INTRODUCTION
Animals that excavate hard substrates (known as bioeroders) play an important functional role in many communities by modifying the physical environment and facilitating its use by other organisms. Bioerosion is a widespread behaviour, occurring in at least twelve marine phyla (Warme, Reference Warme and Frey1975). In most instances, boring into hard substrates has evolved to provide protection and a stable domicile. However, in the gutless worms belonging to the genus Osedax (Figure 1), the hard substrate itself is used as a food source. All described species in the siboglinid genus Osedax subsist on the skeletons of deceased, decomposing vertebrates on the seafloor (Rouse et al., Reference Rouse, Goffredi and Vrijenhoek2004, Reference Rouse, Worsaae, Johnson, Jones and Vrijenhoek2008; Glover et al., Reference Glover, Källström, Smith and Dahlgren2005, Reference Glover, Wiklund, Taboada, Avila, Cristobo, Smith, Kemp, Jamieson and Dahlgren2013; Fujikura et al., Reference Fujikura, Fujiwara and Kawato2006). These worms have soft root-like tissues that release enzymes and acid secretions to invade and erode the bones (Higgs et al., Reference Higgs, Glover, Dahlgren and Little2011a; Tresguerres et al., Reference Tresguerres, Katz and Rouse2013). The trunk section of the worm's body, which extends into the water, is crowned with long respiratory palps (Huusgaard et al., Reference Huusgaard, Vismann, Kühl, Macnaugton, Colmander, Rouse, Glover, Dahlgren and Worsaae2012). Initial studies suggest that Osedax species obtain nutrition from the bone via heterotrophic symbionts that are housed in their root tissues (Goffredi et al., Reference Goffredi, Orphan, Rouse, Jahnke, Embaye, Turk, Lee and Vrijenhoek2005, Reference Goffredi, Johnson and Vrijenhoek2007; Verna et al., Reference Verna, Ramette, Wiklund, Dahlgren, Glover, Gaill and Dubilier2010), although the precise mechanisms of this process remain unclear (Fujikura et al., Reference Fujikura, Fujiwara and Kawato2006; Katz et al., Reference Katz, Klepal and Bright2010). In addition to nutrition, the borings protect the ovaries and provide a refuge into which the reproductive females can partially or wholly retract (Glover et al., Reference Glover, Källström, Smith and Dahlgren2005). Borings are only created by females, since all species investigated to date exhibit extreme male dwarfism, with males living as paedomorphs inside the female tube (Rouse et al., Reference Rouse, Worsaae, Johnson, Jones and Vrijenhoek2008).

Fig. 1. Osedax rubiplumus: (A) i [in] situ on whale phalanx at time of collection; (B) dissected specimen (courtesy of Greg Rouse). Scale bar: B, 1 cm.
To date, only the borings of Osedax mucofloris Glover et al., Reference Glover, Källström, Smith and Dahlgren2005 have been documented in detail (Higgs et al., Reference Higgs, Glover, Dahlgren and Little2010, Reference Higgs, Glover, Dahlgren and Little2011a), whilst those of two other species have been shown in profile (e.g. Kiel et al., Reference Kiel, Goedert, Kahl and Rouse2010; Higgs et al., Reference Higgs, Little, Glover, Dahlgren, Smith and Dominici2012). In this study we examine the range of morphology in borings for nine Osedax species and use micro computed tomography to investigate how each species excavates the bone. Our goals are to examine the diversity of Osedax borings to help improve our knowledge about their morphological taxonomy, to test the hypothesis that the local diversity of Osedax at whale-falls is linked to their ability to differentiate their niches based on boring behaviour and to further improve our knowledge about their trace fossils. We present three-dimensional reconstructions of borings with quantitative details regarding cross-sections, volume and surface area, where possible, for each species.
MATERIALS AND METHODS
Provenance of samples
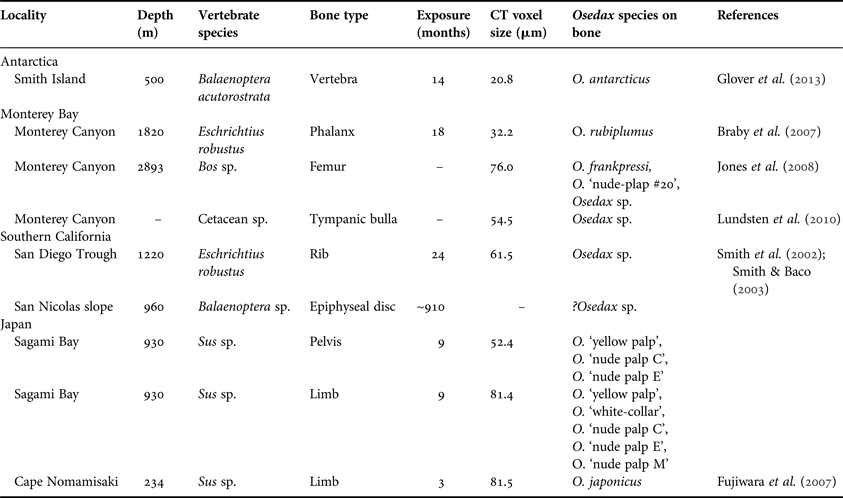
Most of the bone samples investigated in this study were collected during previously reported expeditions (see references in Table 1), so detailed narratives will only be given here for samples not included in previous reports. Unless otherwise stated, bones were collected using remotely operated vehicles (ROVs) from the skeletons of dead whales that had either arrived on the seafloor naturally or were implanted experimentally. Non-cetacean bones were collected from experimentally deployed bone packages and were used when it was not possible to obtain CITES permits for the shipment of whale bones to the UK.
Table 1. Bone samples analysed in this study. Voxel size indicates resolution of computed tomography (CT) scans.

Samples obtained from the Southern California basins were collected in 1995 and 1998 as part of a multi-decade programme of research on whale-fall ecosystems and details of their collection have been reported elsewhere (Smith et al., Reference Smith, Baco and Glover2002; Smith & Baco, Reference Smith and Baco2003; Schuller et al., Reference Schuller, Kadko and Smith2004). In October 2008, subsamples were taken from frozen and formalin-fixed bones collected during these previous investigations and transferred to a buffered 4% formaldehyde solution for shipment to the UK. Samples were shipped to the UK and stored in 80% ethanol after CITES approval was obtained. Similarly, samples from Monterey Canyon were collected during previously reported expeditions (Braby et al., Reference Braby, Rouse, Johnson, Jones and Vrijenhoek2007; Lundsten et al., Reference Lundsten, Schlining, Frasier, Johnson, Kuhnz, Harvey, Clague and Vrijenhoek2010) and sent to the Natural History Museum, London in 80% ethanol from the Scripps Institute of Oceanography under a CITES exemption programme. Details of whale bones experimentally deployed in Antarctica are given in Glover et al. (Reference Glover, Wiklund, Taboada, Avila, Cristobo, Smith, Kemp, Jamieson and Dahlgren2013).
