INTRODUCTION
Trichinella is a genus of nematode that infects a wide variety of vertebrate hosts. Trichinella sp. infection causes satellite cell proliferation (Matsuo et al. 2000; Wu et al. 2001) and transformation of muscle cell to the nurse cell in the capsule (Jasmer, 1990; Despommier et al. 1990). Muscle cell transformation is likely initiated by excretory-secretory (E-S) products released from the larvae (Ko et al. 1994).
Trichinella spiralis and Trichinella pseudospiralis are independent species in the genus Trichinella. These two species are similar but different in terms of host responses, including morphology of the capsule, immunological responses, and expression of genes and E-S products. Trichinella spiralis induces a typical host capsule but T. pseudospiralis induces a poor capsule (Xu et al. 1997), and the infection causes muscle cell degeneration, which is restricted around the worm in the case of T. spiralis infection, but the affected area spreads over the entire length of the muscle cell in the case of T. pseudospiralis infection (Matsuo et al. 2000; Wu et al. 2001).
To understand the mechanisms of transformation of muscle cells, E-S products of Trichinella sp. have received a great deal of attention. The 43 and 53 kDa glycoproteins in larval excretory-secretory (E-S) products contain a tyvelose-containing antigen (TSL-1 antigen) (Wisnewski et al. 1993; Romaris et al. 2002), which is the major antigen recognized by the host during infection (Appleton et al. 1991). Furthermore, these glycoproteins of Trichinella sp. have been characterized at the molecular level, and it has been shown that these proteins are important for muscle cell transformation, capsule formation and continuation of parasitism due to Trichinella sp. (Nagano et al. 2004).
In addition to these two glycoproteins, the E-S proteins of Trichinella sp. are known to contain some functional proteins such as heat shock proteins (Ko and Fan, 1996), endonucleases (Mak and Ko, 1999; Mak et al. 2000), proteinases (Moczon and Wranicz, 1999), protein kinases (Arden et al. 1997), serine proteinase inhibitor (Nagano et al. 2001), superoxide dismutase (Wu et al. 2006) and glycosidases (Bruce and Gounaris, 2006).
Rcd1 (Required cell differentiation 1) initially identified as a factor essential for the commitment to nitrogen starvation-invoked differentiation in fission yeast, is one of the most conserved proteins found across eukaryotes, and its mammalian homologue is expressed in a variety of differentiating tissues (Okazaki et al. 1998). Recently, the mammalian Rcd1 was shown to be a transcriptional cofactor and critically involved in the commitment step in the retinoic acid-induced differentiation of F9 cells (Hiroi et al. 2002). Haas et al. (2004) identified the murine Rcd1 protein as a cofactor of the c-myb proto-oncogene product, and showed that the c-Myb and Rcd1 proteins physically interact with each other and that the c-myb specific mim-1 promoter is down-regulated by Rcd1.
We have successfully cloned the Rcd1-like gene from the cDNA of T. pseudospiralis muscle larvae and produced recombinant Rcd1-like protein. In this paper we report molecular data of Rcd1-like protein, which may play an important role in parasite establishment.
MATERIALS AND METHODS
Parasites and E-S products
Muscle-stage larvae of T. pseudospiralis (ISS13) from mice at 15 days and 30 days post-infection (PI) and muscle-stage larvae of T. spiralis (ISS413) from mice at 30 days PI were isolated by pepsin-HCl digestion (Nagano et al. 2002). The 15-day PI muscle larvae are susceptible to the regular artificial gastric juice. Therefore they were isolated by the following method. The muscles of the hind limbs of mice of 15 days PI were minced into small pieces. The minced muscle tissues were incubated in PBS at 37 °C for 10 min, which resulted in a mixed suspension of larvae and host cell debris. The larvae were collected from the supernatant after appropriate filtration and differential sinking. The harvested larvae were treated with 0·2% pepsin in 0·2% HCl at 37 °C for 10 sec to remove contaminated tissues, and washed immediately with cold PBS 4 times by repeating centrifugation and re-suspension. Adult worms of T. pseudospiralis were isolated from the infected mice intestines at 6 days PI. Newborn larvae of T. pseudospiralis were isolated from female adult worms according to the method by Takada and Tada (1988). E-S products from 30-day PI muscle larvae of T. pseudospiralis or T. spiralis were prepared by the conventional methods (Wakelin et al. 1994; Wu et al. 1998). Whole muscle tissues were obtained from the hind limbs of the mice that were orally infected with 300 muscle larvae of T. pseudospiralis for 13, 18, 23, 28, 38, 48 and 90 days PI.
