Introduction
Citellinema Hall, 1916 includes 6 species of gastrointestinal nematodes that infect squirrels. They are distributed across the Holarctic with 4 species present in North America and 2 in northeastern Eurasia. The species present in North America include Citellinema bifurcatum Hall, Reference Hall1916; Citellinema quadrivittati Hall, Reference Hall1916; Citellinema columbianum Dikmans, Reference Dikmans1938 and Citellinema grisei Lichtenfels, Reference Lichtenfels1971. The species present in Asia include Citellinema nipponicum Yamaguti, 1941 and Citellinema orientale Schulz, 1933, known from Japan and Siberia, respectively. No other species in the genus are known from southeast Asia, Africa, Central America and South America despite the presence of sciurids in those regions.
The lack of detailed comparative morphological and molecular studies of Citellinema created a gap that led taxonomists to identify most of the specimens of Citellinema collected across North America as C. bifurcatum. In an initial revision of the specimens included in the genus, Dikmans (Reference Dikmans1938) concluded that meristic similarities, especially spicule length, warranted the placement of Citellinema sleggsi Manter, Reference Manter1930 and Citellinema monacis Manter, Reference Manter1930 as junior synonyms of C. bifurcatum. Further examination of other specimens collected across North America, including individuals from 6 different sciurid species from localities in Illinois, Maryland, Michigan, Maine, Minnesota and New York in the USA, and Saskatchewan in Canada allowed Dikmans (Reference Dikmans1938) to conclude that C. bifurcatum has a wide geographic range and diverse host spectrum. Notably, he reported the presence of prominent prebursal papillae observed in specimens collected from a fox squirrel (Sciurus niger L.) from Maryland. Though these features were absent in other North American Citellinema, he did not propose that the specimens represented a distinct species because they were almost identical to C. bifurcatum in meristic traits such as the size of spicules.
In a subsequent evaluation of the specimens that served as the basis for description of C. sleggsi and C. monacis Manter (Reference Manter1930), and then Durette-Desset (Reference Durette-Desset1969) described conspicuous variation in the number of ridges in the synlophe, which led them to consider those 2 species to be valid. Subsequently, Lichtenfels (Reference Lichtenfels1971) emphasized the relevance of the length of the genital cone, the size of the shaft and the configuration of the tip of the lamina as important sources of informative characters. He also emphasized the relevance of characterizing the variability of the number of ridges along the body of individuals before proposing this number as a viable character to differentiate species. Because this number could not be determined for species described based on sole individuals, he rejected the validity of C. sleggsi and C. monacis until a proper characterization of the variation of the synlophe becomes available for these species.
In part, the practice of assigning most specimens to C. bifurcatum reflects the emphasis of the description on the spicules and on the fact that this description was based on a single specimen mounted on a permanent slide. As a consequence, there is neither documentation of the variation in the number of ridges for the synlophe, nor there is information regarding variability in the size of spicules or in the distance from the anterior end to the deirids, nerve ring and excretory pore.
The purpose of this study is to re-examine and re-evaluate the available specimens of Citellinema along the Pacific coast of North America and document the species diversity using morphological and molecular approaches. This character reassessment will permit the proper identification of intra and interspecific variation, leading to increasingly robust documentation of the biodiversity present in the continent. Furthermore, we reassess these characters based on specimens with properly vouchered hosts, which is critical for ensuring that the scope of host diversity is correctly described (Galbreath et al., Reference Galbreath, Hoberg, Cook, Armién, Bell, Campbell, Dunnum, Dursahinhan, Eckerlin, Gardner, Greiman, Henttonen, Jiménez, Koehler, Nyamsuren, Tkach, Torres-Pérez, Tsvetkova and Hope2019). As a result of this analysis, 2 new species of Citellinema from Alaska and British Columbia, collected from Tamiasciurus hudsonicus (red squirrel) from British Columbia, Canada and Alaska are herein described. The description of these species and the characterization of their variability and diversity are important in understanding the interactions of these parasites with their hosts, and with their environment. In the Pacific Northwest the distribution of 3 species of Tamiasciurus Trouessart, 1880 shows evidence of isolation across a historical biogeographic barrier in the Nass River valley (Hope et al., Reference Hope, Malaney, Bell, Salazar-Miralles, Chavez, Barber and Cook2016). We therefore apply a molecular phylogenetic approach to investigate if sciurid-dwelling parasites feature a similar pattern of historic isolation coincident with that of their hosts.
Materials and methods
Specimen sampling
One hundred and forty-two specimens of Citellinema including C. bifurcatum, Citellinema quadrivittati, C. sleggsi and C. monacis were used in this study (Table 1). Specimens were borrowed from the Museum of Southwestern Biology (MSB, University of New Mexico, Albuquerque, New Mexico) and National Museum of Natural History (USNM, Smithsonian Institution, Washington D.C., USA), Northern Michigan University (Marquette, Michigan, USA), Harold W. Manter Laboratory of Parasitology (HWML, University of Nebraska State Museum, Lincoln, Nebraska, USA) and The Cornell University Museum of Vertebrates (Ithaca, New York, USA). Most of these specimens were preserved in 70% ethanol, though some, such as holotypes, were preserved in formalin or mounted on permanent slides after being cleared with lactophenol or glycerin. All of the worms were examined under the dissection and compound microscope to confirm their identity based on appropriate characters. Upon obtaining permission for destructive sampling, a small number of specimens from each lot was cleared and dissected to observe the morphological variability of characters.
Table 1. List of lots of worms examined for the completion of this study indicating their original catalogue numbers from the MSB, National Parasite Collection (USNM), HWML and Northern Michigan University (NMI)

Molecular systematics
A list of the nematode specimens used for DNA extraction and sequencing is detailed in Table 2. DNeasy Blood and Tissue spin columns (Qiagen Inc., Madison, Wisconsin, USA) were used for tissues excised between the mid-body and posterior end of the worm. The rest of the worm was saved as a voucher to be preserved in the Parasite Collection of the MSB. Amplification of the mitochondrial gene cytochrome b (CYTB), cytochrome c oxidase subunit 1 (COI) and a continuous region of rDNA including internal transcribed spacer 1 (ITS1), 5.8S and ITS2 (hereafter, ITS) was completed using primers and protocols described elsewhere (Jiménez et al., Reference Jiménez, Gardner, Navone and Ortí2012; Alnaqeb et al., Reference Alnaqeb, Greiman, Vandegrift, Campbell, Meagher and Jiménez2022).
