Introduction
Non-alcoholic fatty liver disease (NAFLD) has been associated with an excess of fatty acids (FA) accumulation in the liver, which is accompanied by hepatocellular inflammation with or without fibrosis. In the liver, the FA can be stored provided from both serum and local synthesis. Triacylglycerols (TAG) accumulation in the liver has been related to an inadequate lipid metabolism including a deficient FA oxidation and elevated very low-density lipoproteins (LDL).Reference Berlanga, Guiu-Jurado, Porras and Auguet 1 NAFLD could be therefore linked to high levels of reactive oxygen species (ROS).Reference Podrini, Borghesan and Greco 2
The influence of sex on the development of NAFLD is controversial. It has been proposed that high estrogen levels protect against TAG accumulation and inflammation of the liver in humans.Reference Gilsanz, Chung and Kaplowitz 3 Nevertheless, adolescent women have a higher risk for elevated levels of serum total cholesterol and LDL as well as for NAFLD, which is even higher than those for men.Reference Fernandes, Ferraro, de Azevedo and Fagundes Neto 4 , Reference Ayonrinde, Olynyk and Beilin 5 Animal models have been also used to investigate the protective effects of estrogens.Reference Xin, Qin and Wang 6 , Reference Zhang, Liu and Xiao 7 The development of NAFLD provoked by a rich-fructose intake in male and female is poorly understand albeit some reports indicate that inflammation of the liver is more pronounced in adult females.Reference Spruss, Henkel and Kanuri 8
The gestational protein restriction has permanent effects on the metabolism of the offspring, increasing the fetal oxidative stress mediated by ROS,Reference Podrini, Borghesan and Greco 2 developing metabolic and cardiovascular diseases,Reference Jahan-Mihan, Rodriguez, Christie, Sadeghi and Zerbe 9 increasing the serum concentration of leptin in the adult male offspringReference Cervantes-Rodriguez, Martinez-Gomez and Cuevas 10 and triggering the onset of both insulin resistance and hepatic steatosis.Reference Day and James 11 – Reference Szostaczuk, Priego, Palou, Palou and Picó 14 Likewise, a rich-carbohydrate diet has been associated with NAFLD in childhood and adulthood elevating the amount of hepatic TAG and lipogenesis, reducing the β-oxidation, and promoting liver inflammation.Reference Mosca, Della Corte and Sartorelli 15 – Reference Mock, Lateef, Benedito and Tou 17
Metabolic alterations provoked by gestational malnutrition can be aggravated by a post-weaning high-fatReference Souza-Mello, Mandarim-de-Lacerda and Aguila 18 or carbohydrate diet.Reference Cervantes-Rodriguez, Martinez-Gomez and Cuevas 10 Particularly, the histological characteristics and function of the liver seem to be affected by the combination of gestational malnutrition and postnatal high-fat diet.Reference Souza-Mello, Mandarim-de-Lacerda and Aguila 18 In the present study, we tested the influence of the combination of gestational protein restriction and the intake of high sucrose on the development of NAFLD in both males and females at the adulthood. We therefore hypothesized that 5% sucrose consumption promotes oxidative damage and liver fat accumulation in the adult offspring exposed to gestational protein restriction, being these effects different between sexes. This work could be relevant for a better understanding whether the sex can influence on the development of NAFLD and whether this occurs during prenatal age or at the adulthood.
Methods
Animals
In total, 16 Wistar pregnant rats (Rattus norvegicus) weighing 200–240 g from the breeding colony of the Centro Tlaxcala de Biología de la Conducta from the Universidad Autónoma de Tlaxcala, México, were used. Dams were housed alone in polypropylene cages (37×27×16 cm) and maintained under a 12-h light to 12-h dark cycle (with the lights off from 08:00 to 20:00 h) at a constant temperature of 20±2°C and 40% of humidity in our operating room. At birth, the size of litters was adjusted to 10 pups per dam.
