INTRODUCTION
There has been unprecedented attention in recent years to the interplay between mild traumatic brain injury (MTBI) and posttraumatic stress disorder (PTSD) (Bryant, Reference Bryant2001b; Moore, Terryberry-Spohr, & Hope, Reference Moore, Terryberry-Spohr and Hope2006). Much of the debate has focused on the extent to which PTSD can develop after MTBI. Some commentators have argued that people who sustain a MTBI are unlikely to develop PTSD, because they suffer impaired consciousness secondary to the brain injury, and accordingly, do not encode the necessary mental representations of the traumatic experience to cause fear reactions (Sbordone & Liter, Reference Sbordone and Liter1995). In contrast, others have argued that PTSD can occur after MTBI, because following MTBI, people can still have islands of memory for the traumatic experience, and some fear conditioning can occur despite impaired consciousness, and much trauma can occur following resolution of posttraumatic amnesia. For example, once cognitive processing has resumed normal functioning, patients can suffer traumatic experiences as a result of extraction from the site of traumatic injury, medical procedures, or extreme pain (Bryant, Reference Bryant2001a).
There is increasing evidence that PTSD can develop after MTBI (Bryant & Harvey, Reference Bryant and Harvey1998a; Castro & Gaylord, Reference Castro and Gaylord2008; Greenspan, Stringer, Phillips, Hammond, & Goldstein, Reference Greenspan, Stringer, Phillips, Hammond and Goldstein2006; Harvey & Bryant, Reference Harvey and Bryant2000; Hoge et al., Reference Hoge, McGurk, Thomas, Cox, Engel and Castro2008; Levin et al., Reference Levin, Brown, Song, McCauley, Boake, Contant, Goodman and Kotria2001). Intriguingly, there is increasing evidence that MTBI may be associated with increased risk for PTSD; two recent studies of combat troops returning from Iraq or Afghanistan found that sustaining a MTBI (typically as a result of explosions) was associated with increased rates of PTSD (Hoge et al., Reference Hoge, McGurk, Thomas, Cox, Engel and Castro2008; Schneiderman, Braver, & Kang, Reference Schneiderman, Braver and Kang2008). Several explanations have been suggested to explain the increased occurrence of PTSD after MTBI. First, prevailing models of PTSD posit that it develops as a result of impaired functioning of the medial prefrontal cortex, which limits regulation of the amygdala (Charney et al., Reference Charney, Southwick, Krystal, Deutch, Murburg and Davis1993). This model is supported by much neuroimaging evidence that PTSD is characterized by diminished recruitment of the medial prefrontal cortex during fear processing (Lanius, Bluhm, Lanius, & Pain, Reference Lanius, Bluhm, Lanius and Pain2006; Rauch & Shin, Reference Rauch and Shin1997). It has been suggested that damage to the prefrontal networks during the course of the MTBI may compromise the functioning of these networks that are implicated in PTSD (Bryant, Reference Bryant2008). Second, cognitive models of PTSD propose that the extent to which people catastrophize about a traumatic experience and its aftermath is pivotal in fueling subsequent PTSD (Ehlers & Clark, Reference Ehlers and Clark2000). This model presumes that the trauma survivor possesses adequate cognitive resources to engage adaptive cognitive strategies to manage the traumatic experience. MTBI can impair one’s cognitive resources (Landre, Poppe, Davis, Schmaus, & Hobbs, Reference Landre, Poppe, Davis, Schmaus and Hobbs2006) and may compromise one’s capacity to engage in the optimal cognitive strategies to manage the aftermath of a psychological trauma. There is much evidence that inappropriate cognitive strategies after trauma is a major predictor of PTSD (Bryant & Guthrie, Reference Bryant and Guthrie2007; Dunmore, Clark, & Ehlers, Reference Dunmore, Clark and Ehlers1997, Reference Dunmore, Clark and Ehlers2001), and it is possible that people with MTBI have insufficient cognitive resources to engage these cognitive strategies, which results in greater PTSD. Alternately, it is possible that increased rates of PTSD following MTBI in recent military studies may reflect greater trauma exposure in troops who sustain MTBI.
