Introduction
Swiss chard (Beta vulgaris L. var. cicla), also known as ‘stem chard’, belongs to the Chenopodiaceae family, which is closely related to garden beets and spinach. Beets are wild forms of B. vulgaris and can be found along the shores of the Mediterranean. It is grown for its leaves and was first mentioned in literature from Mesopotamia dating back to the 9th century (Oyen, Reference Oyen, Grubben and Denton2004). Archeological investigations showed that it has been cultivated for about 2500 years in China (Shun et al., Reference Shun, Chu, Frese, Maggioni, Frese, Germeier and Lipman2000). The leaves and stalks are the edible parts of Swiss chard and the plant is widely cultivated and consumed throughout northern India, South America (Tindall, Reference Tindall1983), the Mediterranean countries and USA.
Plant breeding programmes and variety selection have primarily been targeted at fundamental agronomic traits namely yield, improving the quality of the edible parts of the species and disease resistance (Davey et al., Reference Davey, van den Bergh, Markham, Swennen and Keulemans2009). In recent years, breeding strategies have focused on increasing vitamin and mineral nutrition and on enhancing the nutrient composition of the plant, which could make significant contribution to human nutrition and health (Grusak and Dellapenna, Reference Grusak and Dellapenna1999). Increasing the nutritional properties of food through plant breeding was proposed as a strategy for combating widespread mineral deficiencies. Traditional varieties, wild or primitive forms of cultivated crops showed an enhanced ability to accumulate some micronutrients in edible parts of the plant (Frossard et al., Reference Frossard, Bucher, Machler, Mozafar and Hurrell2000). Davey et al. (Reference Davey, van den Bergh, Markham, Swennen and Keulemans2009) suggested that exploration of existing micronutrient-rich germplasm or applying of breeding methods can enrich crops in terms of micronutrient levels. The knowledge of genetic variation will ensure that the breeding of crop will improve the mineral composition of cultivar to solve deficiency problems in human nutrition.
There has been little research into the degree of genetic diversity of mineral concentrations using multivariate analysis in the edible parts of several cultivated species. Recent studies have been carried out in order to determine the diversity in wild Vigna species, chenopods and kale (Brassica oleracea L. var. acephala DC.), in respect of mineral composition (Bisht et al., Reference Bisht, Bhat, Lakhanpaul, Latha, Jayan, Biswas and Singh2005; Bhargava et al., Reference Bhargava, Shukla, Srivastava, Singh and Ohri2008; Fadigas et al., Reference Fadigas, Dos Santos, De Jesus, Lima, Fragoso, David and Ferreira2010). Among several vegetable species, leafy vegetables can be grown easily in several climatic conditions and contain high amounts of minerals and vitamins which are essential in reducing malnutrition. Pyo et al. (Reference Pyo, Lee, Logendra and Rosen2004) underlined the fact that previous studies reported that Swiss chard has a high vitamin content (vitamins A, B and C), Ca, Fe and P (Tindall, Reference Tindall1983; Anthony et al., Reference Anthony, Mark and Margot1992; Donald and George, Reference Donald and George1997). Although Swiss chard is consumed in several regions of the world, the mineral and trace element composition of Swiss chard germplasm has not been studied. Moreover, classified qualitative variations among accession using multivariate analyses were not reported while nutritional valuable species and significant variability were indeed reported in vitamin C along with a nutritive value among Swiss chard varieties (Pokluda and Kuben, Reference Pokluda and Kuben2002).
The aims of the research presented herein were (i) to characterize the quantitative nutrient composition of Swiss chard accessions and (ii) to explore the extent of genetic variation in foliage nutritional concentrations of whole Turkish Swiss chard accessions using multivariate analysis.