Pig bone samples were experimentally deployed in January 2010 at 234 m depth near a site of previous whale-fall studies off Cape Nomamsaki, Japan (Fujiwara et al., Reference Fujiwara, Kawato, Yamamoto, Yamanaka, Sato-Okoshi, Noda, Tsuchida, Komai, Cubelio, Sasaki, Jacobsen, Kubokawa, Fujikura, Maruyama, Furushima, Okoshi, Miyake, Miyazaki, Nogi, Yatabe and Okutani2007) and retrieved in May 2010. The bones with Osedax living on them were kept in tanks until 2 June 2010 and then fixed in a buffered 4% formaldehyde solution before being transferred to 70% ethanol. Other samples from Sagami Bay, Japan were deployed in May 2009 and collected 8.5 months later (January 2010). Bones with living Osedax were kept in tanks for 6 months, after which, they were fixed and treated in the same way as those from Cape Nomamsaki. Tissue samples were taken from individual Osedax specimens for genetic analysis prior to fixation. All samples from Japan were then sent to the UK for investigation in July 2010.
Osedax identification
Specimens of Osedax were identified to species level upon collection, based on visually identifiable morphological characters or on nucleic acid sequences obtained from tissue samples (e.g. Vrijenhoek et al., Reference Vrijenhoek, Johnson and Rouse2009). Only seven Osedax species have been formally described (Rouse et al., Reference Rouse, Goffredi and Vrijenhoek2004, Reference Rouse, Worsaae, Johnson, Jones and Vrijenhoek2008; Glover et al., Reference Glover, Källström, Smith and Dahlgren2005, Reference Glover, Wiklund, Taboada, Avila, Cristobo, Smith, Kemp, Jamieson and Dahlgren2013; Fujikura et al., Reference Fujikura, Fujiwara and Kawato2006), but operational taxonomic units (OTUs) have been designated for over 20 other genetically distinct forms (Vrijenhoek et al., Reference Vrijenhoek, Johnson and Rouse2009). Here we use the place-holder names given to these OTUs in previous literature and in GenBank (Table 1; Glover et al., Reference Glover, Wiklund, Taboada, Avila, Cristobo, Smith, Kemp, Jamieson and Dahlgren2013).
CT scanning
All samples were scanned with a Metris X-Tek HMX ST 225 cone beam micro computed tomography (CT) system operated at the Natural History Museum in London. A total of 3142 angular projections were collected at 0.11° angular intervals in a single 360° rotation for each scanned sample. The radial projections were reconstructed into a three-dimensional matrix of isotropic voxels. Voxel dimensions for each sample are given in Table 1. The volumes were reconstructed using CT Pro Version 2.0 (Nikon Metrology, Tring, UK) and rendered using VG Studio Max 2.0 (Volume Graphics, Heidelberg, Germany).
Osedax borings were identified as xenogeneic voids in the bone material that were coincidental with areas colonised by Osedax. Voids were isolated and delineated using the region growing tools of the VG Studio software, based on the grey values of voxels making up the void. Voids were then reconstructed as virtual objects and measurements taken using tools of the VG Studio software.
Petrographic thin sections
Bone samples from an epiphysis disc collected from a whale-fall in the San Nicolas slope (Table 1) could not be adequately CT scanned, owing to the extreme contrast of bone dimensions in perpendicular planes. Instead bone samples were sent for petrographic thin sectioning at the University of Leeds, as described by Higgs et al. (Reference Higgs, Little, Glover, Dahlgren, Smith and Dominici2012).
RESULTS
In this section we report on the morphology of Osedax borings found in the bone samples listed in Table 1. We begin with those species that were positively identified and have been formally described, then proceed to describe borings created by species that were positively identified, but are not yet formally described. Finally, we show borings created by Osedax species that could not be positively identified, but appear to show unique boring morphologies. Borings are described according to the terminology set out by Pirrone et al. (Reference Pirrone, Buatois and Bromley2014).