There is evidence that impaired memory for the event may be protective against developing PTSD. One recent study of 228 motor vehicle accidents administered a questionnaire that indexed the extent to which patients with MTBI recalled details of the traumatic accident (Gil, Caspi, Ben-Ari, Koren, & Klein, Reference Gil, Caspi, Ben-Ari, Koren and Klein2005). This study found that the less patients recalled of their traumatic event, the less likely they were to develop PTSD. This finding raises questions concerning the role of memory for a traumatic event in the genesis of PTSD after MTBI. Accordingly, the current study aimed to (a) index the prevalence of PTSD in patients who did and did not sustain a MTBI after a traumatic injury, (b) assess the frequency of each PTSD symptom across MTBI and no-MTBI patients, and (c) evaluate the relationship between posttraumatic amnesia (PTA) and PTSD symptoms. We hypothesized that whereas PTSD would occur at least as often in MTBI and no-TBI patients, that reexperiencing symptoms would be inversely related to PTA length because fear conditioning of events involving the trauma would be limited by the reduced encoding of the experience.
METHOD
Participants
Randomized admissions to four level 1 trauma centers across three states in Australia were recruited into the study between April 2004 and February 2006. The study was approved by the Research and Ethics Committee at each hospital. Inclusion criteria included mild traumatic brain injury or no brain injury (MTBI: American Congress of Rehabilitation Medicine, 1993); aged between 16 and 70 years of age; could understand and speak English proficiently; and had a hospital admission of greater than 24 hours following traumatic injury. MTBI was defined as a documented injury to the head, impaired consciousness for less than 30 minutes, post-traumatic amnesia of less than 24 hours, and a Glasgow Coma Scale score in the range 13–15 (American Congress of Rehabilitation Medicine, 1993). This information was obtained through medical records and interviews of the patient. Individuals were excluded from the study if they had moderate or severe head injury; were currently psychotic or suicidal; were non-Australian visitors, or under police guard. Individuals who met entry criteria were randomly selected using an automated, random assignment procedure, stratified by length of stay based on medical practitioners estimated projected length of stay of each patient. This approach was adopted to ensure that we did not differentially recruit patients who had longer hospital stays because they may be more accessible. Acute assessments were conducted an average of 7.2 days (SD = 9.6) after injury. Following written informed consent, trained researchers conducted clinical interviews assessing past psychiatric history, current PTSD, and depression. They obtained permission to follow-up patients 3 months after hospital admission with a telephone interview for a second clinical interview assessing PTSD.
Trained researchers assessed PTSD symptoms during the hospital admission and at 3 months post-injury using the Clinician Administered PTSD Scale-IV (CAPS-IV; Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Charney and Keane1998). The CAPS assesses each PTSD symptom on 4-point scales of frequency and intensity of each symptom, and each question was anchored to responses to the recent traumatic injury. The CAPS possesses good sensitivity (.84) and specificity (.95) relative to the Structured Clinical Interview for DSM-IV Disorders (SCID) PTSD diagnosis, and also possesses sound test-retest reliability (.90). At the 3-month assessment, patients were again assessed for PTSD using the CAPS-IV, which was administered over the telephone. Diagnosis of PTSD was determined by the 2–1 scoring rule (occurrence of symptom required scores of at least 2 for symptom frequency and 1 for symptom intensity) (Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Charney and Keane1998). We followed previous studies by omitting item 8 of the CAPS (“Inability to recall an important aspect of the trauma”) from the scoring process of PTSD because of high levels of mild traumatic brain injury (MTBI) in the sample and the difficulty differentiating organic from psychogenic amnesia (O’Donnell, Creamer, Bryant, Schnyder, & Shalev, Reference O’Donnell, Creamer, Bryant, Schnyder and Shalev2003). Information regarding demographic, hospital admission, and injury-related factors were obtained from medical records and trauma registries from each of the hospitals. Injury information included the Injury Severity Score (ISS), which is a measure of overall injury severity (American Association for Automotive Medicine, 1990), cause of traumatic injury, hospitalization length, and presence of mild traumatic brain injury.