Table 2. Comparative presentation of diagnostic features used to identify species of Citellinema

Raw sequences were assembled in Sequencher v.3.5 (Gene Codes Corporation, Ann Arbor, Michigan, USA). Annotated sequences were complemented with sequences from relevant sister taxa published elsewhere (Zaleśny et al., Reference Zaleśny, Hildebrand, Paziewska-Harris, Behnke and Harris2014) (Table 2), aligned and trimmed in Mesquite v.3.40 (Maddison, Reference Maddison2018) equipped with Clustal v.2.1 (Sievers et al., Reference Sievers, Wilm, Dineen, Gibson, Karplus, Li, Lopez, McWilliam, Remmert and Söding2011) applying default settings for coding genes where the invertebrate genetic code was used to identify the coding regions of mitochondrial genes. Non-coding DNA regions were aligned enforcing a gap open penalty of 15, a gap extension penalty of 6 and re-aligned by hand. Selection of the models of evolution for each dataset, description of the parameters used for the phylogenetic reconstruction using maximum likelihood (ML) and estimation of both branch support and posterior probability are described elsewhere (Alnaqeb et al., Reference Alnaqeb, Greiman, Vandegrift, Campbell, Meagher and Jiménez2022). A complete list of sequences generated in this study is presented in Table 3 including their accession numbers. The aligned matrixes are universally available at Open SIUC (https://opensiuc.lib.siu.edu/zool_data/17).
Table 3. Correlational table including accession numbers available in the data repository of the National Center for Biotechnology Information, species identities and scientific collection numbers

Genetic distance was calculated separately for each gene as implemented in Phylogenetic Analysis Using Parsimony (PAUP*) v.4.0a (Swofford, Reference Swofford2002). To assess the monophyly of the new species both mtDNA and ribosomal nuclear DNA genes were analysed separately. These analyses included a reduced dataset, in which only operational taxonomic units with all 3 genes (ITS, Cytb and COI) were included. Species limits were established using the diagnosability of clades. These limits were compared against results of a tree-based Poisson Tree Process (PTP) model using the tree topology that included the specimens of Citellinema herein described and other species in the family (Alnaqeb et al., Reference Alnaqeb, Greiman, Vandegrift, Campbell, Meagher and Jiménez2022).
Morphological examination
Nematodes were cleared using lactophenol and compared against type and voucher specimens described in Table 1. For examination of the variation of the synlophe, transversal sections were prepared for 2 males and 2 females at mid-body, and at the anterior and posterior ends. Drawings were made using an Olympus BX50 microscope (Olympus Co. Ltd., Tokyo, Japan) equipped with a drawing tube. For morphometrical characters, the range is given first followed by the mean and coefficient of variation.
Results
Phylogeny
In the phylogram (Fig. 1) based on a combination of ribosomal nuclear DNA (ITS) and mtDNA (COI and CYTB), the clade of Citellinema is placed as the putative sister group to Heligmosomoides and Heligmosomum with a bootstrap support value of 100%. Within the clade of Citellinema, a western (yellow/light rectangle) and an eastern (blue/dark rectangle) clade form reciprocally monophyletic groups. Monophyly of these 2 clades is consistent in the phylogenetic trees of nDNA (Fig. 2) and mtDNA (Fig. 3). The topologies of both ML and Bayesian inference are congruent. The average uncorrected p-distances and Jukes–Cantor genetic distance between both clades is 7% for mtDNA while it is 4% for ITS. Intraspecific genetic distance for mtDNA is 0.07% in the eastern clade (blue/dark rectangle) and 1.6% in the western clade (yellow/light rectangle); for nDNA, this distance is 0.04% in the eastern clade and 0% in the western clade. The PTP analysis revealed a support value of 0.887 for the eastern clade and 0759 for the western clade.

Fig. 1. ML phylogeny based on concatenated nDNA (ITS1, 5.8 and ITS2) and mtDNA (CYTB and COI) sequences. Numbers on branches indicate bootstrap support values (ML) followed by posterior probabilities (Bayesian) for major nodes. Tips are labelled with species names, followed by museum catalogue numbers and GenBank accession numbers as appropriate (Table 3).

Fig. 2. ML phylogeny based on the ribosomal nuclear DNA (ITS) sequences. Numbers on branches indicate bootstrap support values (ML) followed by posterior probabilities (Bayesian) for major nodes. Tips are labelled with species names, followed by museum catalogue numbers and GenBank accession numbers as appropriate (Table 3).

Fig. 3. ML phylogeny based on mtDNA (CYTB and COI) sequences. Numbers on branches indicate bootstrap support values (ML) followed by posterior probabilities (Bayesian) for major nodes. Tips are labelled with species names, followed by museum catalogue numbers and GenBank accession numbers as appropriate (Table 3).
