Introduction
The meaning of the term ‘seed quality’ and the standard required depends on the requirements of the user. Growers of wide-spaced crops that are sown to a stand, such as maize and sugar beet, expect high and reliable emergence close to 100%, as do vegetable transplant producers. For gene banks, longevity and the maintenance of genetic integrity are the paramount quality attributes.
The universal quality standard is the germination test performed according to rules in which the count of normal seedlings is the criterion of germination (ISTA, 2010). The practical expression of quality does, in some production circumstances, fall short of what is expected from high-germinating lots, the production units that are sampled and tested, traded and used in many countries. A more complete picture of quality is given by comparisons of germination progress curves. These are indicative of rate of germination over time, as determined by periodic counts of radicle emergence, and give a relative assessment of what has been referred to as ‘vigour’. This attribute can differ greatly among seed lots with acceptably high levels of germination in the standard test.
Evaluation of quality beyond the standard test is the subject of this review. Other aspects of quality, such as genetic identity and purity and freedom from pathogens and pests, are also important but are not included. Vigour tests have, for the most part, been developed empirically, but all tests, we believe, can be explained in terms of an ageing/repair hypothesis. Evidence is presented for this hypothesis to provide a rationale for most of the tests that have been, and are being, developed and standardized internationally for commercially available seed lots on a range of crops.
Germination progress curves, rate and performance
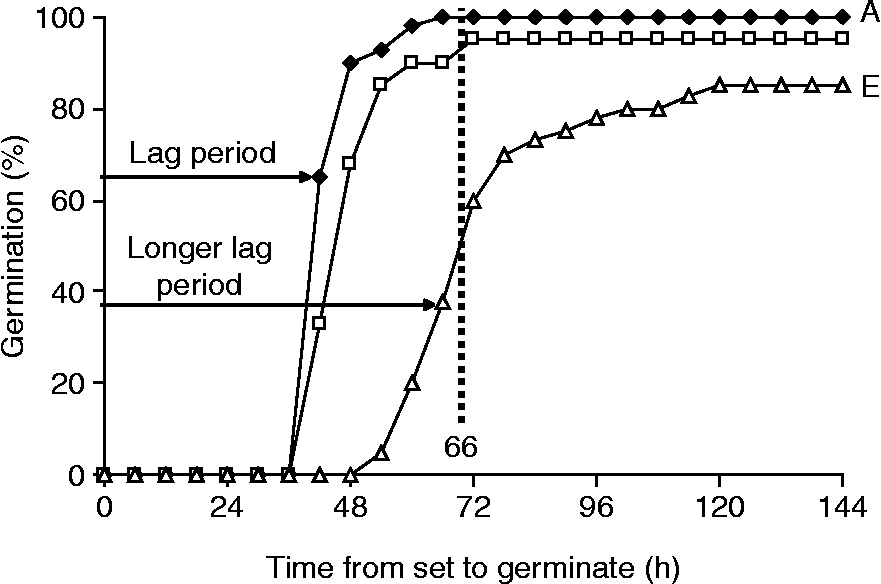
The significance of germination curves can be illustrated by reference to three seed lots of maize that differ in vigour and field emergence, but have similar high standard germinations (>90%; Fig. 1). Lot E, with the lowest vigour and emergence is characterized by a delay in the start of germination and a greater spread over time compared to lot A. These differences can be summarized by the mean germination time (MGT), first proposed by Ellis and Roberts (Reference Ellis, Roberts and Hebblethwaite1980). This is the average delay from the start of imbibition to the protrusion of the radicle, which results from cell expansion, probably facilitated by cell wall loosening, and precedes cell division in many species. A quicker comparison of MGTs, and time courses, can be provided by single counts at appropriate times, e.g. 66 h at 20°C in maize (Fig. 1). The prediction of MGT by single counts of germination for maize (Matthews et al., Reference Matthews, Beltrami, El-Khadem, Khajeh-Hosseini, Nasehzadeh and Urso2011; R 2 = 0.95), cucumber (Mavi et al., Reference Mavi, Demir and Matthews2010; R 2 = 0.97), radish (Demir, unpublished data; R 2 = 0.94) and cotton (Matthews, unpublished data; R 2 = 0.96) supports the general applicability of this comparative measure.