Infected sera and antisera
Infected sera were obtained from BALB/c mice infected with 300 larvae of T. pseudospiralis or T. spiralis for 30 days PI. Polyclonal antibodies against E-S products from 30 day PI muscle larvae of T. pseudospiralis or T. spiralis were produced in outbred Wistar rats injected intradermally with approximately 200 μg of E-S products and complete Freund's adjuvant. This was followed by 4 booster injections of 100 μg of the protein, mixed with incomplete Freund's adjuvant, administered at 2-week intervals. Polyclonal antibody against the recombinant protein was produced in BALB/c mice adopting the similar method with an injection dose of the recombinant proteins of 100 μg.
Preparation of T. pseudospiralis cDNA library
Construction of a cDNA library from T. pseudospiralis muscle larvae was performed according to our previous method (Nagano et al. 2001). In brief, the mRNA was extracted from muscle larvae of T. pseudospiralis at 30 days PI. The cDNA was prepared using a Timesaver cDNA synthesis kit (GE Healthcare Bio-Sciences Co., Piscataway, NJ, USA), ligated into a λ ZAP II vector (Stratagene, La Jolla, CA, USA), and packaged in Gigapack Gold III packaging extract (Stratagene) as described in the manufacturer's instructions.
Cloning of cDNA and DNA sequencing
The T. pseudospiralis cDNA library was immunoscreened with sera from mice infected with T. pseudospiralis according to the conventional methods (Sambrook et al. 1989). Positive clones were selected and converted to plasmids by in vivo excision, and each plasmid was propagated in an Escherichia coli SOLR strain. The recombinant plasmid isolated from the E. coli SOLR strain was sequenced using ABI PRISM BigDye Primer Cycle Sequencing kits (Applied Biosystems Japan, Tokyo, Japan) and an ABI PRISM 3100 genetic analyser (Applied Biosystems Japan). The DNA sequences were assembled and analysed using the DNASIS software (Hitachi Software Engineering, Tokyo, Japan). The BLAST network service was used to search the DNA and protein database at the National Center for Biotechnology Information.
Amplification of the 5′ end of Rcd1-like gene and DNA sequencing
The 5′ ends of Rcd1-like gene were amplified by PCR using the cDNA library of T. pseudospiralis muscle larvae as template. Primers were designed as follows: 5′- CGC TCT AGA ACT AGT GGA TC-3′ (forward: λ ZAP II vector specific primer), 5′- GGA ATT ATC TCG GTG TTG A -3′ (reverse: T. pseudospiralis Rcd1-like gene specific primer). The PCR mixture (total volume of 50 μl) consisted of 5 μl of cDNA library (1[ratio ]100 dilution), 5 μl of 10×PCR buffer, 4 μl of 2·5 mM for each deoxynucleotide triphosphate, 0·75 U of Taq polymerase, and 5 μl of 5 μM for each primer. DNA was amplified for 35 cycles (each cycle consisted of 35 sec denaturation at 94 °C, 30 sec annealing at 52 °C and 60 sec extension at 72 °C). The PCR product was electrophoresed in 1·5% agarose gel. The band of expected size (approximately 500 bp) estimated from the reported nucleotide sequence of Homo sapiens Rcd1 gene was cut from the gel, and was cloned into pT7Blue T-Vector (Novagen, Madison, WI, USA) and sequenced using ABI PRISM BigDye Primer Cycle Sequencing kits and an ABI PRISM 3100 genetic analyser. The DNA sequence was assembled and analysed using the DNASIS software.