Description
Citellinema kinsellai n. sp. (Figs 4–17 and 40B)
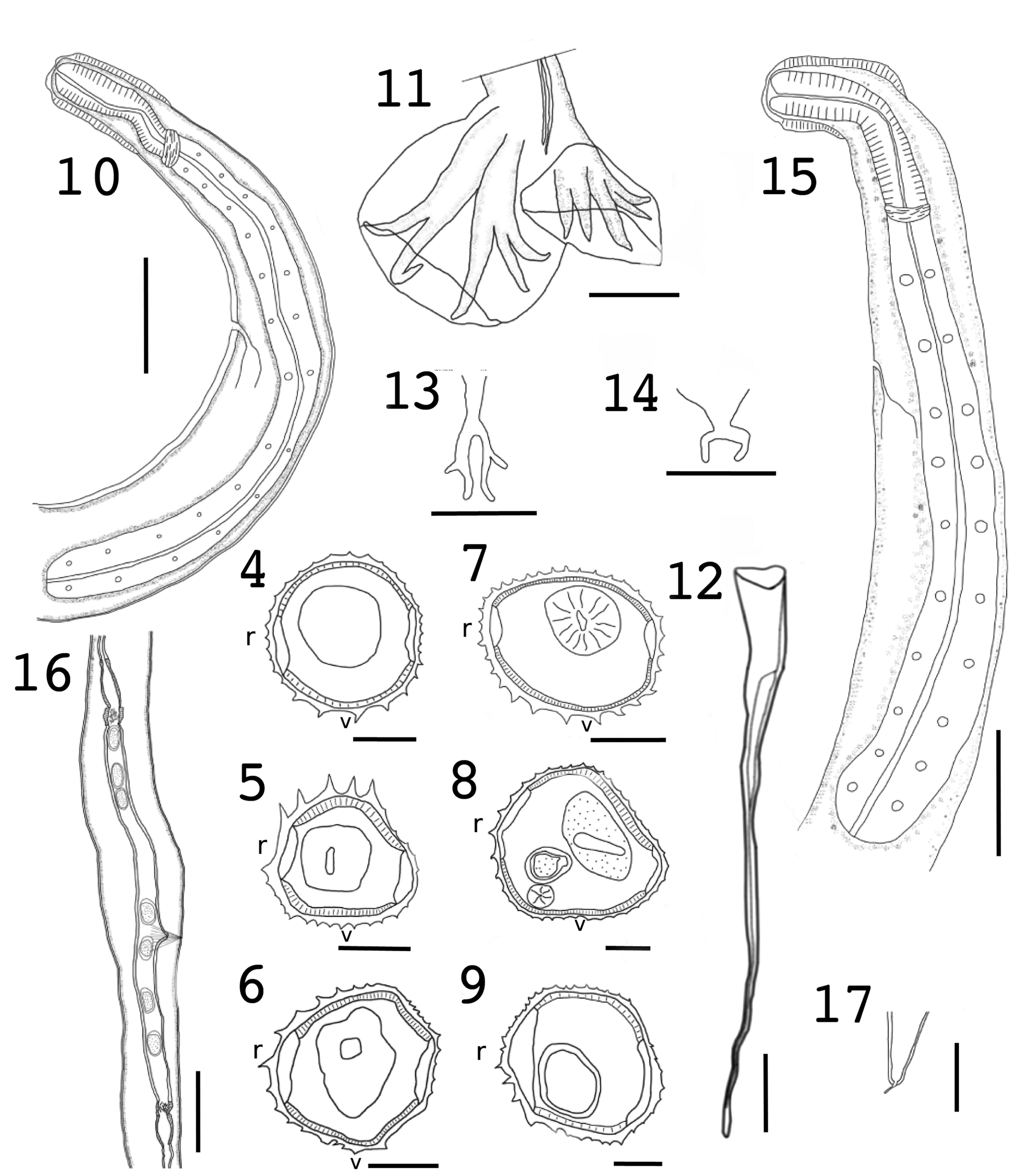
Figs 4–17. Citellinema kinsellai n. sp. from the red squirrel, Tamiasciurus hudsonicus. Synlophe sections at anterior end (4), mid-body (5) and posterior end (6) of a male. Synlophe sections of a female at level of oesophagus (7), mid-body (8) and posterior end (9). Scale bar for Figs 5–10 = 50 μm. (10) Anterior end of male showing cephalic vesicle, oesophagus and nerve ring; scale bar = 100 μm. (11) Detail of a caudal bursa; scale bar = 50 μm. (12) Detail of the spicule; scale bar = 50 μm. (13) Configuration of the dorsal ray; scale bar = 50 μm. (14) Detail of the genital cone; scale bar = 50 μm. (15) Anterior end of female showing cephalic vesicle, oesophagus and nerve ring; scale bar = 100 μm. (16) Posterior end of female showing vulva and ovejector and 2 uterine branches; scale bar = 100 μm. (17) Detail of the tail and terminal spine; scale bar = 50 μm.
Synlophe based on 2 males and 3 females: Cuticle with longitudinal ridges; ventral ridges are larger than dorsal ridges. Male with 31 ridges (18 dorsal/13 ventral) at anterior end, 25–29 at mid-body and 25 (15 dorsal/10 ventral) at posterior end (Figs 4–6). Females with 32 ridges (18 dorsal/14 ventral) at anterior end, 33–37 at mid-body and 36–37 at posterior end (Figs 7–9).

Figs 18–30. Citellinema meagheri n. sp. from the red squirrel, T. hudsonicus. Synlophe sections at anterior end (18), mid-body (19) and posterior ends of a male (20). Synlophe sections at level of nerve ring (21), mid-body (22) and posterior end (23) of a female; scale bar for Figs 19–24 = 50 μm. (24) Anterior end of male showing cephalic vesicle, oesophagus, nerve ring and excretory pore; scale bar = 100 μm. (25) Caudal bursa; scale bar = 100 μm. (26) Detail of the spicule; scale bar = 50 μm. (27) Genital cone; scale bar = 25 μm. (28) Anterior end of female showing cephalic vesicle, oesophagus, position of the nerve ring; scale bar = 100 μm. (29) Detail of the vulva and ovejector; scale bar = 100 μm. (30) Detail of the caudal terminus; scale bar = 25 μm.

Fig. 31. Citellinema manteri n. sp. from the Canadian woodchuck, Marmota monax. Micrograph of the transversal section at mid-body of a male

Figs 32–38. Citellinema manteri n. sp. synlophe sections at anterior (32) and posterior (33) ends of a male; scale bar = 50 μm. Sections of the synlophe at anterior end, close to the level of excretory pore (34), mid-body (35) and posterior end (36) of a female; scale bar = 50 μm. (37) Anterior end of a male showing cephalic vesicle, oesophagus and nerve ring; scale bar = 100 μm. (38) Detail of the bursa; scale bar = 200 μm.

Fig. 39. Spicule of Citellinema bifurcatum from the Wyoming ground squirrel, Urocitellus elegans.

Fig. 40. Comparison of the spicules of (A) C. bifurcatum, (B) C. kinsellai and (C) C. meagheri; scale bar = 50 μm.
Holotype: Length 10.67 mm. Width at mid-body 113. Cephalic vesicle 116 long × 48 wide. Body with 5 coils. Excretory pore, nerve ring and deirids located at 448, 182 and 282, from anterior end, respectively. Oesophagus 576 long and 46 wide at posterior end. Inflection of testis 350 from posterior end. Bursa is asymmetrical with 2–3 arrangement. Spicules sub-equal, proximal end vase-shaped transitions into a cylindrical shaft, lamina relatively wide near the shaft with walls that run very close from one another tapering towards the end (Fig. 12). Right spicule 468 long, 19 wide at proximal end, bifurcation 60 from proximal end representing 12.8% of body length. Left spicule 484 long, 18 wide. Spicule length represents 4.4% of body length. Dorsal ray divided at about mid-length into 2 branches. Genital cone with 2 prominent lyre-shaped papillae.
Male paratypes based on whole mounts of 7 individuals unless otherwise noted: Length 9.35–12.81 mm (10.14 mm, 10.93%). Width at mid-body 108–125 (117, 5.38%). Cephalic vesicle 102–120 (110, 5.17%) long × 48–57 (51, 5.2%) wide. Body with 2–7 (5, 38.3%, n = 12) coils. Excretory pore, nerve ring and deirids located at 321–488 (432, 14.32%), 214–364 (292, 19.97%, n = 5) and 330 (n = 1), from anterior end, respectively. Oesophagus 580–717 (643, 6.46%) long and 45–55 (50, 7.46%) wide at posterior end (Fig. 10). Inflection of testis 307–389 (248, 11.78%, n = 2) from posterior end. Bursa asymmetrical with 2–3 arrangement (Fig. 11). Spicules subequal, with features seen in holotype; 436–489 (466, 3.95%, n = 8) long (Figs 12 and 40B), representing 3.8–4.6% of length of body. Width at proximal end 17–27 (20, 14.67%, n = 20) wide. Bifurcation 65–97 (73, 11.28%, n = 15) from proximal end, representing 14.9–19.8% of spicule. Dorsal ray divided at about mid-length into 2 branches (Fig. 13). Genital cone with 2 prominent lyre-shaped papillae (Fig. 14).