Materials and methods
Experimental site and plant materials
The experiment was conducted at the experimental field of Ege University, Agriculture Faculty, Department of Horticulture, Izmir, Turkey. The experimental field is located at 38°28′N latitude, 27°15′E longitude and at an altitude of 25 m above sea level. To evaluate the quantitative variation among Swiss chard accessions based on the nutritional composition of foliage, the germplasm was collected in several regions of Turkey. A total of 54 Swiss chard (B. vulgaris subspecies cicla) accessions, with 52 accessions and two cultivars as reference, one local and one foreign, were collected from Turkey and Germany (Supplementary Table S1, available online only at http://journals.cambridge.org). The seeds of 52 accessions which represent the whole Swiss chard genetic resources collections of Turkey were obtained from the national state gene bank of Aegean Agricultural Research Institute, Izmir, Turkey.
Experimental set-up
The study was carried out in the autumn of 2007 and the winter of 2008. The seeds were sown in the first week of October in each of these years using 4.5 m2 plots which contained 20 plants per plot. The experimental design was a randomized complete block design with three replications per accession. The soil of the experimental site was sandy loam soil with the following characteristics: pH 7.58, organic matter content of 1.10%, CaCO3 1.45%. Primary and secondary nutrient contents of the soil were: total N = 0.110%, available P = 5.67 mg/kg, K = 319.8 mg/kg, Ca = 4300 mg/kg, Mg = 174.2 mg/kg, Na = 56.4 mg/kg, Fe = 19.61 mg/kg, Cu = 2.18 mg/kg, Zn = 0.90 mg/kg, Mn = 4.36 mg/kg. Each accession was sown by hand and the cultural practices of the region were employed. Irrigation was provided through furrows weekly until the beginning of the rainy season. No chemical fertilizers, fungicides or insecticides were applied during cultivation, and weeds were controlled mechanically by hand until the plants had reached harvest maturity. Plant samples were harvested by hand when leaves were at full maturity and ready to be eaten.
Chemical analysis and data collection
The edible leaf parts (lamina and petioles) were washed using distilled water and dried at room temperature in order to remove external moisture. They were then placed in paper bags and oven-dried at 65°C for 24 h. The dried plant samples were ground in a blender for mineral composition determination. The total amount of N in the leaf samples was determined by the modified Kjeldahl method; P with colorimetry in wet digested samples, K, Ca and Na, with flame photometry (Eppendorf, Hamburg, Germany) and Mg, Fe, Zn, Cu and Mn using atomic absorption spectrometry (SpectrAA-220 FS; Varian, Mulgrave, VIC, Australia). Appropriate calibration controls (calibration curve method with commercial certified ICP (Inductively coupled plasma), multi-element standard solution; Merck, Darmstadt, Germany) were applied to each set of measurements. N, P, K, Ca, Mg, Na, Fe, Cu, Zn and Mn concentrations were calculated on a dry-weight basis, and NO3 and NO2 concentrations were determined colorimetrically according to the method of Balks and Reekers (Reference Balks and Reekers1955) on a fresh-weight basis.
Statistical analysis
The raw data obtained from leaf mineral composition of each accession were analyzed in triplicate, and 12 traits were recorded (NO2, NO3, N, P, K, Ca, Mg, Na, Fe, Cu, Zn and Mn). Principal component analysis (PCA) was carried out for quantitative data and the total amount of variation was calculated as the sum of extracted eigen values. PCA techniques can be used to reduce the information of a multidimensional data set in what can be displayed in a scatter plot with only two or three axes. The major part of variance of the data set comes to lie on the first, second and third axes (Friedt et al., Reference Friedt, Snowdon, Ordon and Ahlemeyer2007). In addition, the varimax factor rotations were applied in factor analyses in order to make the interpretation of the factors to be considered relevant and in order to maximize the loading of the variables in factors (Fadigas et al., Reference Fadigas, Dos Santos, De Jesus, Lima, Fragoso, David and Ferreira2010) using mathematics. Estimates of Euclidean dissimilarity coefficients were used to assess the relationships between accession and hierarchical clustering of accession which was performed using Ward's minimum variance method (Rabbani et al., Reference Rabbani, Iwabuchi, Murakami, Suzuki and Takayanagi1998). This method produces clusters with roughly the same amount of observation (Sokal and Michener, Reference Sokal and Michener1958). All data analyses were performed using the software package Statistica 6 (Statsoft, Inc., 2004).