Osedax rubiplumus
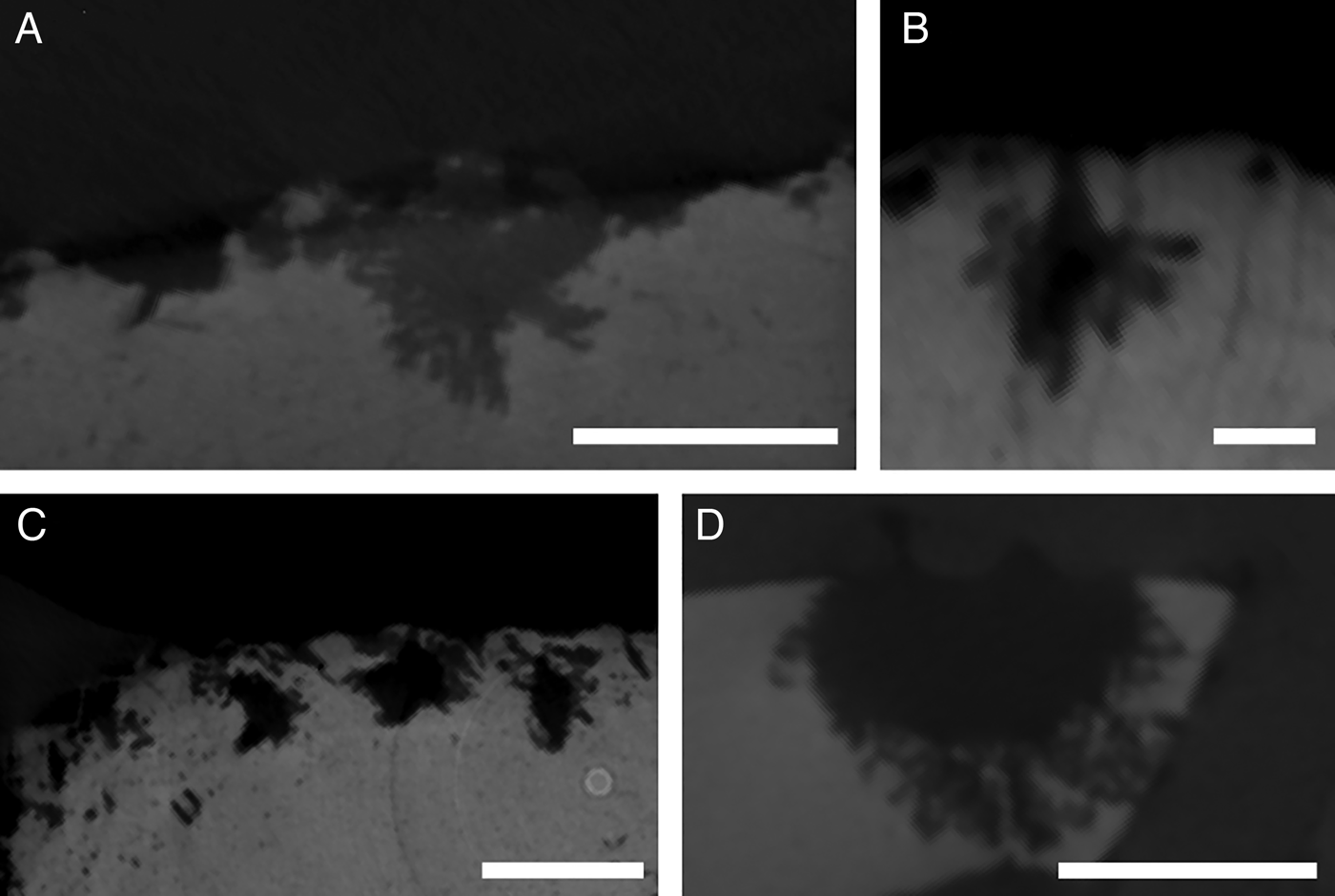
Five borings made by Osedax rubiplumus Rouse et al., Reference Rouse, Goffredi and Vrijenhoek2004 were documented from a single cetacean carpal bone. Individuals in this sample were identified visually upon collection, based on their size and brilliant red palps (Figure 1A). The O. rubiplumus borings are all approximately spherical and appear to expand just below the external bone surface (Figure 2A–C). Some borings have a defined tube section before expansion into the bones begins, but this feature is short when present, making up a small proportion of the boring. The bulk of the boring consists of a chamber section ranging in diameter from 3.2 to 4.6 mm. The surficial openings (apertures) of the borings are 1.3–1.6 mm across.

Fig. 2. Micro computed tomography scans of Osedax borings: (A–C) O. rubiplumus; (D–F) O. frankpressi; (G–I) O. japonicus; (J–L) Osedax ‘yellow palp’; (M–R) Osedax ‘nude palp #20’. Borings are shown as three-dimensional reconstructions (A, D, G, J, M and P), in longitudinal section (B, E, H, K, N and R) and transverse cross-section (C, F, I, L, O and R). Scale mesh size: D, G, M and P, 1 mm; J, 5 mm. Scale bars: B, C, E, F, 3 mm; H, I, 4 mm; K, L, 5 mm; N–Q, 1 mm.
Osedax frankpressi
Only one boring of Osedax frankpressi Rouse et al., Reference Rouse, Goffredi and Vrijenhoek2004 was available for analysis, from an experimentally deployed cow bone (Table 1). The large boring consists of a relatively deep tunnel (60% total boring depth) penetrating into the bone, with an irregular chamber extending distally from its base (Figure 2D–F). The edge of the chamber is not smooth but shows a serrated pattern where the bone has been eroded. The round aperture of the boring is 1.3 mm in diameter, which continues as a uniformly thick tube for 3.4 mm into the bone before tapering into a 9.4 mm wide chamber.
Osedax japonicus
Numerous borings of Osedax japonicus Fujikura et al., Reference Fujikura, Fujiwara and Kawato2006 were found in two pig bones and all consistently showed the same morphology, although many had merged. Individual borings are generally hemispherical with an aperture located at the centre of the boring (Figure 2G–I). They lack a tube section and generally sit just underneath the bone surface, covered by a layer of bone <1 mm in thickness. The top of the reconstructed boring shows a corrugated surface of ridges and troughs radiating from the centre of the borings. Several of the borings show a wedge of bone situated just below the aperture of the boring.
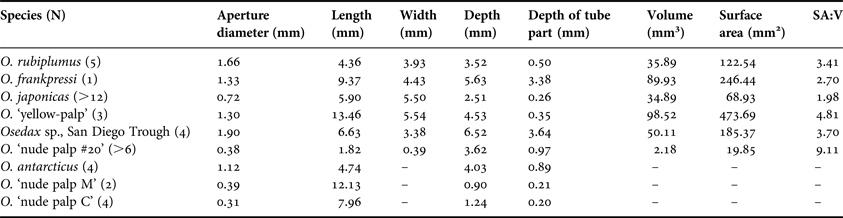
Osedax antarcticus
Small samples (<4 × 4 cm) of whale bone containing several individuals of Osedax antarcticus Glover et al., Reference Glover, Wiklund, Taboada, Avila, Cristobo, Smith, Kemp, Jamieson and Dahlgren2013 were CT scanned, but it was not possible to identify intact individual borings because of the small bone samples. Nevertheless, cross-sections of the borings allow some comparisons to other boring morphologies (Figure 3). Outlines of individual and merged borings show that there is a short tube section leading from the surface aperture (1.1 mm wide) to a globular chamber (4.7 mm across). At its periphery the chamber is divided into distinct lobes that are more pronounced in the denser bone sample. The small size of the samples facilitated high resolution CT scanning (Table 1) that was able to show the hollowed-out bone trabeculae at the margins of the Osedax borings (Figure 3D), as previously described from O. mucofloris borings (Higgs et al., Reference Higgs, Glover, Dahlgren and Little2011a).