Allotype: 19.24 mm long and 162 wide. Body with 9 coils. Cephalic vesicle 123 long × 55 wide. Excretory pore, nerve ring and deirids located at 539, 303 and 241 from anterior end, respectively. Oesophagus 836 long, 42 wide at posterior end. Vulva 2.87 mm from posterior end. Vagina vera 63 long, vestibule 872 long, anterior sphincter 55 long and 65 wide, posterior sphincter 41 long, 55 wide. Anterior infundibulum 168 long, posterior infundibulum 171 long. Ovejector containing mature eggs 62–78 long (69, 5.1%, n = 10) and 38–58 wide (44, 6.3%, n = 10). Tail 60 long with caudal spine 16 long.
Female paratypes based on whole mounts of 14 females: Length 10.97–21.1 mm (18.47 mm, 14.41%, n = 12). Maximum width at mid-body 155–205 (170, 8.5%, n = 11). Cephalic vesicle 71–134 (109, 23%, n = 6) long × 54–69 (61, 10.5%, n = 6) wide. Body with 4–15 (9, 37%, n = 14) coils. Excretory pore, nerve ring and deirids located 371–544 (451, 17.5%, n = 6), 224–373 (287, 19.3%, n = 5) and 392–580 (474, 20.2%, n = 3) from anterior end, respectively. Oesophagus 755–866 (823, 4.8%, n = 6) long × 47–75 (64, 14.7%, n = 6) wide (Fig. 15). Vulva 2.87–3.29 mm (3.12 mm, 5.7%, n = 6) long from the posterior end. Vagina vera 42–76 (64, 18.8%, n = 6) long, vestibule 664–1059 (870, 16%, n = 5), anterior sphincter 45–77 (58, 19.6%, n = 6) long and 47–86 (67, 18.8%, n = 6) wide, posterior sphincter 41–71 (57, 17.3%, n = 7) long and 50–73 (58, 14.4%, n = 7) wide, anterior infundibulum 146–195 (177, 10.5%, n = 6) long, posterior infundibulum 136–263 (179, 22.6%, n = 7) long (Fig. 16). Ovejector containing mature eggs 52–78 (67, 8.4%, n = 68) long, 32–58 (41, 10.2%, n = 68) wide. Immature eggs 37–78 (55, 16.8%, n = 231) long, 22–51 (39, 10.53%, n = 231) wide. Tail 60–134 (101, 26.8%, n = 6) long; caudal spine 14–22 (18, 18.92%, n = 5) (Fig. 17).
Taxonomic summary
Type host: Tamiasciurus hudsonicus (red squirrel) (Rodentia, Sciuridae).
Site of infection: Small intestine.
Symbiotype: MSB:Mamm:266934 (NK232761, collected 31 July 2013).
Type locality: Bob Quinn Lake, Stewart Cassiar Highway 37, British Columbia, Canada (56°44′37.122″N, 129°47′31.1568″W).
Other localities: South Kinaskan Lake, Stewart Cassiar Highway 37, British Columbia, Canada (57°26′33.9432″N, 130°14′23.2548″W); Bob Quinn Lake, Stewart Cassiar Highway 37, British Columbia, Canada (56°44′37.122″N, 129°47′31.1568″W); South Kinaskan Lake, Stewart Cassiar Highway 37, British Columbia, Canada (57°27′51.48″N, 130°13′51.5748″W).
Specimens deposited: Holotype (MSB:Para:32215) and 7 paratypes (MSB:Para:19047); allotype (MSB:Para:32216) and 9 paratype females (MSB:Para:19050).
Specimens examined: MSB:Para:19047, MSB:Para:19050, MSB:Para:24826, MSB:Para:24858, MSB:Para:24859, MSB:Para:24860, MSB:Para:24863, MSB:Para:24864, MSB:Para:24866, MSB:Para:24597, MSB:Para:24599, MSB:Para:24819, MSB:Para:24862; MSB:Para:26332.
Etymology: This species is named in honour of John M. Kinsella, who intensively contributes to the field of nematode taxonomy. Zoobank Registration: urn:lsid:zoobank.org:pub:F6C33748-B11F-4E64-8CE1-A0229F63201E.
Remarks
Citellinema kinsellai n. sp. can be immediately separated from 4 Nearctic species on the basis of the size of the spicules, the ratio of these spicules relative to the body length and the number and distribution of ridges of the male and female (Table 2). The most similar species is C. columbianum; however, the spicules of the latter are over 3 mm in length whereas the spicules of C. kinsellai are 436–529. Furthermore, the spicules in C. columbianum bifurcate at 150 from the proximal end, which represents 4% of the spicule length, while the spicules of C. kinsellai bifurcate at 64–97, representing 14.7–18.7% of the spicule length. The spicules in C. kinsellai are narrower compared to those in C. bifurcatum and in C. quadrivittati (Table 2). In C. kinsellai, the vellum that produces the pattern of bifurcation starts at the anterior conical base and runs to about the middle of the length of the spicules (Figs 12 and 40B). Conversely, the lamina between the filiform processes or walls of the spicule in C. bifurcatum extends further through the length of the spicules (Figs 39 and 40A). These morphological differences support the separation between C. kinsellai and other described species including C. bifurcatum.
Intraspecific genetic distance based on mtDNA is 0.07% and for nDNA it is 0.04%. Because of the morphological differences of the size, proportion and bifurcation of the spicules as well as the characterization of the synlophe and the length of body, we consider C. kinsellai to be a new species.
Description
Citellinema meagheri n. sp. (Figs 18–30 and 40C)
Synlophe (based on 2 males and 3 females): Cuticle with longitudinal ridges; right ventral ridges are large. Male with 26–29 ridges at anterior end, 26–27 at mid-body and 26–27 at posterior end (Figs 18–20). Females with 26–31 ridges at anterior end, 26–28 at mid-body and 27–33 at posterior end (Figs 21–23).
Holotype: Body coiled, length 6.83 mm. Width at mid-body 117. Cephalic vesicle 75 long × 47 wide. Excretory pore, nerve ring and deirids located at 288, 150 and 216 from anterior end, respectively. Oesophagus 503 long and 36 wide at posterior end. Inflection of testis 218 from posterior end. Bursa is asymmetrical with 2–3 arrangement. Spicules sub-equal, proximal end of uniform edge, shaft cylindrical, wider than it is long; lamina fusiform, limited by thick walls, walls approach each other tapering towards the end (Fig. 26). Spicules 485 and 468 long, representing 7.1% of length of body. Width at proximal end 17 and 14 for right and left spicules, respectively. Spicules bifurcate 76 from proximal end, representing 15.6% of length of spicule. Dorsal ray divided at about mid-length into 2 branches. Genital cone with 2 prominent lyre-shaped papillae.