Results
A total of 54 Swiss chard accession samples were collected from several regions of Turkey. Examined collection represents the whole Swiss chard material of the national germplasm and the results displayed significant variations for the primary and secondary nutrient composition. These traits are useful in evaluating germplasm diversity in the nutritional concentration context and for use in further breeding programmes which will focus on improving mineral concentrations in the Swiss chard cultivars, contributing to reduction of nutritional deficiencies in human nutrition.
Primary and secondary plant nutrients
The primary and secondary nutrient compositions of Swiss chard foliage showed a high degree of variation across the accessions. The concentrations ranged within the following limits: ammonium 44.3 and 28.81 g/kg, P 5.99 and 2.38 g/kg, K 49.4 and 28.60 g/kg, Ca 4.74–2.66 g/kg and Mg 9.43–3.33 g/kg (Table 1). The Na content ranged between 5.72 and 2.40 g/kg among the present accessions, Fe ranged between 159.65 and 77.22 mg/kg, and Cu between 16.96 and 7.60 mg/kg. The Zn (65.52–22.31 mg/kg) and Mn (33.89–12.57 mg/kg) concentrations showed a wider range, and TR 56046 and TR 35012 accessions showed twofold higher concentrations in Zn and Mn compared to other accessions. NO3 and NO2 ranged from 496.55 to 262.48 mg/kg and from 0.047 to 0.024 mg/kg, respectively, based on fresh weight.
Table 1 Mean performance of 54 cultivars and accessions of Swiss chard

Principal component analysis
The aforementioned 12 elements were investigated in order to assess the genetic relationships among the accessions and to show the patterns of variation by PCA. High degrees of variation occurred in the first five axes (74.39%) and the value indicated a high diversity in the composition of the examined accessions (Table 2). The first three principal components (PC) accounted for 49.86% of the total variation; the first PC explained 20.75% of the total variation and ammonium (N), NO3 and NO2, which are characters that contribute to the first axis. The PC2 was concerned with Mg, Na and Ca, and explained 14.74% of the total variation. The PC3 exhibited 14.38% of the total variation and consisted of Cu and Zn. K and Fe constituted the third (12.44%), and Mn and P remained on the fifth axis (12.09%). All analyzed parameters except P showed positive association in the five PC axes.
Table 2 Eigen values proportion of variability and minerals contributed to the first five principal components (PCs) of Swiss chard accessions

Cluster analysis
In order to determine the pattern of divergence among Swiss chard accessions, a dendrogram was constructed based on the nutritive elements' concentration using genetic distance values by the Ward's method (Fig. 1). The 54 Swiss chard accessions that had been examined were grouped into five major clusters for the 12 characteristics and cluster means are given in Table 3. Cluster I included 13 accessions and the highest, Cu (13.66 mg/kg) and Zn (47.16 mg/kg), concentrations were recorded in the first cluster while the lowest, K (35.0 g/kg), Ca (3.4 g/kg), Mg (4.8 g/kg) and Fe (87.89 mg/kg), concentrations were also obtained from the same cluster. Cluster II comprised 15 accessions; local cultivar and foreign cultivar also belonged to the first cluster that had the highest P content (4.1 g/kg), while at the same time the lowest, Ca (3.4 g/kg) and Mn (17.50 mg/kg), concentrations. The third cluster included nine accessions with moderate to low concentrations, and also the lowest Na (3.6 g/kg), and the highest ammonium (38.4 g/kg), Fe (120.02 mg/kg), NO3 (423.23 mg/kg) and NO2 (0.04 mg/kg) concentrations among the examined collections. The fourth cluster comprised 13 accessions and this group was qualitatively moderate in terms of the examined parameters, and had the lowest Cu (10.42 mg/kg) and Zn (33.88 mg/kg) concentrations. The fifth cluster included only four accessions having low amounts of ammonium (32.2 g/kg), P (3.0 g/kg), NO3 (304.68 mg/kg) and NO2 (0.028 mg/kg), while the highest amounts of K (39.5 g/kg), Ca (4.3 mg g), Mg (6.4 g/kg), Na (4.9 mg/kg) and Mn (22.83 mg/kg).