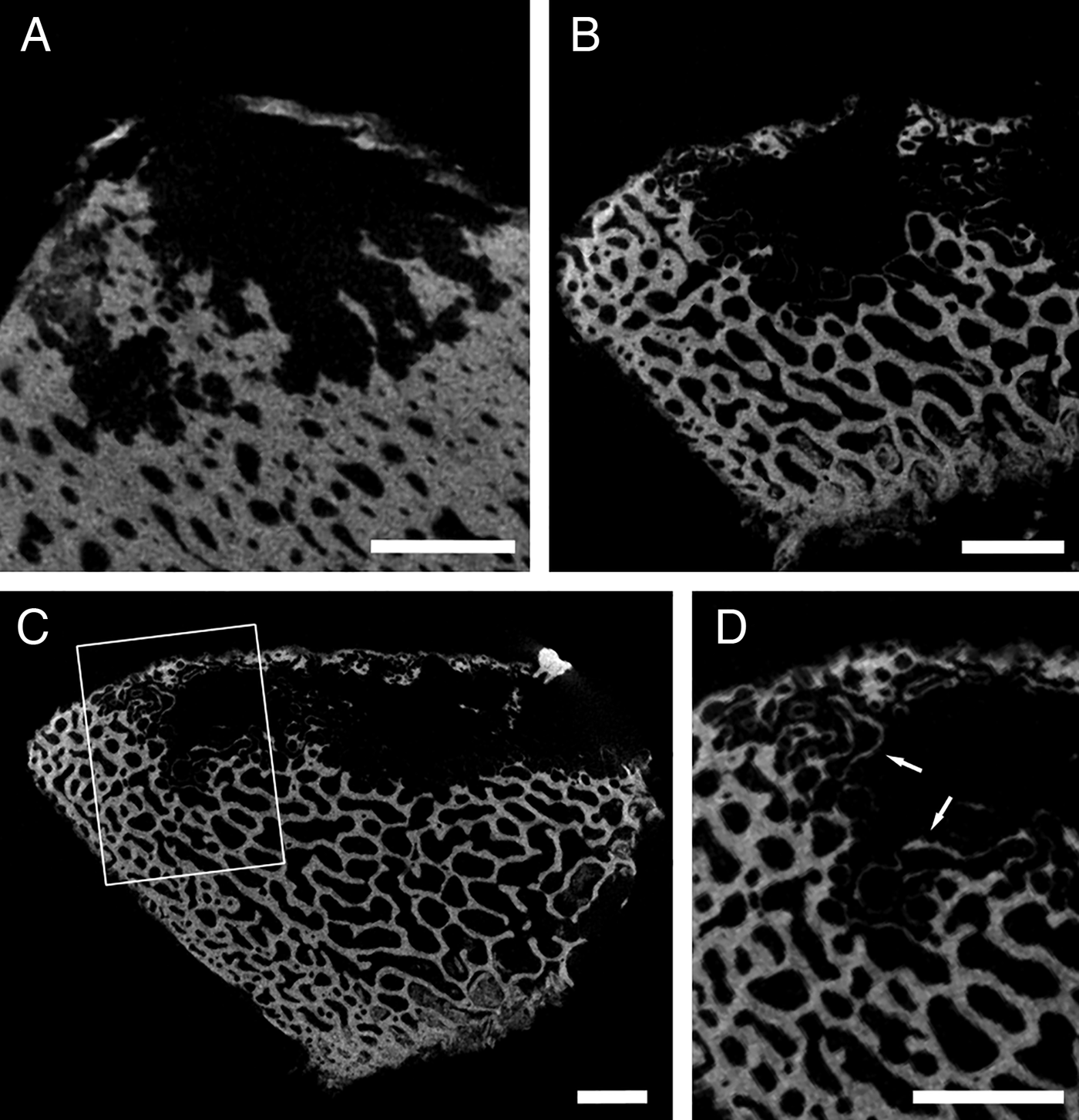
Fig. 3. Osedax antarcticus: (A–C) cross-sections of borings in segments of whale vertebra; (D) enlargement of boxed area in (C), highlighting bone trabecuale that have been hollowed out by root tissues (arrows). Scale bars: A–D, 2 mm.
Osedax ‘yellow-palp’
Osedax ‘yellow-palp’ is an undescribed species known from 980 m in Sagami Bay, Japan. Samples of their borings were found in pig bones and specimens were identified as a genetically distinct OTU (‘Sagami 5’, FM995539 & FM998083 on GenBank). The borings of O. ‘yellow palp’ are predominantly composed of a multi-annexed chamber that expands from a central aperture (Figure 2J–L). The boring extends laterally under the bone surface and is wider than it is deep, giving it a relatively high surface area to volume ratio. The annexes are narrow nearest the central aperture but fan-out distally. CT scans of a small individual show that the boring initially starts out as a shallow flat chamber before branching off into separate annexes.
Osedax ‘nude palp #20’
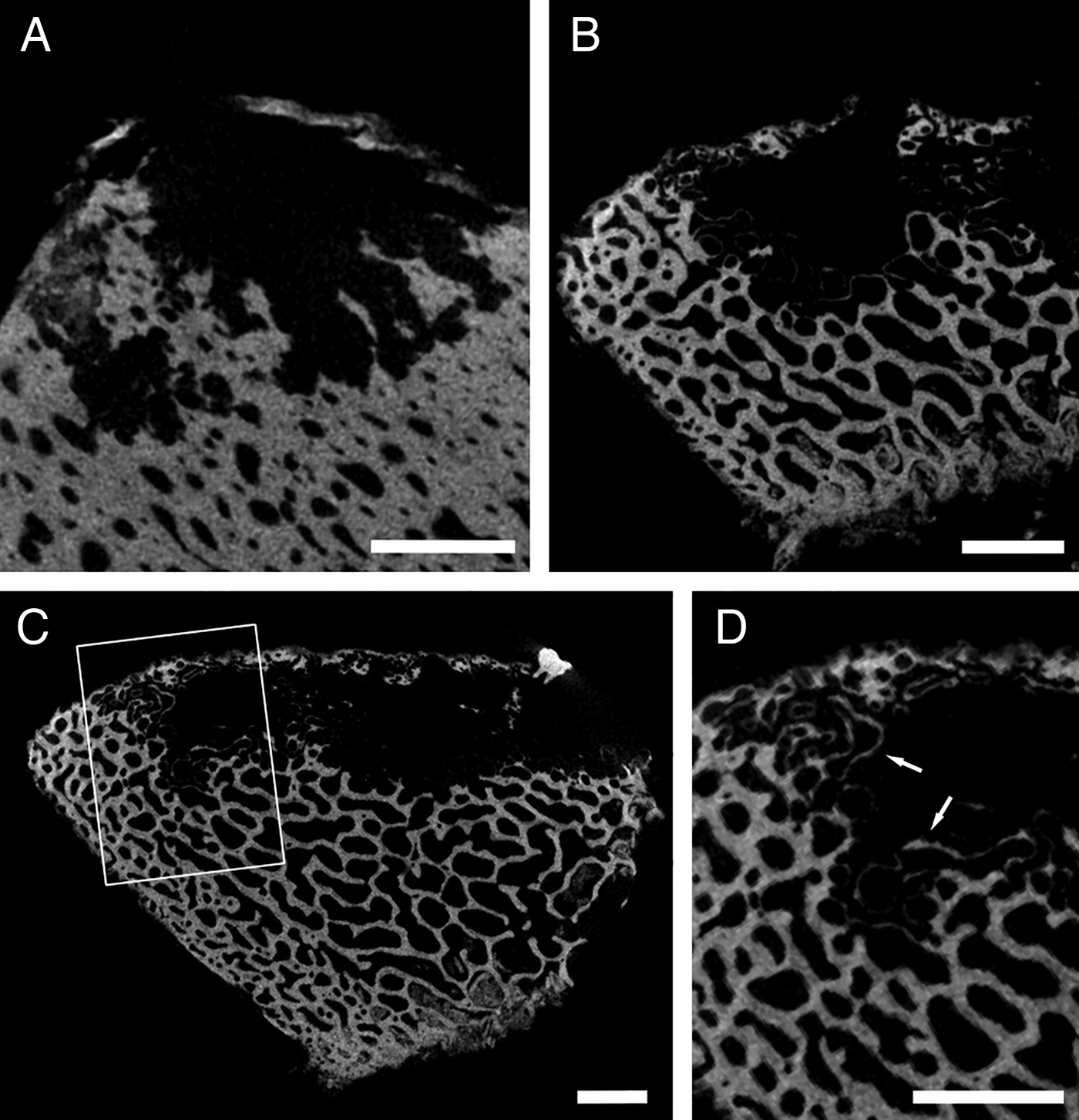
One species of unidentified Osedax that was found on the cow bone from Monterey Canyon, living alongside O. frankpressi (Table 1), was very different in morphology from any other borings examined to date. Based on DNA retrieved from some of the borings, it seems that they were created by Osedax ‘nude palp #20’, another unnamed OTU from the 2893 m site (S. Johnson, unpublished data), but this could not be confirmed visually because of their small size. The boring consisted of a narrow aperture (0.38 mm) that expanded into a laterally broad, but vertically flattened, pouch-shaped chamber (Figure 2M–R). Small micro-tunnels emanated from this chamber, probably representing exploratory root tendrils. Assuming that this is a true representation of root morphology (see Higgs et al., Reference Higgs, Glover, Dahlgren and Little2011a), its surface area to volume ratio would be more than double that of any other species investigated (Table 2).
Table 2. Measurements for the largest boring produced by each Osedax species in this study. SA:V is the surface area to volume ratio of the boring. (N) number of individual borings of each species examined; (–) measurements not possible because no individual borings could be isolated from others.

Osedax ‘nude palp’ species: C, E and M
These three species of Osedax were found in two pig bones from Sagami Bay, Japan (Table 1), but two (C and E) are also known from Monterey Canyon on the opposite side of the Pacific Ocean (Vrijenhoek et al., Reference Vrijenhoek, Johnson and Rouse2009). The three species are similar in external morphology and at the time of collection were thought to represent a single species. Genetic sequencing of several of these individuals revealed three distinct species of Osedax (F. Pradillon, unpublished data). Since a limited number of individuals were sequenced, only a few borings could be definitely attributed to one species and these often occurred in close proximity with each other, so the borings of these three species are presented together. In all instances where borings could definitely be attributed to one particular species the borings were very shallow and laterally expansive, occurring just under the bone (Figure 4). This morphology was observed for all three of the ‘nude palp’ species from Sagami Bay. The aperture of most of these borings was located in the centre of the boring.