Figure 1 Germination progress curves at 20°C of three commercial seed lots of maize.
The rate of radicle emergence measured in the laboratory has been related to performance as seen in the rate of, and final emergence of, commercially available seed lots of maize (Matthews et al., Reference Matthews, Beltrami, El-Khadem, Khajeh-Hosseini, Nasehzadeh and Urso2011), cucurbits (Mavi et al., Reference Mavi, Demir and Matthews2010) and oilseed rape (McLaren et al., Reference McLaren, Cockerell and Matthews2010). MGT describes not only the timing of the start of germination but the spread over time, which relates to the variation between lots in mean emergence time (MET) and seedling size in vegetables (Demir et al., Reference Demir, Ermis, Mavi and Matthews2008) and maize (Khajeh-Hosseini et al., Reference Khajeh-Hosseini, Lomholt and Matthews2009). This is confirmation of the general proposition by Ellis (Reference Ellis1992) that seedling size is determined by the length of time from radicle emergence to assessment.
Physiological age of seeds and performance
Two ISTA validated tests, accelerated ageing (AA) for soybeans and controlled deterioration (CD) for Brassica spp. subject seeds to rapid deterioration by holding them at a high moisture content (mc) and temperature (ISTA, 2010). In both tests seed lots that are at different points on the slow initial decline in the seed survival curve and which have high and similar standard germinations are clearly separated in germination tests after the imposed deterioration. The tests reveal the initial physiological age of the seeds. The term ‘physiological age’ is used since the initial extent of deterioration results not just from the time from harvest but also from the effects of maturity and desiccation damage at harvest, and is greatly influenced by pre- and post-harvest moisture and temperature conditions. Assessments of deterioration using accelerated ageing have been related to emergence and storage potential in many species (Baalbaki et al., Reference Baalbaki, Elias, Marcos-Filho and McDonald2009), but only validated for soybean (ISTA, 2010).
When applied to commercial seed lots of maize (Matthews et al., Reference Matthews, Beltrami, El-Khadem, Khajeh-Hosseini, Nasehzadeh and Urso2011), pepper (Demir et al., Reference Demir, Ermis, Mavi and Matthews2008), cucurbits (Mavi et al., Reference Mavi, Demir and Matthews2010) and cotton (Matthews, unpublished data) these tests (either AA or CD) demonstrated that the physiologically aged, more deteriorated lots were slower to germinate to the radicle emergence stage. Delayed radicle emergence (high MGT) is known to be one of the first signs of deterioration in artificially and naturally aged seeds.
Evidence for an ageing/repair hypothesis
The observation that the time from the start of imbibition to radicle emergence, referred to as the lag period, was longer in rye embryos that had aged, along with experiments over several years, led to the suggestion that DNA damage was repaired during this period and was an essential first step leading to germination (Osborne,Reference Osborne1983). Earlier, electron microscopy of aged maize embryos revealed evidence of damage to membranes in organelles, including mitochondria, early in imbibition, which was repaired before visible germination occurred (Berjak and Villiers, Reference Berjak and Villiers1972).
More recently, Matthews and Khajeh-Hosseini (Reference Matthews and Khajeh-Hosseini2007) suggested that the extent of deterioration of seed lots determines the length of the lag period, or MGT. In maize, a 24 h period of hydration followed by drying back reduced the lag period when the seeds were subsequently germinated (Matthews and Khajeh-Hosseini, Reference Matthews and Khajeh-Hosseini2007) and priming of Brassica seeds by aerated hydration reduced the lag period and was accompanied by an increase in germination after CD, indicative of reduced deterioration (Thornton and Powell, Reference Thornton and Powell1992). This would explain the close correspondence of the level of deterioration, as revealed by ageing tests, and MGT described earlier.