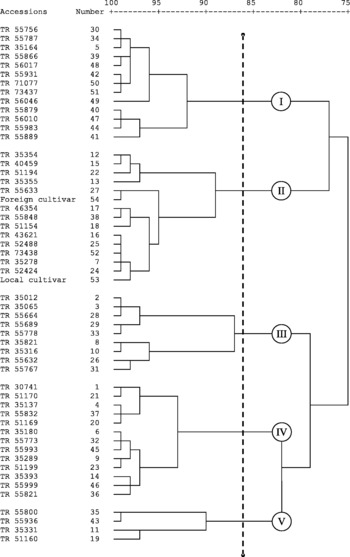
Fig. 1 Genetic relationship among 54 accessions of Swiss chard based on nutritive elements by Ward's clustering method.
Table 3 Mean values for five clusters and maximum (Max), minimum (Min), standard deviation (Std dev.) values for 12 nutritive elements

In order to visualize the relationships of accessions based on the concentrations of nutritive elements, the first three PCs were shown in 3D screen plot since it is difficult to group accessions based solely on the PC axes (Fig. 2). In addition, principal co-ordinate analysis represented the relationship among the nutritional concentrations and each mineral is plotted based on its PC score for each of the first three axes (Fig. 3).

Fig. 2 Patterns of relationships among 54 Swiss chard accessions due to the first three principal co-ordinates.

Fig. 3 PCA of the 12 nutritive elements among Swiss chard accessions.
Discussion
Over the past few years, one of the main objectives of plant breeding in modern agriculture has been to increase productivity by increasing yield (Grusak and Dellapenna, Reference Grusak and Dellapenna1999) and observed that high-quality produce in terms of shape, colour, appearance, size and weight, however, the application of breeding strategies in order to improve yield and quality of the crop has resulted in a decline of between 5 and 40% in mineral, vitamins and protein content in most foods particularly vegetables (Davis, Reference Davis2009). On the other hand, consumers' preferences have begun to change during the last few years and most people now focus not only on the visual-quality properties of a product but increasingly on taste, aroma and nutritional value in fruit and vegetables as well. Today, it is widely encouraged to devote more attention to flavour and to the nutritional quality of fruits and vegetables (Kader, Reference Kader2008). People are seeking the health benefits of fresh fruits and vegetables (Borah et al., Reference Borah, Baruah, Das and Borah2009) and consumer-oriented quality traits are becoming increasingly more important for plant breeders.
Even though morphological and molecular genetic diversity has been widely investigated in most plant species, mineral concentration levels in vegetables have received very little concern in the biodiversity context. Multivariate analysis is widely used in classifying germplasm, assessing genetic diversity or analyzing genetic relationships among breeding materials (Mohammadi and Prasanna, Reference Mohammadi and Prasanna2003; Bhargava et al., Reference Bhargava, Shukla, Srivastava, Singh and Ohri2008). In addition, the PC and hierarchical clustering pattern has been applied in order to evaluate, characterize and classify germplasm based on mineral composition, their concentration and accumulation in several plant species such as bean (Phaseolus vulgaris L.), onion (Allium cepa L.) and kale (Chope and Terry, Reference Chope and Terry2009; Fadigas et al., Reference Fadigas, Dos Santos, De Jesus, Lima, Fragoso, David and Ferreira2010; Santos et al., Reference Santos, Ferri, Santos, Faria, Oliveira and Thung2010). There is a lack of detailed information on Swiss chard's nutritional composition and its diversity. Furthermore, no significant efforts have been made to assess the genetic diversity for the mineral composition of Swiss chard accessions using multivariate analysis techniques.