Total RNA isolation and reverse transcription (RT)
Total RNA was isolated from the parasite samples using TRIZOL (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. To diminish genomic DNA contamination, the isolated RNA was treated with RQ1 RNase-free DNase (Promega, Madison, WI, USA).
Reverse transcription was performed using a SuperScript III Reverse Transcriptase (Invitrogen) according to the manufacturer's instructions. In brief, the 20 μl reaction volume consisted of 3 μg of the sample RNA, 1 μl of 0·5 μg/μl Oligo (dT)12–18, 1 μl of 10 mM dNTP Mix, 4 μl of First-Stand Buffer, 1 μl of 0·1 M DTT, 1 μl of RNase Inhibitor, and 1 μl of SuperScript III Reverse Transcriptase. The reaction mixture was incubated at 50 °C for 60 min and then inactivated by heating at 70 °C for 15 min.
Quantification by real time RT-PCR
The transcription levels of Rcd1-like gene in various developmental stages of T. pseudospiralis were determined by real-time PCR using the iCycler iQ Real-Time Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Oligonucleotide primers were designed based on the sequence of Rcd1-like gene of T. pseudospiralis determined in the present study (GenBank Accession number: DQ403808; 5′-AAT CCT CCA CGA CTG ACT GC-3′/5′-CAG TTT TCA CCA GTG CTC CA -3′). Primers for a constitutively expressed standard gene G3PDH (glyceraldehyde 3-phosphate dehydrogenase) were designed from the reported nucleotide sequence of G3PDH gene of T. pseudospiralis (GenBank Accession number: AF452243; 5′- GGT GCT GAT TAC GTT GTT GA -3′/5′- TCG ACT GTC TTT TGG GTT G C -3′).
The cDNA templates were prepared from the total RNA of various developmental stages of T. pseudospiralis, including from adult worms, newborn larvae, isolated 15-day PI muscle larvae, isolated 30-day PI muscle larvae, infected muscle tissues of 13, 18, 23, 28, 38, 48 and 90 days PI, and non-infected muscle tissues. Adult worms of T. pseudospiralis produce larvae from 5 days PI to 9 days PI. Therefore the estimated age ranges of larvae are as follows: 13, 18, 23, 28, 38, 48 and 90 day PI muscle larvae are 6±2, 11±2, 16±2, 21±2, 31±2, 41±2 and 83±2 days old after birth, respectively.
First, an optimal real-time RT-PCR condition for Rcd1-like gene and G3PDH gene was established using the SYBR Premix Ex Taq Kit (TAKARA BIO Inc., Tokyo, Japan) according to the manufacturer's instructions. Twenty μl of the reaction solution consisted of 2 μl of the template (1[ratio ]10 dilution), 10 μl of SYBR Premix Ex Taq, 0·4 μl of 10 μM of each primer and 0·4 μl of ROX Reference Dye. PCR amplification was performed as follows: pre-denature for 1 cycle at 95 °C for 30 sec, and 45 cycles at 95 °C for 5 sec, 56 °C for 30 sec and 72 °C for 30 sec. Melting curve analysis was done at 65 °C to 95 °C with 0·1 °C /sec temperature transition.
Specific external controls were constructed for the target gene. The PCR fragment of each gene was cloned into a pT7Blue T-Vector. The recombinant plasmid was introduced into competent cells of E. coli JM109 strain. The plasmid DNA was isolated from E. coli using a FlexiPrep Kit (GE Healthcare Bio-Sciences). Ten-fold serial dilutions (101 to 107 copies/2 μl) of the plasmid were used to generate standard curves for the genes.