Diet
Details of the maternal diet, breeding and management have been published elsewhere.Reference Cervantes-Rodriguez, Martinez-Gomez and Cuevas 10 Briefly, 16 female rats were mated with male breeders; the presence of seminal plug in the vagina was used to designate the day 0 of pregnancy. Pregnant rats were randomly fed with a Control (C, n=8) or Restricted (R, n=8) diet consisting of semi-purified diet containing 20% (C) or 10% casein (R); both diets were isocaloric (4 kcal/g). Hematocrit was measured at the end of pregnancy as an indicator of malnutrition in mothers.Reference Golub, Hogrefe and Tarantal 19 Each litter was weaned at postnatal day 21 and separated for sex. Sex ratio was maintained as close to 1:1 as possible. After weaning, all litters were fed with Chow Purina 5001 rodent diet and had continuous access to tap water. At 12 weeks of age, two females and two males of C litters (n=8 each) were randomly assigned to continue receiving the standard control diet and tap water or the standard control diet plus 5% sucrose (sugar food gradeReference Cervantes-Rodriguez, Martinez-Gomez and Cuevas 10 ) diluted in the drinking water. Moreover, two females and two males of R litters (n=8 each) were fed with the standard control diet and drunk tap water or 5% sucrose in the drinking water. After 10 weeks, 12-h fasted rats were decapitated using a rodent guillotine (Harvard-Apparatus, Holliston, MA, USA). Litters were used as the unit of analysis. The final sample size for each measured variable was shown on the footnote of results.
Liver and hepatocyte measurements
Livers were removed and weighed. The relative liver weight was expressed as percentage. A half of the tissue was frozen to −40°C for subsequent analyzes and the middle portion of the major lobe of the liver was fixed in neutral formalin (10% formaldehyde, 0.1 M phosphate buffer, pH 7) for 24 h at room temperature. Tissue was embedded in Paraplast X-tra (Sigma-Aldrich, St. Louis, MO, USA) and 7-μm thick longitudinal sections were obtained using a microtome (Leica RM2135, Germany), mounting four sections per slide. One series of slides from each animal were stained with hematoxylin and eosin. Photomicrographs of liver sections were done with a digital camera (Tuczon Olympus, USA) with reference to the central hepatic vein (one at the left side and another at the right side). The cross-sectional area (CSA) of hepatocytes was measured on six selected fields at 100× per animal using Axio Vision Rel 4.6 (Zeiss Software Inc.). The averaged CSA of hepatocytes was calculated for each rat and averaged per litter.Reference Rodríguez-Castelán, Corona-Pérez and Nicolás-Toledo 20
Liver fat content
Liver fat content was measured using the method reported by Folch et al.Reference Folch, Lees and Sloane Stanley 21 with some modifications. Briefly, frozen liver samples (1 g, approximately) were disrupted in 5 ml of chloroform–methanol (2:1; v/v; J. T. Baker, México) using an electronic homogenizer (Tissue Tearor; Biospec Products Inc., México). Organic and inorganic layers were separated by adding 2 ml of 0.7% NaCl (NaCl J. T. Baker). Samples were mixed and centrifuged at 1500 g at 4°C for 20 min. The organic phase was carefully transferred to a new tube, evaporated and weighed. The fat percentage was reported as g/g of wet liver weight.