Fig. 4. Osedax ‘nude palp’ species: (A) boring made by O. ‘nude palp M’ (arrowed); (B, C) borings made by O. ‘nude palp C’; (D) pig pelvis showing overlapping borings of O. ‘nude palp’ species (C) and (E) (arrowed). Scale bars: A–C, 2 mm.
Additional Osedax borings from the San Diego Trough
Higgs et al. (Reference Higgs, Little, Glover, Dahlgren, Smith and Dominici2012) briefly described Osedax borings in the rib of a gray whale from the San Diego Trough off Southern California from an undescribed species of Osedax. It has not been possible to obtain sufficient genetic information from these specimens to positively identify them, since they were preserved in formalin.
Unlike most other Osedax borings, those seen in this bone penetrate deeply into the cancellous region of the bone (Figure 5). There is a long tube section (3.64 mm in the specimen illustrated in Figure 5) that passes through the dense cortical bone layer before expanding into a pouch-shaped (sensu Bromley, Reference Bromley and Donovan1994) chamber section (Table 2). The chamber is substantially wider than the tube section in one axis but nearly identical in width when viewed on the perpendicular axis (Figure 5B, C).

Fig. 5. Undetermined Osedax sp. boring from San Diego Trough: (A) three-dimensional reconstruction; (B) longitudinal section; (C) Transverse cross section. Scale mesh spacing: A, 1 mm. Scale bars: B, C, 5 mm.
Additional Osedax borings from Monterey Canyon
Another large boring found in the same bone as Osedax frankpressi & O. ‘nude palp #20’ (Table 1), is assumed to belong to a separate Osedax species, having a distinct hemispherical morphology (Figure 6D). Unlike the O. mucofloris or O. japonicus borings however, these had many fine micro-tunnels emanating from the main chamber, which densely penetrated the surrounding bone. It can be assumed that expansion of the boring by this species of Osedax occurs when these many micro-tunnels grow together and merge.

Fig. 6. Undetermined Osedax spp. borings from Monterey Canyon: (A–C) cetacean tympanic bulla (ear bone); (D) cow femur. Scale bars: A, C and D, 5 mm; B, 1 mm.
A similar pattern of small micro-tunnels extending from the main chamber was also identified in Osedax borings in a whale ear-bone (tympanic bulla) from Monterey Canyon (Figure 6A). Other Osedax borings in this bone showed similar structure, but also have defined tube sections leading from the surficial aperture (Figure 6B, C). The chamber of the borings found in the ear bone tend to be globular, but are more irregular than those from other bone. There is no information on the species of Osedax in this bone.
Additional borings from San Nicolas slope
Inspection of the bones recovered from a natural whale-fall discovered on the San Nicolas slope revealed small holes in surface of an epiphyseal vertebral disc similar to those produced by Osedax. Thin sections of bone that bisected these holes revealed areas of bone trabeculae that had been hollowed out (Figure 7), as observed in CT scans of bone inhabited by O. antarcticus specimens described above (Figure 3D).