The transcription level was represented as estimated copy numbers of target gene/104G3PDH copies. Three independent experiments were performed and 4-well repeats were measured for each sample. The values were expressed as mean±S.D.
Expression and purification of recombinant protein
The genes encoding full-length Rcd1-like protein was amplified by PCR from 30-day PI muscle larvae cDNA using oligonucleotides primers with Bam HI and EcoR I restriction enzyme sites added (underlined) to assist cloning into the pTrcHis expression vector (Invitrogen). Primers for the amplification of the Rcd1-like gene were designed as follows: 5′- CGG GAT CCC GAT GTC GGC CGA CTC CGA ATC- 3′ (forward), 5′- GGA ATT CCT TAT CGT TCA CGG GTA ACA GCC – 3′ (reverse). The PCR product was digested with Bam HI and EcoR I, and cloned into pTrcHis vector. The recombinant plasmid was transformed into an E. coli DH5α strain, and the expression of a polyhistidine-containing recombinant protein was induced by adding isopropyl β-D-thiogalactopyranoside at a final concentration of 1 mM at 37 °C for 3 h. The induced cells were harvested and disrupted by sonication in 20 mM Tris-HCl buffer (pH 8·0). Recombinant protein expressed as inclusion bodies was solubilized completely with 6 M urea in 20 mM Tris-HCl buffer (pH 8·0), and then subjected to a His Trap kit (GE Healthcare Bio-Sciences) for affinity purification of histidine-tagged proteins according to the manufacturer's instructions. For the refolding of recombinant protein, urea was removed from the sample protein solution by stepwise dilution of urea with 20 mM Tris-HCl buffer (pH 8·0) in His Trap chelating column according to the method of Colangeli et al. (1998). The recombinant protein was eluted with 500 mM imidazole, and then imidazole was removed from the sample with a PD-10 column (GE Healthcare Bio-Sciences).
Western blotting analysis
Twenty μg of E-S products, or 0·4 μg of recombinant protein were electrophoresed on 12% SDS-PAGE, electro-transferred to nitrocellulose sheets, and immunostained with sera from mice infected with T. pseudospiralis or T. spiralis (1[ratio ]100 dilution), antibody against E-S products of T. pseudospiralis or T. spiralis (1[ratio ]100 dilution), or antibody against the recombinant protein (1[ratio ]100 dilution). Goat anti-mouse or rat IgG alkaline phosphatase-conjugate (Sigma Chemical Co., St Louis, MO, USA) was used as the second antibody, and the alkaline phosphatase developed in 5-bromo-4-chloro-3-indolyl-p-toluidine salt and nitroblue tetrazolium.
Immunocytolocalization
Skeletal muscle tissues from mice infected with T. pseudospiralis or T. spiralis for 30 days PI and adult worms of T. pseudospiralis were fixed with 4% paraformaldehyde. Sections of 4 μm in thickness were incubated with the antibody against recombinant protein or normal mouse sera (1[ratio ]100 dilution) for 1 h at 25 °C. After washing, the sections were treated with the HistoStain SP kit (ZYMED Laboratories Inc., South San Francisco, CA, USA) according to the manufacturers' instructions.
Nucleotide sequence Accession number
Nucleotide sequence reported in this article is available in the GenBank™, EMBL and DDBJ databases under the Accession number DQ403808.
RESULTS
Molecular characterization of the Tp8 clone
Primary screening of a cDNA library (approximately 100000 plaques) with sera from mice infected with T. pseudospiralis resulted in 25 positive clones. Plaques were picked up and rescreened with sera from mice infected with T. pseudospiralis, and 10 positive clones were converted to a plasmid by in vivo excision. Differences of length and restriction patterns using the restriction enzyme of inserted fragments showed that 2 of 10 positive clones overlapped each other (data not shown). One clone out of the 2 clones was sequenced and its amino acid sequence was deduced.