Male paratypes based on whole mounts of 3 individuals unless otherwise noted: Length 6.83–7.42 mm (7.04 mm, 3.88%). Width at mid-body 105–139 (120, 11.81%). Cephalic vesicle 47–55 (51, 12.87%, n = 2) long × 47–55 (51, 7.44%, n = 2) wide. Body with 1–5 (3, 42.16%, n = 5) coils. Excretory pore and nerve ring located at 251–288 (266, 5.94%) and 150–200 (168, 13.37%) from anterior end, respectively. Oesophagus 503–669 (572, 12.27%) long and 36–48 (44, 12.09%) wide at posterior end (Fig. 24). Inflection of testis 261–277 (269, 3.07%, n = 2) from posterior end. Bursa is asymmetrical with 2–3 arrangement (Fig. 25). Spicules 437–507 (478, 5.34%, n = 4) long (Figs 26 and 40C), representing 6.3–6.8% of length of body. Width at proximal end 14–19 (16, 11.32%, n = 7) wide. Spicules bifurcate 48–76 (65, 18.65%) from proximal end, representing 10.9–14.9% of length of spicule. Genital cone with 2 prominent lyre-shaped papillae (Fig. 27).
Allotype: Body coiled, length 16.23 mm; maximum width 121. Cephalic vesicle 94 long × 60 wide. Excretory pore, nerve ring and deirids located at 262, 224 and 333 from anterior end, respectively. Oesophagus 682 long, 50 wide at posterior end. Vulva located at 2682 from posterior end. Vagina vera 62 long, vestibule 768, anterior sphincter 66 × 68, posterior sphincter 44 × 45, anterior infundibulum 152 long, posterior infundibulum 125 long. Ovejector containing mature eggs 61–68 long (64, 4.16%, n = 6), 31–43 wide (38, 11.81%, n = 6). Immature eggs 47–86 (59, 17.4%, n = 11) long, 35–43 (39, 7.41%, n = 11) wide. Tail 72 long; caudal spine 21.
Female paratypes based on whole mounts of 6 individuals: Length 11.17–17.48 mm (15.36 mm, 11.3%). Width at mid-body 114–175 (145, 17.25%). Cephalic vesicle 63–114 (92, 24.5%, n = 5) long × 44–58 (52, 9.77%) wide. Body with 5–12 (7, 40%, n = 4) coils. Excretory pore, nerve ring and deirids located at 262–414 (314, 17.5%, n = 3); 161–216 (197, 15.64%, n = 1) and 468 (n = 1) from anterior end, respectively. Oesophagus 639–822 (680, 11.4%, n = 5) long × 47–72 (56, 16.97%, n = 5) wide (Fig. 28). Vulva located at 2.47–2.98 mm (2.63 mm, 8.94%, n = 4) from posterior end. Vagina vera 50–71 (62, 12.95%, n = 5) long, vestibule 740–1355 (955, 24.72%, n = 5), anterior sphincter 48–66 (59, 10.67%, n = 4) long × 30–47 (41, 16%, n = 5), posterior sphincter 35–58 (48, 17.6%, n = 5) long × 40–65 (50, 20.17%, n = 5), anterior infundibulum 120–122 (121, 1.3%, n = 2) long, posterior infundibulum 136–164 (153, 7.4%, n = 4) long (Fig. 29). Ovejector containing mature eggs 61–101 (69, 13.88%, n = 26) long and 25–46 (38, 11.4%, n = 23) wide. Immature eggs 37–69 (57, 13.8%, n = 64) long and 25–47 (38, 13.6%, n = 64) wide. Tail 118 (n = 1) long; caudal spine 15–21 (19, 16.5%, n = 2) long (Fig. 30).
Taxonomic summary
Type host: Tamiasciurus hudsonicus (red squirrel) (Rodentia, Sciuridae).
Site of infection: Small intestine.
Symbiotype: MSB:Mamm:195556 (NK152789, collected 28 July 2009).
Type locality: St. John's Harbor, Zarembo Island, Alexander Archipelago, Petersburg Quad, Alaska, USA (56°25′49.4″N, 132°58′26.8″W).
Other localities: Salmon Run Campground, Haines, Alaska (59°17′51.8″N, 135°30′54.4″W and 59°17′52.6″N, 135°30′52.8″W).
Specimens deposited: Holotype (MSB:Para:32212) and 4 paratypes (MSB:Para:25568); allotype (MSB:Para:32212) and 2 paratype females (MSB:Para:24829). Additional paratypes MSB:Para:24828; MSB:Para:24830.
Etymology: This species is named in honour of Shawn Meagher, colleague dedicated to the study of nematode evolution. Zoobank Registration: urn:lsid:zoobank.org:pub:F6C33748-B11F-4E64-8CE1-A0229F63201E.
Remarks
Citellinema meagheri n. sp. can be separated from the 5 Nearctic species of Citellinema known mainly on the basis of the variation in the number of ridges of the synlophe, their overall smaller size, the size/ratio/width of spicules and their width at the shaft. Additionally, the female can be separated from other species based on the larger length of the oesophagus and the location of the vulva from the posterior end and the size of infundibulum (Table 2). The bifurcation pattern in the spicules among C. meagheri is different from C. bifurcatum in that it is less pronounced. In C. meagheri, the lamina is conspicuously different from the shaft and it extends until about the middle of the length of spicules (Figs 26 and 40C). From that level and toward the posterior end, the two filiform processes that limit the lamina come very close to each other. In contrast, the lamina limited by the filiform processes in C. bifurcatum is more conspicuous in that it is evident towards the posterior end of the spicules (Figs 39 and 40A).
Differences between C. meagheri and C. kinsellai include the range in the number of ridges in synlophe at the anterior end of the male, which is 26–29 in C. meagheri and 31 in C. kinsellai. Additionally, the range in body size is conspicuously different (6.83–8.57 mm in C. meagheri compared to 9.35–12.81 mm in C. kinsellai). The size and ratio of spicules in C. meagheri (437–510) represent approximately 5.9% of the body length whereas it represents approximately 4.5% of the body length in C. kinsellai (436–519). The shape and configuration of the lamina in the spicules are different between C. meagheri and C. kinsellai.
In females, the length of the oesophagus also differentiates specimens of C. meagheri from C. grisei, since these are 668–822 and 575–676 long, respectively. The ovejector represents approximately 7.8% of body length in C. meagheri while it is about 11.2% of the body length in C. kinsellai. These proposed morphological differences support the proposal of C. meagheri as a valid species.