Posttraumatic amnesia (PTA) is defined as the period of disturbed memory function and disorientation following neurotrauma (Forrester, Encel, & Geffen, Reference Forrester, Encel and Geffen1994). Length of PTA in the current study was assessed retrospectively following a similar strategy to Gronwall and Wrightson (Reference Gronwall and Wrightson1980). Specifically, participants were asked to describe the event during which they received their injury, starting from just before the injury occurred. They were then asked “And what happened then?” until a continuous memory is evident. Witnesses, ambulance, and medical records were consulted to set the times of the accident and subsequent events. The duration of PTA was defined as the elapsed time between the return of continuous memory and the accident.
Data Analysis
The likelihood of developing PTSD in MTBI and no-TBI participants was analysed with logistic regression that controlled for ISS. One-way analyses of covariance (ANCOVAs) were conducted between CAPS total scores and cluster scores that controlled for ISS. To determine the association between PTA length and PTSD symptoms, we calculated Pearson Product partial correlations between PTA length and CAPS scores that controlled for the influence of ISS (in recognition of the higher ISS scores in MTBI patients) with a Bonferonni-adjusted alpha of .001.
RESULTS
Participant Characteristics
There were 1593 participants approached, and 1167 agreed to participate (73%). Participants included 791 males and 280 females of mean age 37.59 years (SD = 13.98). Four hundred and fifty-nine experienced a MTBI and the mean ISS was 10.98 (SD = 8.11). Table 1 presents participant characteristics. Four hundred and seventy-eight (43%) experienced a MTBI (including patients with extracranial hematomas and contusions). A normal cerebral CT scan was reported in 346 (72.4%) of MTBI participants; intracranial abnormalities were observed in 54 (11.3%) patients, which suggests they sustained a “complicated” traumatic brain injury (Williams, Levin, & Eisenberg, Reference Williams, Levin and Eisenberg1990). Cerebral CT scan was not performed in 78 (16.3%) individuals. The mean PTA length was 2.38 hours (SD = 1.60). More patients with a MTBI than with no TBI suffered a motor vehicle accident (74.1% vs. 56.6%), (χ2 = 36.52, df = 1, p < .0001). The mean Injury Severity Score (ISS, American Association for Automotive Medicine, 1990) was 10.87 (SD = 7.94); patients with a MTBI (M = 13.74, SD = 8.89) had higher ISS scores than patients with no TBI (M = 8.78, SD = 6.51), t (df = 1127) = 10.4, p <. 0001. Participants spent an average of 12.38 (SD = 12.93) days in hospital; there was no difference in number of days spent in hospital between MTBI (M = 12.96, SD = 13.79) and No-TBI (M = 11.80, SD = 12.08) patients, t (df = 1127) = 1.5, p = .14. Individuals who refused to participate in the current study did not differ from participators in gender (χ2 = 1.50, df = 1, ns), length of hospital admission, t (1571) = .92, ns, or ISS, t (1571) = 1.46, ns.
Table 1. Demographic characteristics of the sample

At the 3-month follow-up assessment, 157 patients could not be contacted or declined to participate; 920 were interviewed by telephone, representing 85% of the initial sample. Patients at the follow-up assessment did not differ from those who did not participate in terms of age, length of hospital stay, injury severity score, MTBI status, or PTA length. Participants who were lost to follow-up (M = 22.89, SD = 20.31) had higher CAPS scores at baseline than those who were retained (M = 17.44, SD = 15.87), t (1136) = 3.88, p < .001.