Fig. 7. Petrographic thin section through a boring in whale bone from San Nicolas slope. Hollowed out trabeculae (arrow) near external surface of the bone.
DISCUSSION
Osedax worms are not physiologically restricted to exploiting whale bones, and experimental studies have shown them living on the bones of fish (Rouse et al., Reference Rouse, Goffredi, Johnson and Vrijenhoek2011), cows (e.g. Jones et al., Reference Jones, Johnson, Rouse and Vrijenhoek2008), pigs (Vrijenhoek et al., Reference Vrijenhoek, Collins and Van Dover2008a) and even birds (R. Vrijenhoek, personal observation). Borings found in a similar array of fossil vertebrate bones have been attributed to Osedax (Kiel et al., Reference Kiel, Goedert, Kahl and Rouse2010, Reference Kiel, Kahl and Goedert2012). Previous investigations of Osedax borings have established computed tomography as an ideal method of studying these features (Higgs et al., Reference Higgs, Glover, Dahlgren and Little2011a, Reference Higgs, Little, Glover, Dahlgren, Smith and Dominici2012). The results presented here are the first systematic examination of the borings of multiple Osedax species using CT scanning. Below, we discuss the results with reference to the autecology, synecology and palaeontology of this diverse genus of polychaete worms.
Factors driving morphological diversity in Osedax borings
The range of boring morphologies illustrated above shows that Osedax borings are highly variable between species and previous studies have shown that the boring morphology is also variable within a species (Higgs et al., Reference Higgs, Glover, Dahlgren and Little2011a). Disentangling this intraspecific variation from interspecific variation is dependant on identifying the factors that govern the growth of root tissues in each species. A detailed study of borings created by Osedax mucofloris in whale bones showed that boring morphology was primarily determined by bone structure: borings were consistent on the same bone but highly variable when compared between different bone types (Higgs et al., Reference Higgs, Glover, Dahlgren and Little2011a). This phenomenon is also observed here for Osedax japonicus, O. rubiplumus, O. ‘nude palp C’, O. ‘nude palp E’, O. ‘nude palp #20’ and the Osedax specimens on the San Diego Trough whale bone. In all of these cases the morphology of borings within a particular species is consistent when examined from the same bone, generally confirming the role of bone structure in determining the morphology of Osedax borings.
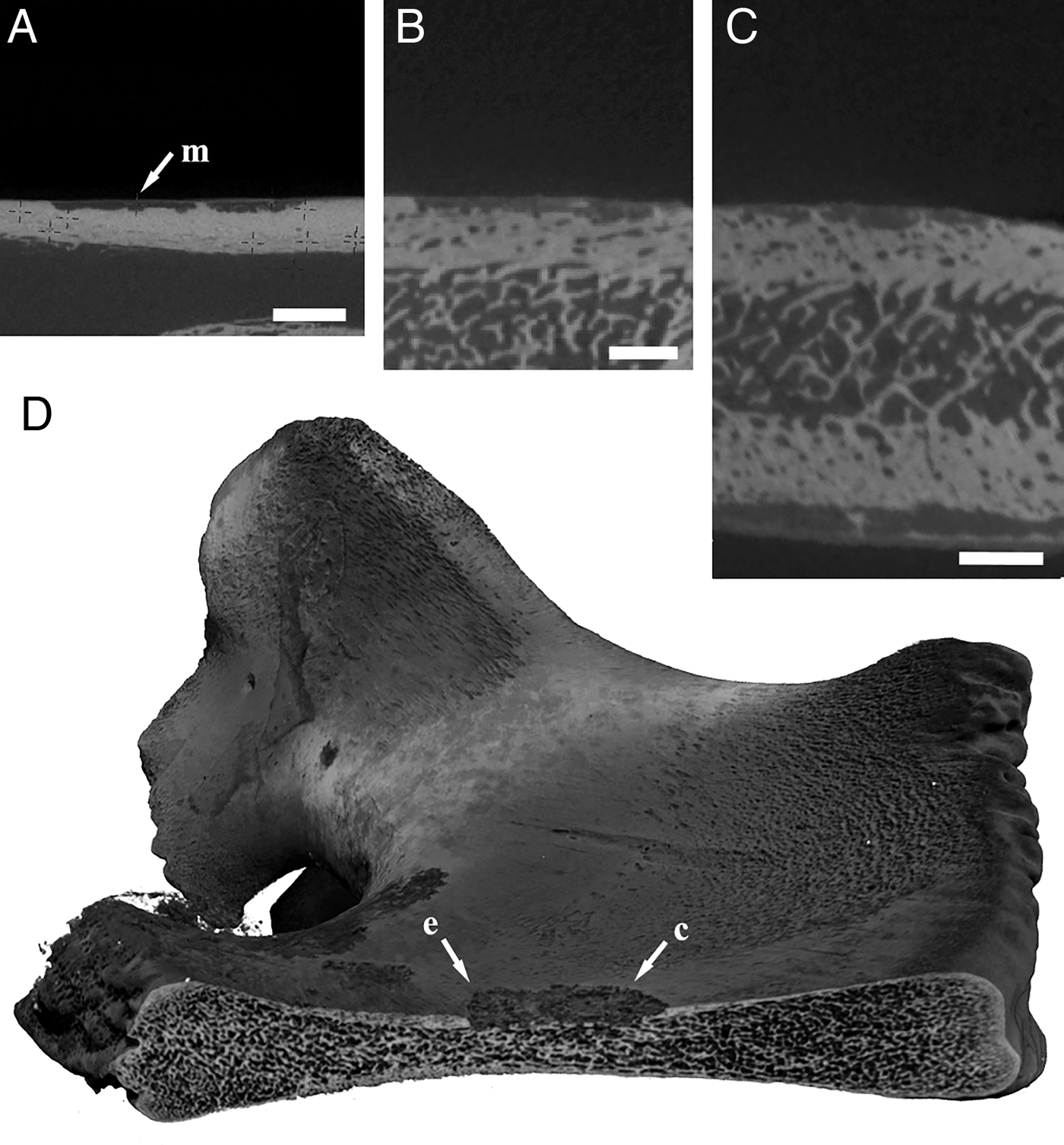
Where multiple species co-exist on the same bone, each species may display a different boring morphology, showing that there are also species-specific factors that influence boring morphology. For example, the cow bone collected from Monterey Canyon housed one specimen of Osedax frankpressi and multiple individuals of two undescribed species. All three species displayed contrasting boring morphologies (Figures 6D & 8B). One of the undescribed species (O. ‘nude palp #20’) was more prevalent on this bone and all individuals of this species showed the same boring morphology. The consistent differences in boring morphology between species may lie in the detailed mechanisms of how they erode the bone.