3-nitrotyrosine (3-NTyr) immunostaining
Other slides from the middle portion of each liver were deparaffinized and incubated in microwave-heated 10 mM sodium citrate pH 6 to retrieve antigens. Endogenous peroxidases were quenched with 0.3% hydrogen peroxide (H2O2; Sigma-Aldrich) during 30 min. Liver sections were blocked with 5% normal goat serum diluted in phosphate buffered saline (PBS) for 1 h to prevent non-specific binding of antibodies. The primary antibody was a mouse monoclonal anti-3-NTyr (MAB 5404; Chemicon Millipore, USA) used at a 1:200 dilution and incubated overnight at 4°C. Sections were incubated for 2 h at 4°C with a biotinylated goat anti-mouse IgG (1:250; Vector Labs, USA). Sections were rinsed with PBS and the immunostaining was developed according to the Vectastain ABC kit directions (Vector Labs, USA). Immunostained sections were counterstained with cresyl violet. Sections were dehydrated and mounted using a histological resin. Liver sections were observed under an optical microscope, photographed and the 3-NTyr immunoreactivity was considered as indicator of the presence of oxidative stress. To comparative ends, we used homologous liver sections that were simultaneously processed to estimate the resultant immunoreactivity. We calculated the percentage area of positive staining using a true color image analysis system Axio Vision Rel 4.6 (Zeiss Software Inc.) applying a segmentation method with fixed thresholds.Reference Willemse, Nap, Henzen-Logmans and Eggink 22
Serum concentration of malondialdehyde (MDA)
Lipid peroxidation was assessed by estimating the serum MDA concentration by using the thiobarbituric acid spectrometry assay. Thus, 200 µl of serum was reacted with 1.5 ml of 20% acetic acid (Sigma-Aldrich), 1.5 ml of 0.8% thiobarbituric acid (Sigma-Aldrich) and 0.6 ml of ultrapure water. This mixture was incubated for 60 min. Later, samples were cooled in ice water and added with 1 ml of 2% KCl (w/v; Sigma-Aldrich) and 5 ml of a mixture of butanol/pyridine (15:1 v/v; Sigma-Aldrich). Samples were vortexed and centrifuging at 2000 g for 10 min. Supernatant was collected and read at 532 nm on a spectrophotometer and MDA levels were calculated using its extinction coefficient (1.54×105 cm−1/M) and expressed as nanomols per 100 μl.
Statistical analysis
Both maternal protein intake and hematocrit are means±s.e.m. Two-tail unpaired Student-t tests were done to compare these between the C and R mothers. Offspring data were analyzed using a three-way analysis of variance to identify significant differences among effects of maternal diet, trial water and sex. Thus, protein and total and carbohydrate intake, liver weight, hepatocyte CSA, liver 3-NTyr immunoreactivity and the serum MDA concentration were used as dependent variables, and maternal diet, trial water and sex were used as fixed factors. The second-order interaction maternal diet×trial water×sex was tested in each analysis. The first-order interactions maternal diet×trial water, maternal diet×sex and trial water×sex were then considered and Tukey post-hoc tests were done to determine the statistical differences that could explain the second-order interaction. Data for these interactions are least squares means ± s.e.m. Otherwise, only effects of each separated factor were considered. Correlation analyses were performed using non-parametric Pearson’s tests. For all cases a P<0.05 was accepted as statistically significant. Statistical tests were carried out with the program JMP 4 (version 4.0.4) and graphs made in Graph Pad Prism (version 5.01 for Windows).
Results
Maternal protein intake and hematocrit
Along pregnancy, protein intake for the R mothers was lower than for the C mothers (18.9±1.6 v. 37.1±1.3 g/100 g body weight; P=0.0001). The same was true for the hematocrit measured at the end of pregnancy (40±0.002% v. 53±0.03%; P=0.01).
Offspring protein and total carbohydrates intake during trial water
The interaction maternal diet×sucrose trial×sex did not affect the protein and total carbohydrates intake (Table 1). The same was true for the maternal diet×sucrose intake, maternal diet×sex and sucrose intake×sex interactions as well as the maternal diet alone (Table 1). The sucrose administration decreased the protein intake, whereas increased that of total carbohydrates. The intake of both nutrients was higher in males than in females (Table 1).
Table 1 Offspring protein and total carbohydrates intakes during trial drink (post 10 weeks)

Relative liver weight and hepatocyte CSA
Neither the interaction maternal diet×sucrose trial×sex nor sucrose trial×sex interaction affected the relative liver weight (Table 2). Otherwise, the interaction maternal diet×trail water and sex×trial water altered the relative liver weight (Table 2). Thus, the liver of the offspring from R mothers drinking 5% sucrose was heavier than the offspring from C mothers drinking tap water (Table 2). The effect of 5% sucrose intake on the relative liver weight was higher in females than in males.