The clone contained a cDNA of 846 bp with a poly-A tail of 15 nucleotides. The 5′ end of the cDNA was identified resulting in the addition of another 480 bp to generate the sequence of the full-length cDNA. The full-length cDNA clone designated Tp8, consisted of 1326 bp with a single open reading frame of 909 bp which was terminated by TAA stop codon at position 975. The cDNA had 63 bases of 5′ -untranslated nucleotides, and 351 bases of 3′ -untranslated nucleotides. The clone encoded a protein of 303 amino acid residues with a molecular mass of 34187 Da (Fig. 1).

Fig. 1. Alignment of the deduced amino acid sequence of Trichinella pseudospiralis Rcd1-like protein open reading frame, with that of Rcd1 protein from Homo sapiens (GenBank under Accession number BAA13508), Rcd1-like protein from Apis mellifera (GenBank under Accession number XP_395846), Rcd1 protein from Schizosaccharomyces pombe (GenBank under Accession number BAA13507), Rcd1-like protein from Caenorhabditis elegans (GenBank under Accession number NP_498048), and Rcd1-like protein from Arabidopsis thaliana (GenBank under Accession number NP_196802). Amino acid residues conserved in all 6 sequences are indicated by asterisks. Highly conserved sequences are indicated in shaded boxes. The identities with T. pseudospiralis Rcd1-like protein are represented along the margins. Gaps are represented by dashed lines. The numbers along the margin designate the positions of amino acid residues.
A BLAST search revealed significant similarity with Rcd1-like proteins. For example, the Tp8 protein was 66% identical to the Rcd1 protein from Homo sapiens (GenBank under Accession number BAA13508), 65% identical to the Rcd1-like protein from Apis mellifera (GenBank under Accession number XP_395846), 58% identical to the Rcd1 protein from Schizosaccharomyces pombe (GenBank under Accession number BAA13507), 57% identical to the Rcd1-like protein from Caenorhabditis elegans (GenBank under Accession number NP_498048), and 55% identical to the Rcd1-like protein from Arabidopsis thaliana (GenBank under Accession number NP_196802) (Fig. 1).
Quantification by real-time RT-PCR
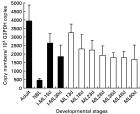
The transcription level of Tp8 gene in various developmental stages of T. pseudospiralis, including adult worms, newborn larvae and different stages of muscle larvae, was determined by real-time RT-PCR. The transcription level of Tp8 gene in adult worms was highest (3935 copies/104G3PDH copies) among all the stages (P<0·01). The transcription level in newborn larvae was lowest (464 copies/104G3PDH copies) (P<0·01) (Fig. 2).

Fig. 2. Quantitative analysis of transcription of Tp8 gene in various developmental stages of Trichinella pseudospiralis. Total RNA was isolated from adult worms (Adult), newborn larvae (NBL), pepsin-HCl isolated 15-day-old muscle larvae (I-ML15d), 30-day-old muscle larvae (I-ML30 d), infected muscle tissues at 13 (ML13d), 18 (ML18d), 23 (ML23d), 28 (ML28d), 38 (ML38d), 48 (ML48d) and 90 (ML90d) days post-infection. The transcription level was determined with real-time PCR and was presented as estimated copy numbers within 104G3PDH copies. Four-well repeats for each sample were measured, and 4 independent experiments were performed. The value was expressed as mean±S.D. The closed bar represents the samples from isolated worms. The open bar represents the samples from infected muscle tissue.
Among the different stages of muscle larvae (13, 18, 23, 28, 38, 48 and 90 days PI), the transcription level was decreased according to the number of days after infection, from 3258 copies at 13 days PI to 1696 copies at 90 days PI (Fig. 2). The transcription level in muscle larvae at 13 days PI was significantly higher than that at 28, 38 or 90 days PI (P<0·05). The transcription level in non-infected muscle tissues was 0 copy/104G3PDH copies.