Description
Citellinema manteri n. sp. (Figs 31–38)
Synlophe (based on 1 male and 1 female): Cuticle with longitudinal ridges. Male with 33 ridges (14 dorsal/19 ventral) at anterior end, 39 (19 dorsal/20 ventral) at mid-body and 38 (19 dorsal/19 ventral) at posterior end (Figs 31–33). Females with 24 ridges (11 dorsal/13 ventral) at anterior end, 39 (18 dorsal/21 ventral) at mid-body and 42 (21 dorsal/21 ventral) at posterior end (Figs 34–36).
Males based on 5 worms unless otherwise noted: Length 7.42–10.45 mm (8.47 mm, 13.9%); width at mid-body 139–175 (157, 13.8%). Cephalic vesicle 97–128 (112, 13.4%) long × 55–63 (59, 11.7%) wide. Body with 4–5 (4.5, 17.6%) coils. Excretory pore and nerve ring located at 251–295 (273, 34.7%, n = 4) and 200–263 (231, 10.7%), from anterior end, respectively. Deirids not seen. Oesophagus 669–787 (728, 10.7%, n = 4) long and 48–55 (51, 11.9%, n = 4) wide at posterior end (Fig. 37). Inflection of testis 277 (n = 1) from posterior end. Bursa is asymmetrical with 2–3 arrangement (Fig. 38). Spicules sub-equal, conical proximal end and tapering shaft; lamina features conspicuous filiform processes that run posteriad gradually approaching each other. Spicules 427–507 (467, 13%, n = 7) long, 19–22 wide at proximal end; spicular length represents 4.85–5.75% of length of body. Spicules bifurcated at 53–84 (71, 20%, n = 4) from proximal end, representing 12.4–16.5% of length of spicule. Dorsal rays and genital cone unknown.
Females based on 5 individuals unless otherwise noted: Length 16.65–19.88 mm (17.76 mm, 7.43%), maximum width 162–194 (180, 7.6%). Cephalic vesicle 89–124 (102, 12%, n = 3) long × 64–69 (66, 4.5%, n = 3) wide. Body with 9–12 (9, 13.3%) coils. Excretory pore and nerve ring located at 544–582 (564, 2.9%, n = 4) and 232–288 (256, 9.2%) from anterior end, respectively. Deirids not seen. Oesophagus 684–876 (755, 13.8%, n = 3) long × 63–77 (71, 7.7%, n = 4) wide. Vulva located at 3.41 mm (n = 1) from posterior end. Vagina vera 43–45 (58, 2.5%, n = 3) long, vestibule 299 (n = 1), anterior sphincter 66 (n = 1) long and 89–90 (90, 0.74%, n = 2) wide, posterior sphincter 64–82 (73, 14.3%, n = 2) long and 56 (n = 1) wide, anterior infundibulum 90–122 (106, 17.6%, n = 2) long, posterior infundibulum 65 (n = 1) long. Ovejector containing mature eggs 62–84 (73, 8.9%, n = 27) long and 34–48 (41, 8.4%, n = 27) wide. Immature eggs 39–48 (43, 9.8%, n = 3) long and 33–36 (35, 4.7%, n = 3) wide. Tail 153–225 (189, 18.8%, n = 3) long with caudal spine 18–21 (13, 18.3%, n = 2) long.
Taxonomic summary
Host: Marmota monax (Canadian woodchuck) (Rodentia, Sciuridae).
Site of infection: Small intestine.
Locality: Thunder Bay, Ontario, Canada.
Specimens examined: 10 syntypes (USNM:1374038 = USNPC:078608.01).
Etymology: This species is named in honour of Dr Harold W. Manter, founder of the parasitological collection of the University of Nebraska State Museum and pioneer in the taxonomic assessment of Citellinema. Zoobank Registration: urn:lsid:zoobank.org:pub:F6C33748-B11F-4E64-8CE1-A0229F63201E.
Remarks
Based on the number of cuticular ridges in the synlophe the species is different from all other species in the genus (Table 2). At mid-body the number of ridges is 39 for both male and female; the species with greater similarities to C. manteri would include C. monacis, which features 41 ridges at mid-body. However, the spicules of the latter range between 280 and 330, whereas the spicules in C. manteri range between 427 and 507. Furthermore, the distances of the anterior end to excretory pore are other morphological characters that can be used to differentiate C. manteri from the other species of the genus as shown in Table 2. Finally, the right spicule of C. manteri appears narrower than all the examined species (Table 2).
Dikmans (Reference Dikmans1938) considered C. monacis to be a junior synonym of C. bifurcatum. His interpretation was based on the limited morphological characteristics offered by Manter (Reference Manter1930). Subsequent researchers supported the use of the name C. bifurcatum for parasites found in the woodchuck, citing the lack of clarity in the solution of this taxonomic problem (Fleming et al., Reference Fleming, Georgi and Caslick1979). Our characterization allows the comparison of intraspecific morphological variability. It is apparent that parasites infecting woodchucks feature a greater number of ridges in the synlophe than any of the other species thus far characterized. We posit that the differences in the number of ridges and spicule size among worms collected in Maine and Thunder Bay, Ontario represent different species. Size variation of the spicule and total body length resulting from long-term preservation has been documented even for species in this genus; nevertheless, the differences are seldom equivalent to 1/3 of the length of the spicule as observed in this case. Consequently, we reject the proposed hypothesis of C. monacis being a junior synonym of C. bifurcatum posited by Dikmans (Reference Dikmans1938).
Redescriptions
Citellinema bifurcatum Hall, Reference Hall1916 (Figs 39 and 40A).
Synlophe: Unknown.
Redescription of holotype based on slide consisting of anterior and posterior ends: Length unknown. Approximate width at mid-body 166. Cephalic vesicle 38 wide. Excretory pore and nerve ring situated at 328 and 165, from anterior end, respectively. Deirids not seen. Oesophagus 695 long and 70 wide at posterior end. Inflection of testis not evident. Bursa is asymmetrical with 2–3 arrangement. Spicules unequal, manubrium or proximal end cup-shaped; proximal end fused with cylindrical shaft; lamina starts immediately after shaft, characterized by the presence of 2 bifurcating filiform processes or walls. Lamina divided into 2 parts: the first part is as long as the proximal end and it is limited by conspicuous filiform processes or walls; the second part is characterized by these filiform processes or walls gradually approaching each other towards the distal end (Fig. 39). Right spicule 334 long, bifurcation starting 70 from proximal end, representing 20.9% of length of spicule (Figs 39 and 40A). Left spicule 392 long, with bifurcation starting 87 from proximal end, 22.1% of length of spicule. Width of right and left spicules at proximal end 30 and 33, respectively. Dorsal rays and genital cone not seen.