The general observation that the ranking of the rates of germination of seed lots differing in physiological age are maintained at different temperatures also points to deterioration as an underlying cause of delayed radicle emergence. In maize, consistent ranking of the MGT of seed lots at both 13 and 20°C (Matthews et al., Reference Matthews, Beltrami, El-Khadem, Khajeh-Hosseini, Nasehzadeh and Urso2011) has been reported. At the lower temperature, where metabolic repair would be slower, the more deteriorated lots germinated particularly slowly. Furthermore, in 17 commercial seed lots of pepper, the ranking of the MGTs remained the same at five temperatures and were related to the extent of deterioration (Demir et al., Reference Demir, Mavi, Kananoglu and Celikkol2010). The suboptimal temperature (15°C) for MGT had a much greater effect in slowing germination in the most deteriorated seed lot (MGT = 13.0 d) compared to the least deteriorated lot (MGT = 7.5 d). Slower metabolic repair at non-optimum temperatures may have resulted in an increased delay (MGT) in the most deteriorated seeds because of a greater need for repair. These differences in MGT between seed lots of the same cultivars highlight the importance of distinguishing between the effects of seed quality and genotype in plant breeding when selecting for tolerance to stress during germination and crop establishment.
Most vigour tests can be explained in terms of an ageing/repair hypothesis (Fig. 2). Ageing tests (AA and CD) are measures of the extent of deterioration and are indicative of emergence and longevity in storage (Powell and Matthews, Reference Powell and Matthews2005). The greater increase in abnormal seedlings in deteriorated lots in the cold test (Baalbaki et al., Reference Baalbaki, Elias, Marcos-Filho and McDonald2009) may result from incomplete metabolic repair during the initial cold, and sometimes anaerobic, period. In the cool germination vigour test, such as that used for cotton at 18°C, reduced seedling length in deteriorated lots would result from delayed radicle emergence, especially at suboptimum germination temperatures, leading to less time for growth before assessment, as described earlier. This might also be the case for all so-called seedling growth tests.

Figure 2 Interpretation of vigour tests in terms of an ageing/repair hypothesis. AA, accelerated ageing; CD, controlled deterioration; EtOH, ethanol; MGT, mean germination time. (A colour version of this figure can be found online at http://journals.cambridge.org/ssr).
A new vigour test has been proposed for canola (Brassica napus L. var. napus), in which high levels of ethanol generated by anaerobic respiration early in germination identify deteriorated, low-vigour lots (Buckley and Buckley, Reference Buckley and Buckley2009), and was explained as the result of impaired mitochondrial activity in deteriorated seeds. Differences in ethanol production might also relate to the duration of the lag phase of oxygen uptake, when oxygen diffusion is restricted through the seed coat, leading to use of the anaerobic pathway. This lag phase is extended in deteriorated seeds by a delay in radicle emergence (Bettey and Finch-Savage, Reference Bettey and Finch-Savage1996). Therefore, ethanol assessments, taken at the same time, might be made during the lag phase (before radicle emergence) in deteriorated lots, but after radicle emergence in relatively undeteriorated lots, when ethanol levels have fallen.
Physiological age and solute leakage
Solute leakage from seeds into soak water, measured as electrical conductivity, is a validated vigour test for grain legumes (Fig. 2), first accepted into the ISTA Rules for garden peas in 2001, followed by Phaseolus vulgaris in 2009 and soybeans in 2010. Although there is a small increase in leakage from living cells in the early stages of deterioration, much of the leakage in germinable grain legume seeds, aged naturally and artificially, is associated with dead areas of tissue on the abaxial surface of the cotyledons (Powell et al., Reference Powell, Matthews, Oliveira and Coaker1985). This effect of ageing is exacerbated by rapid water uptake and so-called imbibition damage (Fig. 2).