PTSD Symptom Clusters
At the follow-up assessment, 90 (9.4%) patients met criteria for PTSD (MTBI: 50, 11.8%; No-TBI: 40, 7.5%); MTBI patients were more likely to develop PTSD than no-TBI patients, after controlling for injury severity (adjusted odds ratio: 1.86, 95% confidence interval, 1.78–2.94). Table 2 reports the mean total CAPS scores, as well as scores for each cluster for MTBI and no-TBI participants. Analyses of covariance (ANCOVAs) that controlled for ISS indicated that patients with MTBI had higher CAPS scores at baseline t(1048) = 6.98, p < .01, and at follow-up, t(918) = 11.91, p < .001, than those without MTBI. ANCOVAs of symptom cluster score on the CAPS indicated that at baseline MTBI participants scored higher than no-TBI participants on the avoidance cluster, F(1, 1048) = 14.38, p < .001. At follow-up, MTBI participants scored higher than no-TBI participants on the avoidance, F(1, 918) = 11.54, p < .001, and arousal, F (1, 918) = 11.71, p < .001, clusters.
Table 2. PTSD clusters according to MTBI status

Note
Standard deviations appear in parentheses.
Posttraumatic Amnesia and PTSD
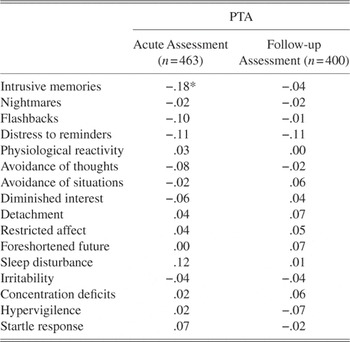
Pearson Product correlations were conducted between PTA length and CAPS total scores. There was no association between PTA length and CAPS scores at the acute assessment (r = .07, p = .16) or at 3 months (r = .06, p = .16). PTA length was correlated with each specific PTSD symptom (combined frequency and intensity score) at the baseline and follow-up assessment, with a Bonferonni-adjusted alpha of .001 (see Table 3). Longer PTA was associated with less severe intrusive memories at the acute assessment (r = .18, p = .001).
Table 3. Pearson Product partial correlation coefficients between reexperiencing symptoms and PTA, controlling for Injury Severity Score, in MTBI participants

Note
*p <.001. PTA = post-traumatic amnesia, MTBI = mild traumatic brain injury. Symptoms are scored as combined frequency and intensity score for each Clinician Administered PTSD item. Amnesia is excluded because of a confound with loss of consciousness.
DISCUSSION
Consistent with previous reports from military cohorts (Hoge et al., Reference Hoge, McGurk, Thomas, Cox, Engel and Castro2008), we found that patients with MTBI were more likely to develop PTSD than those without TBI; although we controlled for ISS in analyses, this conclusion needs to be qualified by recognition that MTBI patients were more severely injured, which may account for the more severe PTSD. If MTBI does result in increased rates of PTSD, it may be attributed to impaired emotion regulation resulting from damage to the medial prefrontal cortex (Bryant, Reference Bryant2008), impaired cognitive strategies that limit management of stress reactions (Ehlers & Clark, Reference Ehlers and Clark2000), or additional stressors that may occur after the MTBI. We also note that an inherent problem in studying PTSD after MTBI is the problem in differential diagnosis between the two conditions. Some of the arousal (e.g., concentration difficulties, insomnia, irritability) and avoidance (e.g., reduced interest, detachment) PTSD symptoms are also common postconcussive symptoms, and it is very difficult to discern whether these symptoms are attributed to MTBI or PTSD (Bryant, Reference Bryant2001b). We attempted to reduce the confound by eliminating amnesia as a symptom in the calculation of PTSD, because amnesia is a defining feature of MTBI. It is worth noting, however, that reexperiencing symptoms, which cannot be readily attributed to MTBI, occurred as often in MTBI as no-TBI participants.