Table 2 Weight and morphometry of liver

The hepatocyte CSA was not modified by the interactions maternal diet×sucrose trial×sex nor by maternal diet×trial water, maternal diet×sex and trial water×sex (Table 2). In contrast, the offspring from R mothers and the sucrose administration increased the hepatocyte CSA in females and males (Table 2).
Fat liver content
The interaction maternal diet×trial water×sex was statistically significant for the fat liver content (F=4.9794, P=0.0308; Fig. 1a and 1b). Statistical analyses showed this was driven for the interaction maternal diet×sex (F 3,28=27.81, P<0.0001; Fig. 1c) but not for the interaction maternal diet×trial water (F 3,28=3.12, P=0.084) nor the trial water×sex (F 3,28=0.31, P=0.5798). Tukey post-hoc tests indicated a significant increase (P<0.0001) in the liver fat content of the female offspring from R mothers, which did not occur for the case of males (Fig. 1c). Furthermore, the female offspring from R mothers showed higher a liver fat content (P<0.0001) that its male counterpart. Thus, the increment in fat liver amount promoted by drinking 5% was exacerbated in the female offspring from R mothers. In contrast, the extent in which the fat liver content was elevated in males was similar than in the offspring from R and C mothers.
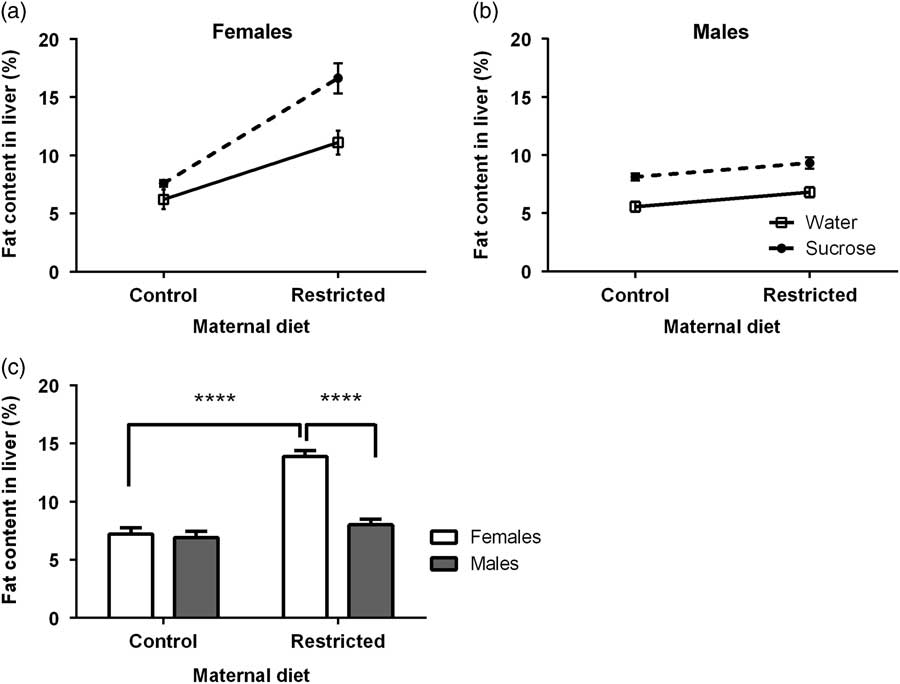
Fig. 1 Effect of maternal diet and 5% sucrose consumption at adulthood on the liver fat content (as percentage, %) of the female (a) and male (b) offspring. Data are least squares means±s.e.m. (a–c). Data were analyzed by non-repeated measures three-way analysis of variance (seven litters for each maternal diet and trial water per sex). The maternal diet×trial water×sex interaction was statistically significant (P=0.0308). Only the interaction maternal diet×sex was retained as statistically significant (P<0.0001). Tukey post-hoc tests were conducted to determine significant differences among data (c). ****P<0.0001.