Fig. 8. Cow femur from Monterey Canyon colonized by multiple Osedax species; (A) experimentally implanted bone in situ, with the gelatinous tubes of Osedax ‘nude palp #20’ visible on the bone; (B) computed tomography scan showing relative position of a single large O. frankpressi boring (arrows) and multiple borings of Osedax ‘nude palp #20’ (other voids in the bone matrix).
In addition to phylogeny and bone structure, ontogenetic development of root tissues can lead to changes in the morphology of Osedax borings such that complex boring morphologies develop from simpler ones. For example, the multi-annexed borings produced by large individuals of Osedax ‘yellow-palp’ are more complex than the simple hemispherical borings created by smaller individuals. Similarly, the simple bulb shaped chamber created by the small individuals of O. rubiplumus shown above lacks the discrete root projections figured in the original description of the species, which develop as individuals mature (Greg Rouse, personal communication). The ontogenetic development of complexity contrasts with the simple hemi-ellipsoidal shape of O. mucofloris borings, which remains consistent in shape over a range of sizes, indicating that there is little ontogenetic variation in this species (Higgs et al., Reference Higgs, Glover, Dahlgren and Little2011a).
Resource partitioning and coexistence of Osedax species
Vertebrate skeletons are spatially and temporally patchy resources in the deep-sea, which are degraded by Osedax worms over time, eventually leading to the demise of the Osedax populations living on them. The bones are often densely populated and it is common for multiple Osedax species to occupy the same skeleton, or even the same bone in close proximity (see Table 1). For example, one whale skeleton monitored in Monterey Canyon had seven distinct species of Osedax living on it concurrently, with six of them coexisting for several years (Lundsten et al., Reference Lundsten, Schlining, Frasier, Johnson, Kuhnz, Harvey, Clague and Vrijenhoek2010). Assuming limiting resources, classic niche theory suggests that these species must differ in some ecological trait in order to coexist, i.e. niche differentiation (Chesson, Reference Chesson2000). Just as plants may segregate their root systems in soil to avoid competition for resources (Schenk et al., Reference Schenk, Callaway and Mahall1999; Schenk, Reference Schenk2006), it may be hypothesized that Osedax root tissues will be segregated spatially within a single bone when resources are limiting so as to access different parts of the resource spectrum. Root tissues could be segregated by depth within the bone or by type of bone tissue. At least three of the bones used in this study contained multiple species, allowing us to test this hypothesis.
The cow bone from Monterey Canyon contained three species of Osedax, each with a distinct boring morphotype. The root tissue of Osedax ‘nude palp sp. #20’ and that of the unidentified species were both located just below the surface of the bone, whereas that of O. frankpressi sits deeper into the bone, at the base of a relatively long tube (Figure 8). Although only one individual of O. frankpressi was available for study with CT, deep-penetration of their roots into the bone is commonly observed (Greg Rouse, personal communication). Similarly, the roots and ovisac of the Osedax species from the San Diego Trough sit at the base of a long tube section that penetrates deep into the spongy (and lipid-rich) part of the bone (Figure 5; Table 2). This is in stark contrast to the root growth of O. mucofloris, which closely follows the cortical (collagen-dense) part of the bone near the surface (Higgs et al., Reference Higgs, Glover, Dahlgren and Little2010, Reference Higgs, Glover, Dahlgren and Little2011a). These two contrasting boring types show that there may be different spatial niches within a bone, occupied by different Osedax species, although differences in size and tissue distribution between cow and whale bones of various sizes could also conceivably influence burrow morphologies. An even more extreme example of spatial differentiation has apparently evolved in another undescribed OTU, Osedax ‘spiral’, which exploits bones that are buried in the sediment (Braby et al., Reference Braby, Rouse, Johnson, Jones and Vrijenhoek2007). In this species, the root tissues are highly filamentous and bear more of a resemblance to their botanical analogues.
The distinction between Osedax species in a bone is not always clear-cut. At least four species co-occurred on the pig bones from Sagami Bay, and no distinction could be made between the borings of the closely related Osedax ‘nude palp’ species (Figure 9A–C). Borings of Osedax ‘yellow palp’ were noticeably deeper than those of the other species and their roots seemed to undercut those of the ‘nude palp’ species (Figure 9C). Additionally, the Osedax ‘white collar’ boring is not at all distinguishable from those of the Osedax ‘nude palp’ species. Caution must be exercised in interpreting niche differentiation in cow bones, however, because cow bones are unlikely to be natural habitat for Osedax. Nonetheless, taken together, these examples suggest that some degree of spatial niche differentiation exists among different Osedax species occupying a particular bone, but the diversity of spatial niches appears to be far less than the diversity of Osedax species. In other words many species still display spatial niche overlap. However, we cannot say from the present data whether this niche overlap persists when resources are limiting (e.g. at very high population densities), and in natural habitats such as adult whale bones, where the full potential niche dimensionality is likely to be present.

Fig. 9. Pig limb bone colonized by multiple Osedax species: (A) computed tomography reconstruction showing species marked with crosses; (B) transverse cross-section through bone; (C) longitudinal cross-section through bone. np-C, O. ‘nude palp C’; np-E, O. ‘nude palp E’; np-M, O. ‘nude palp M’; wc, O. ‘white collar’; yp, O. ‘yellow palp’. Scale bars: B and C, 5 mm.