In the present study, a total of 54 accessions were evaluated and the results indicated that significant differences were present among Swiss chard accessions and remarkably high concentration of nutrients. Dzida and Pitura (Reference Dzida and Pitura2008) showed that concentration of nutrients in the edible parts of Swiss chard depends on N rates and fertilizers. They also reported higher Ca (10.1–13.9 g/kg), P (5.3–11.9 g/kg) and K (64.5–92.3 g/kg) concentration whereas similar Mg (6.4–9.4 g/kg) concentration on a fresh-weight basis compared to our germplasm composition. Rozycki et al. (Reference Rozycki, Baigorria, Freyre, Bernard, Zannier and Charpentier1997) mentioned that a higher variation exists in nutrient composition between cultivars, non-hybrid and wild forms of Swiss chard compared to modern varieties. Pokluda and Kuben (Reference Pokluda and Kuben2002) pointed out significant differences in the agronomic properties and nutritional value among 12 Swiss chard cultivars. In the present study, a great deal of diversity is obtained among Swiss chard accessions in terms of the investigated parameters. Nutritional value and chemical composition of plant tissue may greatly vary depending on cultivation, fertilization (Dzida and Pitura, Reference Dzida and Pitura2008) and soil composition (Davey et al., Reference Davey, van den Bergh, Markham, Swennen and Keulemans2009). Chope and Terry (Reference Chope and Terry2009) explained the variations occurring among values of mineral concentration in onions by pre-harvest growing conditions, post-harvest treatment, analytical methods and genotype. In our study, the experiment was carried out in same-soil conditions, and same-cultivation practices were applied in all plots. Therefore, the great variability observed among accessions can be partly responsible for a genetically diverse population. The mineral composition differences between Swiss chards of different origins and varieties have been reported upon (Rozycki et al., Reference Rozycki, Baigorria, Freyre, Bernard, Zannier and Charpentier1997). Genetic variation caused by the mineral concentration differentiation within genotypes was also underlined in onions, kale, beans and Brassica rapa L. (Wu et al., Reference Wu, Schat, Sun, Koornneef, Wang and Aarts2007; Chope and Terry, Reference Chope and Terry2009; Fadigas et al., Reference Fadigas, Dos Santos, De Jesus, Lima, Fragoso, David and Ferreira2010; Santos et al., Reference Santos, Ferri, Santos, Faria, Oliveira and Thung2010). These genetic differences were reported in Fe and Zn contents of common bean seeds over different seasons and environments (Beebe et al., Reference Beebe, Gonzalez and Rengifo1999). Harrison and Bergman (Reference Harrison and Bergman1981) showed significant differences for leaf P, Ca, Mn, Cu and Zn concentrations in three cabbage cultivars. Bozokalfa et al. (Reference Bozokalfa, Yağmur, Ilbi, Eşiyok and Kavak2009) reported genetic variations for several nutrient compositions in wild (Diplotaxis tenuifolia) and cultivated rocket salad (Eruca sativa L.) accessions. Bhargava et al. (Reference Bhargava, Shukla, Srivastava, Singh and Ohri2008) revealed that the heavy-metal concentration in Chenopodium varied greatly among accessions and species and explained this result by the genetic ability of accumulation of heavy metals.
The results presented here demonstrate that Swiss chard accessions collected from Turkey were classified into five major clusters by multivariate analysis. The clustering pattern and principal co-ordinates obtained indicated that there was no relationship between geographic origin and their concentrations of the populations. No groups among Swiss chard accessions and variables transformed into new co-ordinates in a multi-dimensional space represented by three PC axes.