Western blotting analysis of Tp8 recombinant protein
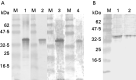
The recombinant Tp8 protein migrated at approximately 37 kDa, and was immunostained positively with sera from mice infected with T. pseudospiralis (lane 1, Fig. 3A), sera from mice infected with T. spiralis (lane 2, Fig. 3A), with antibody against E-S product of T. pseudospiralis muscle larvae (lane 3, Fig. 3A), and with antibody against E-S product of T. spiralis muscle larvae (lane 4, Fig. 3A). Although the expected molecular mass of the recombinant Tp8 protein is 34·2 kDa, it migrated at approximately 37 kDa due to the possession of 2·5 kDa plasmid vector proteins.

Fig. 3. (A) Western blotting analysis of recombinant Tp8 protein with sera from mice infected with Trichinella pseudospiralis (lane 1), with sera from mice infected with T. spiralis (lane 2), with antibody against T. pseudospiralis excretory-secretory (E-S) product (lane 3) and with antibody against E-S product of T. spiralis muscle larvae (lane 4). (B) Western blotting analysis of E-S product of T. pseudospiralis from 30-day PI muscle larvae (lane 1) and E-S product of T. spiralis from 30-day PI muscle larvae (lane 2) stained with antibody against recombinant Tp8 protein. M: molecular mass standard, size in kDa is shown on the left side.
The antibody against recombinant Tp8 protein stained proteins migrating at 34 kDa in the E-S product of T. pseudospiralis from 30-day PI muscle larvae (lane 1, Fig. 3B), and in E-S product of T. spiralis from 30-day PI muscle larvae (lane 2, Fig. 3B).
Cytolocalization of the Tp8 protein in infected skeletal muscle
As shown in Fig. 4, positive immunostaining was predominantly found within the stichocyte of muscle larvae of T. pseudospiralis at 30 days PI (Fig. 4A) or within the stichocyte of adult worms of T. pseudospiralis (Fig. 4B), and within the stichocyte of muscle larvae of T. spiralis at 30 days PI (Fig. 4C). This positive staining, however, was not observed in muscle larvae of T. pseudospiralis at 30 days PI (Fig. 4D) or in adult worms of T. pseudospiralis (Fig. 4E) when stained with normal mouse sera.

Fig. 4. Immunocytochemical staining of muscle larvae of Trichinella pseudospiralis at 30 days PI (A), adult worms of T. pseudospiralis (B), and muscle larvae of T. spiralis at 30 days PI (C) with antibody against recombinant Tp8 protein. Immunocytochemical staining of muscle larvae of T. pseudospiralis at 30 days PI (D), adult worms of T. pseudospiralis (E) with normal mouse sera.
DISCUSSION
Rcd1 was discovered initially as a key factor required for nitrogen starvation-induced sexual development in fission yeast (Okazaki et al. 1998). Rcd1 is highly conserved among eukaryotes, and at least budding yeast, nematodes, fruit flies and mammals contain its homologue with >60% amino acid identity. The mammalian homologue of Rcd1 is expressed in a variety of differentiating tissues, though its function is not well known (Okazaki et al. 1998; Gregory et al. 2000). In this study we have cloned Rcd1-like gene (Tp8 gene) from a cDNA library of T. pseudospiralis muscle larvae. The Tp8 gene is most likely an Rcd1 gene because the predicted amino acid sequence of the Tp8 protein had an identity of approximately 60% to Rcd1-like protein of H. sapiens, A. mellifera, S. pomb, C. elegans or A. thaliana.