The three bones analysed here only provide a snapshot in time and cannot provide information on temporal dynamics of recruitment, population growth and resource utilization that may facilitate co-existence of species (e.g. Leibold et al., Reference Leibold, Holyoak, Mouquet, Amarasekare, Chase, Hoopes, Holt, Shurin, Law, Tilman, Loreau and Gonzalez2004). At the regional scale, bathymetric segregation plays a role for many of the Osedax species living in Monterey Bay, California (Lundsten et al., Reference Lundsten, Schlining, Frasier, Johnson, Kuhnz, Harvey, Clague and Vrijenhoek2010). At the local scale, temporal segregation also plays a role, as Osedax species exhibit changing dominance as bones decompose, with some species disappearing altogether (Braby et al., Reference Braby, Rouse, Johnson, Jones and Vrijenhoek2007; Pradillon et al., Reference Pradillon, Kawato, Kubokawa, Fujiwara, Fujiwara and Takeoka2009; Lundsten et al., Reference Lundsten, Schlining, Frasier, Johnson, Kuhnz, Harvey, Clague and Vrijenhoek2010). The composition of nutritional symbionts associated with various Osedax species also change over time, but the symbionts do not appear to be linked to particular hosts (Salathé & Vrijenhoek, Reference Salathé and Vrijenhoek2012). Heterogeneity in the skeletal resource (see Higgs et al., Reference Higgs, Little and Glover2011b) may provide another mechanism for niche differentiation, but given the strong role of dispersal and recruitment in shaping the composition of Osedax communities (Rouse et al., Reference Rouse, Worsaae, Johnson, Jones and Vrijenhoek2008; Vrijenhoek et al., Reference Vrijenhoek, Johnson and Rouse2008b) it is also likely that neutral processes are important in maintaining Osedax species coexistence. Neutral theory assumes that all species are functionally equivalent and that community structure is primarily shaped by stochastic demographic mechanisms (Hubbell, Reference Hubbell2005). It was initially assumed that neutral and niche theories were mutually exclusive, but current opinion posits a continuum where ‘niche and neutral processes combine to generate coexistence’ (Adler et al., Reference Adler, Hillerislambers and Levine2007). We suggest that such a combination of mechanisms may explain why spatial niche differentiation is not more evident here, given the high global and local diversity of what appear to be functionally similar Osedax species coexisting on vertebrate carcasses in space and time.
Osedax borings as trace fossils
Detailed information on the morphology of borings is essential for documenting the fossil record of Osedax (Kiel et al., Reference Kiel, Goedert, Kahl and Rouse2010; Higgs et al., Reference Higgs, Little, Glover, Dahlgren, Smith and Dominici2012) and elucidating their evolutionary history (Vrijenhoek et al., Reference Vrijenhoek, Johnson and Rouse2009), as the soft bodied animals themselves are unlikely to be preserved in the geological record. The diverse array of borings illustrated here greatly expands the range of borings known to be created by Osedax, allowing more detailed information to be gleaned from the fossil record. For example, fossil borings in fish bones shown by Kiel et al. (Reference Kiel, Kahl and Goedert2012) are almost identical in size and shape to those of O. japonicus shown here. Additionally, the consistency of boring morphology by different Osedax species in the same bone (see above) allows palaeontologists to estimate the minimum number of species that were living on a fossil bone where different boring morphotypes are present; i.e. multiple ichnospecies of trace fossil found on the same bone probably represent multiple biological species.
Osedax traces in fossil bones may take several forms depending on how much the borings have been degraded before burial (Higgs et al., Reference Higgs, Little, Glover, Dahlgren, Smith and Dominici2012). In cancellous bone with only a thin layer of cortical bone, borings are likely to collapse and appear as shallow chambers (sensu Pirrone et al., Reference Pirrone, Buatois and Bromley2014) in the bone surface (Figures 4D and 9A; see also Higgs et al. (Reference Higgs, Glover, Dahlgren and Little2011a), figure 6A). In dense cortical bone borings are more likely to be preserved intact and simply appear as small round holes in the bone surface. Borings created by species that lack a substantial tube section (such as O. japonicus) may also collapse, leaving deep, hemispherical chambers in the dense bone. Such features may be caused by a number of organisms, so more detailed information is needed to identify Osedax in fossil bones (Higgs et al., Reference Higgs, Little, Glover, Dahlgren, Smith and Dominici2012). The peculiar way in which Osedax hollow out individual bone trabeculae (Figures 3D & 7) as part of the boring process was first shown by Higgs et al. (Reference Higgs, Glover, Dahlgren and Little2011a) for O. mucofloris. This pattern of bioerosion is also shown here for O. antarcticus and has been associated with other Osedax borings (Higgs, Reference Higgs2012). The hollowed out trabeculae may be taken as indicators of Osedax activity and offer additional diagnostic evidence when assignment of fossil borings to Osedax is not straightforward (see discussion in Higgs et al., Reference Higgs, Little, Glover, Dahlgren, Smith and Dominici2012). Furthermore, it may provide a relatively cheap and quick way of identifying Osedax activity in fossil bones, since micro-CT technology is not widely available at low cost. A similar pattern of bone erosion was also observed on fossil whale bone from the Miocene (Amano et al., Reference Amano, Little and Inoue2007; Figure 2E), although no other signs of Osedax traces were described in this instance.
The presence of hollowed out trabeculae on bones from the San Nicolas slope indicates that Osedax was once present on this carcass, but had died out by the time that it was first sampled over fifty years after the carcass arrived on the seafloor (Schuller et al., Reference Schuller, Kadko and Smith2004). Exactly why the Osedax population died out is not clear, but may be related to natural succession following the diminishing collagen resources in the surficial portions of the bone as the dense parts of bones were eaten away. Resource exhaustion by Osedax may explain the apparent disparity in rates of decomposition between the large San Nicolas skeleton and those of juvenile whale skeletons of Monterey Canyon that were estimated to degrade or disappear in under 10 yr (Lundsten et al., Reference Lundsten, Schlining, Frasier, Johnson, Kuhnz, Harvey, Clague and Vrijenhoek2010). Factors such as bone size and degree of bone calcification are also likely to be important for bone resistence to Osedax boring and the time scales of whale-skeleton persistence at the deep seafloor (Smith & Baco, Reference Smith and Baco2003).
ACKNOWLEDGEMENTS
Drs Richie Abel and Russell Garwood provided guidance in CT scanning. Valuable advice and fieldwork assistance was provided by Dr Greg Rouse (Scripps Institution of Oceanography) during the project. The authors are grateful to Iris Altamira (University of Hawaii, supported by NSF funding) and Harim Cha (Scripps Institution of Oceanography) for preparing shipments of whale bones and obtaining the necessary CITES documentation.
FINANCIAL SUPPORT
This work was funded by a CASE studentship from the Natural Environment Research Council UK (NE/G523755/1) to N.D.H. Writing of the paper was facilitated by a Postdoctoral Research Fellowship to N.D.H. from the Marine Institute, Plymouth University. This is publication no. 9126 from SOEST, University of Hawaii at Manoa. The manuscript was greatly improved by thoughtful comments and suggestions from Robert Boessenecker and an anonymous referee.