Leaf vegetables play a considerable role in the human diet and their consumption increases every day due to the fact that they contain significant nutritional sources and minerals (Kuhnlein, Reference Kuhnlein1990). Improving of cultivars in terms of targeted macro and micro nutritional composition continues to increase. Davey et al. (Reference Davey, van den Bergh, Markham, Swennen and Keulemans2009) informed that in order to reduce micronutrient malnutrition (particularly vitamin C, Fe and Zn deficiencies) in a number of countries, a comprehensive breeding programme appears to be required for improving food crops that are rich in micronutrients. In the light of this, it should be noted that the more the Swiss chard that is consumed, it can be recognized for a notably high source of dietary fibre, vitamin A, vitamin C, vitamin E, vitamin K, riboflavin, vitamin B6, Ca, Fe, Mg, P, K, Na, Cu, Mn, thiamin and Zn.
This is the first comparative study on genetic diversity in foliage of Swiss chard accessions, available nutritional concentration and their diversity among Swiss chard accessions collected in Turkey for their mineral concentration. The present research showed a wide variability among 54 Swiss chard accessions in minerals, NO3 and NO2 concentration; and the extent of variation indicates that there is a high potential for developing enriched Swiss chard varieties for different mineral elements to provide additional nutrients in diets. The present results, besides expressing the importance of nutritional concentration in Swiss chard, also allow nutritionally rich accessions to be used in breeding programmes aimed at obtaining cultivars with high nutrient concentrations. A hierarchical agglomerative procedure separates accessions into five clusters and some accessions having high concentrations of elements are placed in different clusters. The results show that several accessions have the potential to participate in base material for further breeding programmes in order to improve the nutritional quality of Swiss chard germplasm.
Today in most countries, plant breeding and improved crop management focus on developing more nutrient-dense, stable food crops (Zhang et al., Reference Zhang, Song, Yan, Tang, Zhao, Zhang, He, Zou and Ortiz-Monasterio2010) in an attempt to solve nutritional problems. Furthermore, to reduce the widespread micronutrient deficiencies among the human population, nutritionally improved cultivars should be available and their consumption should be encouraged. Thus, it is very important to identify germplasm with high levels of targeted nutritive elements in the selection programme in order to enhance the nutritional composition of Swiss chard through conventional plant breeding.
Conclusion
There is an increasing amount of research aimed at improving cultivars in terms of targeting nutritional composition in order to reduce micronutrients deficiencies (Welch, Reference Welch2000; Welch and Graham Reference Welch and Graham2004). A comprehensive breeding programme seems to be required in order to improve food crops rich in micronutrients. The consumption of nutrient-enriched species or cultivars such as Swiss chard, which has beneficial health effects, may reduce nutrient deficiencies in diet and genotypic selection and may reduce dietary micronutrient deficiencies (Wu et al., Reference Wu, Schat, Sun, Koornneef, Wang and Aarts2007). In view of this, Swiss chard is a good source in terms of several health-benefit compounds and provides high amounts of vitamins A, C, E and K, as well as Fe, Mn, Mg, K and dietary fibre. In addition, Swiss chard has been cultivated in many parts of the world for year-round availability at a low cost and is widely used in many traditional dishes (Gao et al., Reference Gao, Han and Xiao2009). Identification of Swiss chard accessions may contribute to valuable scientific knowledge in terms of new breeding programmes for the improvement of targeted health-promoting minerals. The objective of this study presented here was achieved and the study concluded that it is possible to identify genetic differentiation among Swiss chard accession for some nutritional elements; and the results provided show great variability among the accessions. This valuable information demonstrates the potential nutritional value of Swiss chard and the extent of nutritional diversity. In addition, the results highlight the potential of germplasm for breeding programmes to increase the total quantity of mineral elements in the edible parts of the plants and underlined TR 35331, TR 56046 accessions that could be used as gene sources due to their high levels of K, Ca and Zn.
Acknowledgements
The authors thank Aegean Agricultural Research Institute Gene Bank, Izmir, Turkey for providing the seed samples. Part of this work was supported through funds from the Ege University Scientific Research Fund under grant number 2005-TTUM-002.