3-NTyr immunostaining
The proportion (as percentage) of 3-NTyr immunostaining in liver sections was assessed as an indicator of protein oxidation (Fig. 2a and 2b). This variable was not modified by the interactions maternal diet×trial water×sex (F 3,20=0.7426, P=0.3935), maternal diet×trial water (F 3,20=0.4697, P=0.4967), maternal diet×sex (F 3,20=0.0517, P=0.8212) and trial water×sex interactions (F 3,20=0.9728, P=0.3553). The same was true for the sex factor (F 1,12=2.5345, P=0.1185). In contrast, the maternal diet (F 1,12=24.1600, P<0.0001) and trial water (F 1,12=10.388, P=0.0024) affected the percentage of 3-NTyr immunostaining. Thus, both female and male offspring from R mothers that had drunken 5% sucrose showed a high hepatic 3-NTyr immunostaining as compared with the offspring of C mothers (Fig. 2c and 2d).
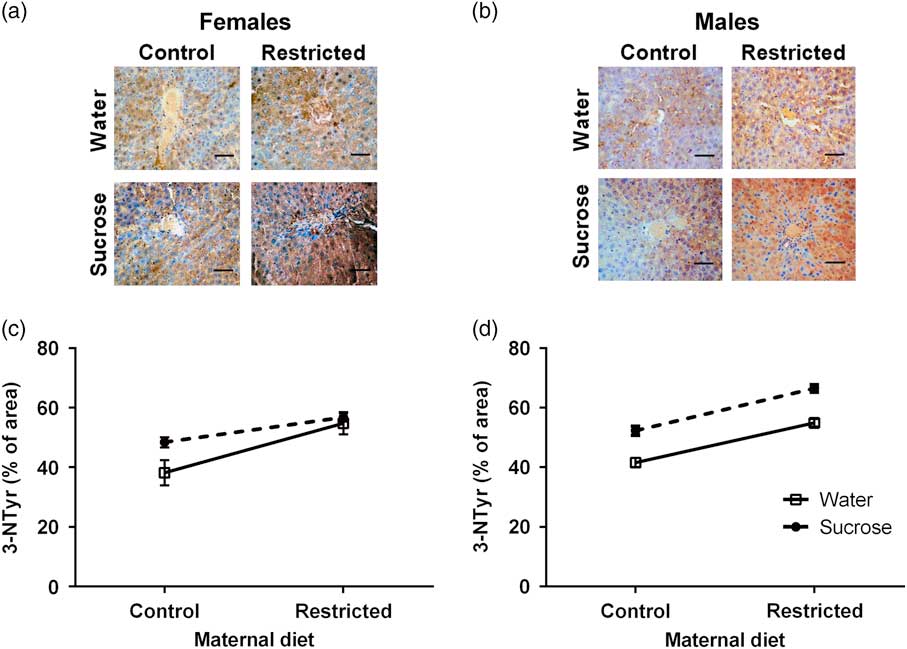
Fig. 2 Effect of maternal diet and 5% sucrose consumption at adulthood on the percentage (%) of hepatic 3-nitrotyrosine (3-NTyr) immunostaining for the female (a,c) and male (b,d) offspring. Data are expressed as least squares means±s.e.m. Non-repeated measures three-way analysis of variance (ANOVA) was done to determine significant differences. Data were analyzed by three-way ANOVA (seven litters per combination of maternal diet and trial water per sex). The maternal diet×trial water×sex was not statistically significant (P=0.4). The same was true for the other interactions analyzed. Scale bar is 50 µm.