Female: Unknown.
Taxonomic summary
Type host: Spermophilus elegans (=Urocitellus elegans) (Wyoming ground squirrel) (Rodentia, Sciuridae).
Site of infection: Small intestine.
Type locality: Waldon, Colorado, USA.
Specimens examined: A single slide of holotype (USNM:1324365 = USNPC:016176.01).
Remarks
The holotype is the single specimen available from the type series as designated by Hall (Reference Hall1916). The present re-description indicated slight meristic differences in the body size, length of the oesophagus and size of spicules. Hall (Reference Hall1916) provided measurements for body size, length of the oesophagus and spicules size, which are 6.8 mm, 535 and 360 (5.2% of the body length), respectively. Since the specimen is fragmented, it was not possible to measure the length of the body, yet we measured a slightly longer oesophagus, 695, and subequal spicules of sizes 334 and 392, respectively. The combined length of the body for the 2 fragments (parts) of the anterior and posterior ends mounted on the slide was 8.21 mm. It is uncertain if the 2 fragments mounted on the slide represent the complete parts and size of the total length of the holotype, or if there is another dissected part that was missing from the specimen before it was mounted.
This specimen is different from other species in the size and width of spicules as shown in Table 2. Because no additional specimens are available other than the holotype we were unable to examine the characteristics of the synlophe in specimens collected from the host and type locality. However, Durette-Desset (Reference Durette-Desset1969) listed the number of ridges in the synlophe in the mid-body of specimens identified as C. bifurcatum that originated from the typical host, these range from 16 to 20 ridges, while it is 20–22 in C. orientale, 29 in C. quadrivittati, 17 in C. nipponicum and 23–27 in C. grisei. Because of the lysis of the holotype specimens of C. bifurcatum slide, we were unable to observe any ridges along the body of the holotype. One should bear in mind that the characterization of the synlophe of C. bifurcatum presented by Durette-Desset (Reference Durette-Desset1969) was conducted at mid-body, but it was not clearly stated if these sections were made using type material. Finally, reconstructing and describing the characteristics of the posterior end, including the bursa, was difficult, since the specimen is mounted in a permanent medium.
Redescription
Citellinema quadrivittati (Hall, Reference Hall1916) Manter, Reference Manter1930
Synlophe: Unknown.
Redescription based on holotype slide: Length 7.24 mm. Width at mid-body 169. Cephalic vesicle 60 wide. Excretory pore and nerve ring situated at 425 and 130 from anterior end, respectively. Oesophagus 215 long and 17 wide at posterior end. Inflection of testis not observed. Bursa is asymmetrical with 2–3 arrangement. Spicules, sub-equal; right and left spicules 623 and 627 long, representing 8.6% of length of body. Proximal end vase shaped followed by cylindrical shaft; lamina bifurcates forming 2 long filiform processes that run parallel to each other towards the distal end of the lamina; one of these processes ends slightly before the other. Bifurcation of lamina 73 from proximal end, representing 11.7% of length of right spicule; in left spicule bifurcation starts 81 from proximal end, representing 12.9% of length. Width of right and left proximal processes 25 and 29, respectively. Dorsal rays and genital cone not seen.
Females: Unknown.
Taxonomic summary
Synonyms: Warrenius quadrivittati Hall, Reference Hall1916.
Type host: Tamias quadrivittatus (Colorado chipmunk) (Rodentia, Sciuridae).
Site of infection: Small intestine.
Type locality: Crested Butte, Gunnison County, Colorado, USA.
Specimens examined: A single slide of holotype (USNM:1324374 = USNPC:016185.01).
Remarks
Hall (Reference Hall1916) proposed Warrenius Hall, 1916 based on Warrenius quadrivittati Hall, Reference Hall1916. The morphological traits used to establish the genus included the size of spicules and the presence of unilateral cervical membrane at the anterior end of the single male examined. The nomenclatural history of Citellinema and Warrenius was efficiently summarized by Dikmans (Reference Dikmans1938). Relative to the diagnostic feature, the presence of the cervical ala is perceived as an artefact due to the width of the cephalic vesicle, which makes it apparently thicker on 1 of the sides, yet thin on the opposite side.
Based on our observations, the type of C. quadrivittati shows slight morphological differences from the data presented in the original description of Hall (Reference Hall1916); in particular in body size, width and spicules’ length. Body length, width and spicules are 7.24 mm; 169 and 623–627 in our re-description compared to 6.21 mm, 112 and 695 reported by Hall (Reference Hall1916).
Furthermore, Durette-Desset (Reference Durette-Desset1969) examined a specimen of Citellinema sp. isolated in Glaucomys sabrinus from Montana; this specimen is very similar to C. quadrivittati, yet the specimen from Montana differs from the holotype on being larger (11.5 mm) and possessing shorter spicules (570 and 575); the proportion of spicule/body length of 5% is however, relatively close to the range of 8–11% established for the holotype of C. quadrivittati (Table 2). The examination of fresher material that allows the establishment of the size variation of the body, the spicules and their proportions should provide an additional morphological element to separate C. quadrivittati from C. bifurcatum as by Hall (Reference Hall1916).
Reidentification
Citellinema sleggsi Manter, Reference Manter1930
The specimens of this lot were determined by a donor to the USNPC. The examination of the material allowed us to determine that they do not belong to Citellinema because the females lack the characteristic cephalic cap and spine; furthermore, the spicules do not feature thick walls limiting the lamina. As a consequence, we cannot make the relevant comparisons against the rest of the species in the genus, precluding the assessment of the independence of this species relative to C. bifurcatum.
Taxonomic summary
Synonyms: Warrenius bifurcatus Sleggs, 1925.
Host: Sciurus carolinensis (eastern grey squirrel) (Rodentia, Sciuridae).
Site of infection: Small intestine.
Locality: Bowie, Prince George's County, Maryland, USA.
Specimens studied: 3 males and 3 females deposited in (USNM:1329987 = USNPC:027851.01).
Discussion
The origins of C. kinsellai and C. meagheri may be closely tied to geographic isolation that also drove speciation in their sciurid hosts. Some of the specimens used in this analysis were collected from localities that are separated by the Nass River, which cuts through the coastal mountains of British Columbia and was shown to be a significant barrier limiting the distribution of species of red squirrels (Hope et al., Reference Hope, Malaney, Bell, Salazar-Miralles, Chavez, Barber and Cook2016). It appears that the parasites of these squirrels may also be limited by this geographical barrier given the strong divergence between the C. kinsellai and C. meagheri clades (Figs 1–3) at both mtDNA and nDNA loci.
