool, each turnover rate would be high, and if the urchins do not move from their pits at all, the turnover rate would be 0. The low turnover rate recorded in this study indicates that sea urchins, especially pit-borrowing ones, move out from their pits in rare cases. Because sea urchins here were monitored at the species level, rather than the individual level, it is likely that the true turnover rate was underestimated. However, the observed low replacement rate suggests that the individual turnover rate may have been as low as 0.60%. Generally, our results suggest that sea urchin populations in these warm temperate tide pools are static in terms of abundance and community structure, barring extreme weather events.
Despite the extremely low inter-pit migration, we observed a small number of sea urchins wandering around the tide pools at night. We suspect that these individuals may have been migrants from the subtidal zone, because sea urchins in the subtidal zone are often caught as bycatch in nocturnal gill netting for lobster (Kobayashi & Tokioka, Reference Kobayashi and Tokioka1976), and larval settlement and juvenile growth processes take place in the pebbly substrate of the subtidal zone (Kobayashi & Tokioka, Reference Kobayashi and Tokioka1976; Fernandez et al., Reference Fernandez, Caltagirone and Johnson2001).
The aboral surface of sea urchin tests is densely armed by long, stout spines, but the oral surface is armed only by short, thin spines, and is subject to attacks by predators such as fish (Sala, Reference Sala1997). Therefore, sea urchins are best protected when they have retreated to the innermost portion of the pit. However, grazing for seaweed necessitates leaving the pit. Thus, there is a trade-off for sea urchins between the need to acquire rich food resources and the risk of both predation and the loss of the pit.
Drastic decrease of sea urchin population and behavioural change after the cold event
We observed a substantial, dramatic population decline following the cold event in 2018: 81% of the sea urchins in the study pools died, and Echinometra sp. B was extirpated from the pools, leading to significant changes in the structure of the sea urchin community. Mass mortality events due to unusually low winter temperatures have occurred in the past at Shirahama, with documented events in 1963 and 1984 (Tokioka, Reference Tokioka1963; Ohgaki et al., Reference Ohgaki, Kato, Kobayashi, Tanase, Kumagai, Ishida, Nakano, Wada and Yusa2019) (as reference data, Figure S2 shows the water temperature data off Wakayama since 1982). In those cases, the intertidal zone was more affected than the subtidal zone, and urchin populations eventually recovered (Tokioka, Reference Tokioka1966; Ohgaki et al., Reference Ohgaki, Kato, Kobayashi, Tanase, Kumagai, Ishida, Nakano, Wada and Yusa2019). In our study, population recovery was slow for the two remaining species and was likely the result of recruitment from the subtidal zone because all recruited individuals were mature sized. After 15 months, pit occupancy had recovered from 16% to only 30%. In addition to the disappearance of Echinometra sp. B, the proportion of E. molaris in the sea urchin community gradually decreased after the cold event, likely due to gradual recruitment of A. crassispina, which has higher mobility than E. molaris, from the subtidal zone. The slow pace of recovery suggests that the subtidal populations of these species may not have been large enough to act as source populations for the intertidal zone. In March 2019, two months before the end of observation period, sea urchins began to be observed in the subtidal zone, suggesting that they may soon join the burrows in the intertidal zone.
The turnover rate of pit occupants increased following the cold event, suggesting that sea urchins were released from a resource limitation (pit limitation) and migrated more frequently between pits. Pits were rarely continuously occupied by a sea urchin in the 15 months following the cold event, suggesting that sea urchins do not have strong fidelity to a specific pit and that migration between pits may be frequent when pits are not limited.
Despite the substantial decline in the sea urchin population, the study area remained devoid of seaweeds throughout the study period. This suggests that the urchin population, even after mass mortality (density of urchins in the whole study area: 14.97 m–2), was high enough to maintain barren conditions. Thus, although sea urchin populations are influenced by temperature, red tides (Ohgaki et al., Reference Ohgaki, Kato, Kobayashi, Tanase, Kumagai, Ishida, Nakano, Wada and Yusa2019) and other biological factors such as predation and disease (Moses & Bonem, Reference Moses and Bonem2001; Ling et al., Reference Ling, Scheibling, Rassweiler, Johnson, Shears, Connell, Salomon, Norderhaug, Pérez–Matus, Hernández, Clemente, Blamey, Hereu, Ballesteros, Sala, Garrabou, Cebrian, Zabala, Fujita and Johnson2015), local sea urchin barrens can persist in intertidal rocky reefs with high pit densities.
Population of the limpet-like snail
The number of pits occupied by B. iridescens declined along with their sea urchin hosts following the cold event. This finding suggests that snails shared the fate of their hosts without migrating to new hosts. However, a consistent rate of commensalism was observed throughout the study, even with frequent inter-pit migration among urchins following the cold event. This snail has been observed following wandering sea urchins in aquaria (Yamamori & Kato, Reference Yamamori and Kato2018), and we suggest that this phenomenon also occurs in our study area. Since the number of individuals surveyed was small, it can only be used as a reference, but the average shell length of B. iridescens living in the burrows of A. crassispina was larger after the cold wave. It suggests that smaller individuals may have been hit particularly hard by the cold wave. Parallel population dynamics between a host and its epizoic symbionts have been reported for isopods and sea urchins in the North-east Pacific (Stebbins, Reference Stebbins1989). However, our observations are surprising because this snail does not live on its host species, but instead follows the host through repeated inter-pit migrations.
Our findings clarified the local population dynamics among sea urchins in warm temperate tide pools with high pit densities. Specifically, we found that sea urchins are limited by the number of pits, and that their abundance and community structure are strongly influenced by low water temperature and recruitment from the subtidal zone. A dramatic population decline in two of the sea urchin species under investigation led to increased inter-pit migration and an associated decrease in trochid snail populations. Finally, despite mass mortality, sea urchin populations in our study area remained high enough to prevent seaweed recovery and maintain barren conditions.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0025315421000680
Acknowledgements
We thank all the staff of the Seto Marine Biological Laboratory for supporting our field survey, Shirahama Aquarium for provision of water temperature data, and Toshiyuki Sakai for advice on statistical analysis.
Financial support
This work is supported by Japan Ministry of Education, Culture, Science, Sports, and Technology Grant-in-Aid for Scientific Research (15H02420, 20H03321; https://www.jsps.go.jp/english/index. html) for Makoto Kato and Grants-in-Aid for Scientific Research Grant number 18J22890 for Luna Yamamori.