Concentrations of serum MDA
The maternal diet×trial water×sex interaction did not affect the concentration of serum MDA (F=0.119, P=0.7314; Fig. 3a and 3b). The same was true for the maternal diet×trail water (F 3,28=3.8009, P=0.0562) and trial water×sex (F 3,28=0.0521, P=0.8204) interactions. In contrast, the maternal diet×sex interaction significantly modified (F 3,28=7.7453, P=0.0073) the concentration of serum MDA. The same was true for the trial water (F 1,14=4.5788, P=0.0367) and sex (F=7.7021, P=0.0075), but not the maternal diet (F=1.4901, P=0.2273). Thus, the female offspring from R mothers showed high values for serum MDA levels as compared with those of males (Fig. 3c).

Fig. 3 Effect of maternal diet and 5% sucrose consumption at adulthood on the serum malondialdehyde (MDA) levels. Data are expressed as least squares means±s.e.m. (a–c). Data were analyzed by non-repeated measures three-way analysis of variance (eight litters per combination of maternal diet and trial water per sex). The interaction maternal diet×trial water×sex was not statistically significant (P=0.7). Only the interaction maternal diet×sex was retained as statistically significant (P<0.007). Tukey post-hoc tests were conducted to determine significant differences among data (c). *P<0.05.
Correlation analyses
As the differences in the liver fat content caused by 5% sucrose intake in the female and male offspring from R mothers, we analyzed its correlation with hepatic 3-NTyr immunostaining and concentrations of serum MDA (Fig. 4). There was a positive correlation between the liver fat content and 3-Ntyr immunostaining for females (P=0.006, r=0.307; Fig. 4a) and males (P=0.006, r=0.548; Fig. 4b). A positive correlation was found between the liver fat content and serum MDA levels for females (P=0.03, r=0.17; Fig. 4c) but not for males (P=0.1, Fig. 4d).

Fig. 4 Liver fat content correlates with local and systemic oxidative parameters. Pearson’s tests were done to determine the correlation between the liver fat content and the percentage (%) of hepatic 3-nitrotyrosine (3-NTyr) immunostaining (a,b) and the liver fat content and serum malondialdehyde (MDA) levels (c,d) in female (a,c) and male (b,d) offspring of mothers fed C or R diet and drinking tap water or 5% sucrose at adulthood.
Discussion
At the end of gestation, a low hematocrit is considered as an indicator of malnutrition.Reference Golub, Hogrefe and Tarantal 19 Present findings suggest that, at least in the first days of life, the offspring from R mothers could present malnutrition increasing the possibility of developing metabolic problems.Reference Jahan-Mihan, Rodriguez, Christie, Sadeghi and Zerbe 9 Present findings show that a high-sucrose intake during adulthood amplifies or, even, reveals some hepatic alterations promoted by the low-protein intake during pregnancy. Importantly, none of the interactions involving sex, maternal diet and sucrose intake affects the intake of both proteins and carbohydrate of the offspring, which in turn were separately influenced by the sucrose intake and sex of the offspring. Strikingly, the increase in the hepatic fat content in females was more pronounced than in males.