Although a longer period of PTA was not associated with different severity of PTSD symptoms overall, it was inversely associated with intrusive memories in the acute phase. This pattern is associated with a previous report that impaired memory of the details of the traumatic event following MTBI was associated with reduced risk of PTSD development (Gil et al., Reference Gil, Caspi, Ben-Ari, Koren and Klein2005); this study found that reexperiencing symptoms was inversely related to the extent to which the individual could recall details of the traumatic experience. Whereas this earlier study was limited by reliance on patients’ self-reports of trauma memory, which may have been confounded by biases in recall (Harvey & Bryant, Reference Harvey and Bryant2001; Southwick, Morgan, Nicolaou, & Charney, Reference Southwick, Morgan, Nicolaou and Charney1997), the current study relied on PTA assessments conducted immediately after the injury. The most parsimonious explanation for this finding is that duration of PTA was associated with reduced encoding or consolidation of the trauma memory, which subsequently resulted in less reexperiencing symptoms in the acute phase. Fear conditioning models suggest that the strength of the conditioned stimulus at the time of the trauma will contribute to subsequent conditioned responses, which include reexperiencing symptoms (Pitman, Reference Pitman1988). There is evidence that the strength of fear conditioning partially depends on the individual’s awareness of the contingency between the unconditioned stimulus and response (Lovibond & Shanks, Reference Lovibond and Shanks2002). Longer duration of PTA may lead to a reduction in reexperiencing symptoms in the acute phase after MTBI, because there are fewer mental representations of the experience that can be subsequently conditioned to the fear response.
Interestingly, the association between PTA length and reexperiencing symptoms was weaker at the follow-up assessment. This pattern suggests that other factors contributed to the occurrence of intrusive memories during the three months after the MTBI. Previous research has indicated that PTSD after TBI can occur as a result of reconstructive memory processes operating in the post-trauma period. Case studies have indicated that patients with severe TBI can develop intrusive memories many months after the TBI as a result of seeing newspaper photographs about their accident, reading police reports of their traumatic injury, and even dreaming about what may have occurred (Bryant & Harvey, Reference Bryant and Harvey1998b). It is possible that the inverse relationship between intrusive memories and PTA length was no longer significant three months after the MTBI, because some patients may have reconstructed memories of the experience, which subsequently developed into intrusive memories or flashbacks. This interpretation is consistent with current models of autobiographical memory, which note the reconstructive nature of personal memories, and place emphasis on the role of current concerns and self-construct in formulating autobiographical memories (Conway & Pleydell-Pearce, Reference Conway and Pleydell-Pearce2000). This interpretation, along with evidence from longitudinal studies following MTBI that demonstrate that MTBI patients do reconstruct their memory for the traumatic events in the months after injury, support the finding that this reconstruction tends to be associated with more severe posttraumatic stress reactions (Harvey & Bryant, Reference Harvey and Bryant2001).
We note several methodological limitations of this study. First, we did not directly assess patients’ memories for details of the traumatic injury, and accordingly, we cannot draw inferences about the role of memory for trauma and subsequent PTSD. Second, we did not assess neuropsychological functioning, and so it is difficult to determine the extent to which impaired cognitive performance contributed to PTSD development. Third, we assessed PTSD symptoms at follow-up via telephone rather than in a face-to-face format; we note, however, that comparisons indicate that telephone and personal interviews compare favorably (Aziz & Kenford, Reference Aziz and Kenford2004). Fourth, we note that PTA may be influenced by analgesic medications in a traumatically injured population.
These findings indicate that survivors of MTBI are at greater risk of developing PTSD than patients without MTBI; the possibility that greater injury severity may be responsible for this increased risk does not reduce the clinical importance of the finding that a proportion of these patients may require clinical intervention. Early intervention of patients with acute stress disorder following MTBI with cognitive behavior therapy has been shown to effectively limit subsequent PTSD, and should be considered for these patients (Bryant, Moulds, Guthrie, & Nixon, Reference Bryant, Moulds, Guthrie and Nixon2003). The finding that longer duration of PTA appears to be somewhat protective against reexperiencing symptoms in the acute phase does not seem to buffer against PTSD at subsequent periods. This pattern suggests that screening protocols for PTSD symptoms following MTBI, regardless of PTA length, may be warranted to increase detection of patients who may benefit from treatment.
ACKNOWLEDGMENTS
This study was supported by an NHMRC Program Grant (300403), a Victorian Trauma Foundation grant (#V-11), and a National Health and Medical Research Council Australian Clinical Research Fellowship (359284).