The lack of Citellinema specimens available for DNA isolation limited the scope of our phylogenetic analysis. Inclusion of specimens of C. bifurcatum and other species such as C. orientale, C. quadrivittati, C. columbianum, C. nipponicum and C. grisei is important for reconstructing the phylogeny of the genus and family; this phylogeny will enable us to better understand the history of diversification in this group. Most specimens deposited in scientific museums are old and either preserved as permanent slide mounts or in formalin. Furthermore, those collections that do exist commonly are represented by a few individual specimens that have been archived, and geographic sampling is poor. Therefore, fresh specimens collected through spatially extensive and site-intensive sampling are required to resolve the relationships among species of the genus Citellinema.
Following the standards of the times, the original description of the type species is incomplete, lacking important morphological characteristics such as the synlophe that were later applied in diagnoses of these strongyles. In the earlier part of the 20th century taxonomists relied on the size of the spicules as the main morphological characteristic to differentiate species. This practice was changed by Durette-Desset (Reference Durette-Desset1969) and Lichtenfels (Reference Lichtenfels1971) who attempted to standardize morphological descriptions by adding information about variability of the synlophe along the body and other structures omitted in the original descriptions.
In addition to discrete synlophe morphologies, we demonstrated considerable levels of genetic divergence between C. kinsellai and C. meagheri. Although the divergence in the nDNA genes was substantially lower than that in the mtDNA genes, it is consistent with the patterns of genetic divergence observed in other groups of nematodes, and genetic distances in the range of 3–4% for mtDNA have been suggested as rough benchmarks for interspecific divergence (Hebert et al., Reference Hebert, Cywinska, Ball and DeWaard2003; Mayer et al., Reference Mayer, Dietz and Kiefer2007; Pyziel et al., Reference Pyziel, Laskowski, Demiaszkiewicz and Höglund2017).
In the recent past, scientists underestimated the diversity of strongyles by not recognizing the predictive value of some portions of the spicules. The spicules in Citellinema appear to be uniform in that there is no conspicuous separation between the proximal end, shaft and lamina, and the narrow lamina is surrounded by walls that confer it the appearance of being bifurcated. Our observations revealed that the proportion of the lamina relative to the proximal end and shaft may be a useful trait to diagnose species in this genus. This determination was made possible by the proper preservation of specimens deposited in scientific collections, even when these specimens were expected to belong to C. bifurcatum, which was historically assumed to be a geographically widespread taxon. We suspect that this expectation of finding an extremely common parasite in squirrels from across North America caused many researchers to make assumptions regarding the identity of nematodes collected from squirrels. Reports of C. bifurcatum that are not supported by properly archived voucher specimens should therefore be viewed with caution. This phenomenon, dubbed the ‘fallacy of expected identification’ (Hoberg and Soudachanh, Reference Hoberg and Soudachanh2021) prevents an accurate documentation of diversity, since no species identity can be tested nor assumed in the absence of the materialistic evidence of the voucher.
Biological collections and specimen archives are the foundation for documenting and understanding patterns and distribution of diversity for complex parasite–host assemblages and for tracking the outcomes accelerating climate and environmental changes in the biosphere (Dunnum et al., Reference Dunnum, Yanagihara, Johnson, Armien, Batsaikhan, Morgan and Cook2017; Colella et al., Reference Colella, Bates, Burneo, Camacho, Carrion Bonilla, Constable, D'Elía, Dunnum, Greiman and Hoberg2021). Our results were possible because primary materials for species of Citellinema were largely derived from extensive field collections linking Alaska, Siberia and regions of the continental USA under the Beringian Coevolution Project over the past 2 decades (Cook et al., Reference Cook, Hoberg, Koehler, Henttonen, Wickström, Haukisalmi, Galbreath, Chernyavski, Dokuchaev and Lahzuhtkin2005, Reference Cook, Galbreath, Bell, Campbell, Carrière, Colella, Dawson, Dunnum, Eckerlin and Fedorov2016; Hoberg et al., Reference Hoberg, Galbreath, Cook, Kutz and Polley2012), which include vouchered records for both parasite and host specimens. These sampling efforts provide holistic information regarding mammal–parasite assemblages across spatial and temporal scales, and have been instrumental in building an increasingly nuanced picture and synthesis for the history of diversity and faunal assembly at northern latitudes during the Late Pliocene and Quaternary (Hoberg et al., Reference Hoberg, Galbreath, Cook, Kutz and Polley2012; Haas et al., Reference Haas, Hoberg, Cook, Henttonen, Makarikov, Gallagher, Dokuchaev and Galbreath2020). Especially critical are archives and repositories with sufficient scope and depth to reveal the limits and occurrence of cryptic diversity as demonstrated in our explorations of Citellinema (e.g. Hoberg et al., Reference Hoberg, Agosta, Boeger and Brooks2015).
Data
The aligned matrices are universally available at Open SIUC (https://opensiuc.lib.siu.edu/zool_data/17). Specimens were returned to the MSB and the USNM. Sequences were deposited in GenBank.
Acknowledgements
We are grateful to Drs J. A. Cook and E. P. Hoberg at the MSB who organized and facilitated extensive field inventories in Alaska, Siberia and the continental USA under the Beringian Coevolution Project. Dr Sara Brant facilitated access to the materials in MSB. Dr Jerzy Behnke provided specimens collected in the UK. Collectors whose tears, sweat and blood made these collections possible include J. W. Brunt, J. A. Cook, N. Dawson, L. Delehanty, N. Dokuchae, A. Hope, A. Lahzuhtkin, S. O. MacDonald, A. Runck, A. Tsvetkova and M. Westover. Drs K. Neubig, E. A. Zieman and Mr C. Williams provided technical assistance and feedback.
Author contributions
H. A. and F. A. J. conceived and designed the study. K. E. G. and F. A. J. helped securing funding. K. E. G., A. V. K. and M. L. C. conducted fieldwork, archived specimens and documented parasite presence. H. A. conducted data gathering. H. A. and F. A. J. performed phylogenetic analyses. H. A., K. E. G., A. V. K., M. L. C. and F. A. J. wrote the article.
Financial support
Alnaqeb was funded by the Saudi Arabian Cultural Mission (SACM). Funding for this research was possible through a grant in aid by the Annual Midwestern Conference of Parasitologists (AMCOP), collection was enabled by the Beringian Coevolution Project funded by NSF-DEB 0196095, 0415668 and 1258010; USDA Forest Service and US Fish and Wildlife Service contracts to J. A. Cook and NSF-DEB 1256943 to K. E. Galbreath. Writing supported in part by NSF-DUE1564969.
Conflict of interest
The authors declare there are no conflicts of interest.
Ethical standards
Collecting and handling of mammals was done in accordance with the approved Animal Care and Use Committee of Southern Illinois University, USA (Protocol 21-017, Assurance Number D16-00044).
