Similar small increases in leakage occur during early ageing of the small-seeded brassicas when seeds remain capable of producing normal seedlings. However, much greater increases in leakage occur later in ageing when seeds produce abnormal seedlings or fail to germinate (Mirdad et al., Reference Mirdad, Powell and Matthews2006; Demir et al., Reference Demir, Ermis, Mavi and Matthews2008). This can be illustrated for single seeds of oilseed rape from a naturally aged seed lot with a standard germination of around 50%. The mean conductivities after 24 h for single seeds that went on to produce normal (22) and abnormal (21) seedlings or were dead (3), were 4.9, 6.9 and 15.1 μS cm− 1 seed− 1 respectively. The abnormal seedlings were produced by seeds in which radicle emergence occurred later than from seeds producing normal seedlings. A greater proportion of abnormal seedlings from later-germinating seeds has been reported in oilseed rape (Khajeh-Hosseini et al., Reference Khajeh-Hosseini, Nasehzadeh and Matthews2010) and maize (Khajeh-Hosseini et al., Reference Khajeh-Hosseini, Lomholt and Matthews2009), which were explained in terms of incomplete metabolic repair. In conductivity tests on bulk samples of 50 seeds, high solute leakage would therefore occur in lots having a higher proportion of dead seeds or seeds that give rise to abnormals. Similarly, it may be that in seeds producing abnormal seedlings, it is the presence of an increased number of severely damaged cells that contributes to the enhanced leakage, rather than impaired membrane integrity in all cells.
International standardization
The promotion and use of internationally standardized methods to achieve uniformity in seed testing is the primary objective of the International Seed Testing Association (ISTA). This has been successfully achieved for the standard germination test. This review has focused on the timing of the emergence of the radicle as a way of devising quicker methods of evaluation. In maize, the timing of radicle emergence has undergone international comparative testing to confirm repeatability within and reproducibility between laboratories (Khajeh-Hosseini et al., Reference Khajeh-Hosseini, Lomholt and Matthews2009; Matthews et al., Reference Matthews, Beltrami, El-Khadem, Khajeh-Hosseini, Nasehzadeh and Urso2011). This has led to the proposal of a vigour test for maize that takes less than 3 d as part of a standard germination test (Matthews et al., Reference Matthews, Beltrami, El-Khadem, Khajeh-Hosseini, Nasehzadeh and Urso2011) and also to the ISTA validation of the method. Similar methods might apply to other species.
There is considerable interest in developing methods and equipment that enable the rapid and automated evaluation of seed quality (Van der Burg, Reference Van der Burg2009). Computer imaging is the technique attracting most attention (Dell'Aquila, Reference Dell'Aquila2007), including the assessment of the first signs of germination (Ducournau et al., Reference Ducournau, Feutry, Plainchault, Revollan, Vigouvoux and Wagner2005). Such methods need to be tested on commercially available seeds in several laboratories before they can be regarded as fit for purpose internationally.
Concluding comments and the future
Seed deterioration from natural ageing during the production and storage of commercial seeds is the major cause of quality differences seen in the standard germination test, in vigour tests and in practical use. Radicle emergence is delayed in deteriorated seeds and evidence suggests that the extent of deterioration determines the length of the delay (lag period) during which metabolic repair of damage caused by deterioration takes place before further germination processes can continue. This delay is measured by the mean germination time of the seed lot or, more conveniently in routine testing, by a single early count.
Research on repair processes in seeds is not extensive compared to other aspects of seed physiology. However, recent work on Arabidopsis mutants has identified DNA ligases that repair double-strand breaks in chromosomes and reduce the delay in radicle emergence, the decline in viability caused by ageing and the incidence of abnormal seedlings (Waterworth et al., Reference Waterworth, Masnavi, Bhardwaj, Jiang, Bray and West2010). How DNA repair is linked to the cell expansion that results in radicle emergence in the many species in which radicle emergence follows cell-cycle activity but precedes cell division, remains to be established. More research on repair in early imbibition could lead to rapid methods of seed quality evaluation and to biochemical and genetic approaches to increasing the efficiency of repair processes to improve longevity and plant establishment.
Acknowledgements
Many colleagues and companies are acknowledged for their co-operation and provision of seeds; Dr Alison Powell, Chair of the ISTA Vigour Committee, is thanked for helpful advice throughout.