Trichinella sp. produces a variety of biologically active proteins, which may or may not be a part of E-S products. Proteins in E-S products likely affect host cells and tissues for respective purposes, and non-E-S proteins likely are engaged in internal reactions within the parasites. On Western blotting, the recombinant Tp8 protein reacted with antibody against E-S products of T. pseudospiralis and with the sera from mice infected with T. pseudospiralis, and the antibody against the Tp8 protein recognized the 34 kDa protein in E-S products. In the present immunocytochemical study, positive immunostaining was found within the stichocytes of muscle larvae and adult worms, which are the principal secretory cells of the parasite and are the main source of E-S proteins in Trichinella sp. Therefore, the Tp8 protein is part of the E-S products of T. pseudospiralis, and is mainly synthesized in the stichocytes, secreted into the host cell, and has been shown to be highly antigenic in infected animals.
The 43 kDa glycoprotein in E-S products of T. spiralis has a potential helix-loop-helix motif (HLH) that resembles HLH domains critical in the function of muscle differentiation factors such as MyoD and myogenin, and antibodies against the 43 kDa recombinant protein strongly stained the nurse cell nuclei (Vassilatis et al. 1992). The gene encoding a 43 kDa glycoprotein was expressed by muscle larvae, either pre-capsule or post-capsule larvae, and the 43 kDa glycoprotein could not be expressed by adult worms (Wu et al. 2002; Nagano et al. 2004). From these results, the 43 kDa glycoprotein may be responsible for muscle cell transformation and capsule formation that occurs immediately after the entrance of muscle cells by the newborn larvae. However, it hardly seems possible that the remarkable phenotypic changes by infection with Trichinella sp. occur due to the 43 kDa glycoprotein alone. Therefore, other factors like transcription factors may be necessary for phenotypic changes.
Hiroi et al. (2002) indicated that mammalian Rcd1 is a transcriptional cofactor and is critically involved in the commitment step in the retinoic acid-induced differentiation of F9 mouse teratocarcinoma cells, at least in part, via forming complexes with retinoic acid receptor and activation transcription factor-2. Recently, the murine Rcd1 protein has been identified as a cofactor of the c-myb proto-oncogene product (Haas et al. 2004). Rcd1 protein is located mainly in the nucleus, and it interacts with c-Myb, and represses the activation of the myeloid c-myb specific mim-1 promoter. It may be inferred from these reports that Rcd1 protein secreted from Trichinella sp. functions as a transcriptional cofactor, and that it interacts with a transcriptional factor like c-Myb. Transcriptional initiation of eukaryotic genes depends on the cooperative interaction of various transcription factors. The Rcd1 protein may be responsible for muscle cell transformation in cooperation with other E-S proteins like the 43 kDa glycoprotein, but the precise function of the Rcd1 protein, however, remains unknown.
Our real time RT-PCR results showed that the Tp8 gene in T. pseudospiralis muscle larvae was expressed significantly higher than in newborn larvae, and the transcription level of the Tp8 gene in muscle larvae before complete stichosome formation (13 days PI) was significantly higher than in muscle larvae after stichosome formation (from 28 days PI to 90 days PI). The Rcd1-like protein secreted from muscle larvae likely affects host cells and tissues for muscle cell transformation. On the other hand, the transcription level of the Tp8 gene reached the highest value in adult worms. Hiroi et al. (2002) showed that Rcd1 was expressed in virtually all tissues of early rat embryo, but its level decreased during the later stages of embryogenesis. The adult worms of Trichinella sp. seem to be more active in embryogenesis than in newborn larvae or muscle larvae.
Based on Western blotting and an immunocytochemical study, antigenic epitopes of Tp8 recombinant protein are also present in E-S products of T. spiralis. It is well known that the two species, T. pseudospiralis and T. spiralis, share E-S products with considerable similarity, in terms of cDNA sequence, molecular mass and antigenicity (Kehayov et al. 1991; Zhang et al. 1993; Vassilatis et al. 1996; Wu et al. 1998, 1999; Nagano et al. 2004). Shared proteins in E-S products including Tp8 protein are likely to play a fundamental role common to the two species of Trichinella sp. and are therefore crucial for worm establishment in the host.
This study was partially supported by a Grant-in-Aid for Scientific Research (17590370) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.