The sucrose intake promotes an increase in the relative liver weight of both female and male offspring of rats fed low-protein diet at pregnancy. This finding agrees with the augmentation in the adipocyte size and adiposity index previously reported.Reference Cervantes-Rodriguez, Martinez-Gomez and Cuevas 10 Data herein supports this notion as the R diet and sucrose intake enlarged hepatocyte CSA in females and males. Other study has suggested that a high hepatocyte area, prompted by a gestational low-protein diet, increases the vacuolization of hepatocytes, fat droplets and glycogen granules.Reference Ramadan, Alshiraihi and Al-karim 23 – Reference Sasaki, Nakagawa and Kajimoto 27 This could be explained in terms of an elevation in the delivery of FA from adipose tissue or the diet, an increased synthesis of FA in the de novo lipogenesis pathway, and-or a decrement in the mitochondrial β-oxidation of FA.Reference Mehta, Van Thiel, Shah and Mobarhan 24 , Reference Musso, Gambino and Cassader 25 . Certainly, de novo lipogenesis inside hepatocytes uses carbohydrates as the major substrate.Reference Neuschwander-Tetri 26 Otherwise, low-protein intake at pregnancy increases adiposity in the offspring.Reference Sasaki, Nakagawa and Kajimoto 27 Furthermore, male and female offspring from mothers fed a junk food diet promotes enlarged hepatocytes with both macro- and microvesicular steatosis.Reference Bayol, Simbi, Fowkes and Stickland 28 Some studies have shown that the FA composition and/or protein content in the maternal diet can affect the hepatic lipid amount and metabolic gene expression,Reference Zhang, Wang and Terroni 29 – Reference Gosby, Maloney and Phuyal 31 as well as hepatic enzymes in the progeny.Reference Maloney, Gosby and Phuyal 32 , Reference Musso, Gambino and De Michieli 33 In general, unbalanced diets may predispose them to both fat accumulation in the liver and subsequent metabolic alterations. Certainly, excessive lipid accumulation in the liver is frequently accompanied by oxidative stress.Reference Berlanga, Guiu-Jurado, Porras and Auguet 1
Findings herein demonstrate the great extent in which fat is accumulated in the liver as an outcome of sucrose intake in the female offspring from mothers fed low-protein diet while this response was absent in the male offspring. In our study we did not measure estrogen levels. It would be relevant as estrogens are responsible for the accumulation of visceral fat depots.Reference Kozakowski, Gietka, Leszczynska and Majos 34 In contrast, it has been suggested that estrogens may play a protective role in the preventing of hepatic steatosis and inflammation in females.Reference Gilsanz, Chung and Kaplowitz 3 – Reference Ayonrinde, Olynyk and Beilin 5 Even, estrogens can have protective properties for the liver in healthy men.Reference Tian, Sun and Pang 35 Although, we did not measure the serum concentration of sex hormones in females and males, it is possible that the production of estrogens could be affected by the gestational protein restriction, favoring the fat liver accumulation.Reference Kamada, Kiso and Yoshida 36 In this regard, it is known that gestational undernutrition impacts on genes involved in folliculogenesis and these changes were underpinned by ovarian oxidative stress.Reference Bernal, Vickers, Hampton, Poynton and Sloboda 37 For its part, a significant increase in the ROS and MDA levels was found in both testes and sperm of the male offspring from protein-restricted mothers.Reference Rodríguez-González, Reyes-Castro and Vega 38 This high oxidative process in males could affect estrogens synthesis in the testis. Furthermore, Western-type diets impair the hepatic testosterone metabolism and its conversion to estrogens,Reference Krawczyńska, Herman and Antushevich 39 which could affect the liver metabolism.
Findings herein show that the hepatic 3-NTyr immunostaining increases by gestational low-protein diet and 5% sucrose intake in adulthood differentially in both females and males. In concordance, patients with NAFLD have a greater systemic nitrosative stress.Reference Musso, Gambino and De Michieli 33 In contrast, serum MDA levels were higher in the female offspring from R mothers. Oxidative stress is able to program to the offspring for metabolic outcomes.Reference Vega, Reyes Castro and Rodríguez Gonzalez 40 Restricted protein diet during gestation and lactation in rats increases the production of ROS and decreases the antioxidant activity levels in heart associated with an increase in the susceptibility to develop metabolic and cardiac diseases in adulthood.Reference Nascimento, Freitas and Silva-Filho 41 Moreover, a high-fat diet induces obesity and oxidative stress that correlate with an increase in the MDA and protein carbonyl levels in various tissues.Reference Noeman, Hamooda and Baalash 42 In this regard, a positive correlation between the percentage of hepatic fat with the serum MDA levels and the expression 3-NTyr in the liver was observed. In general, both oxidative and nitrosative stresses can damage the hepatic function, favor fibrosis and apoptosis of hepatocytes, as well as ischemia of the liver,Reference Lee, Wang, Hsu and Chen 43 – Reference Kattaia, Abd E-Baset, Mohamed, Abdul-Maksou and Elfakharany 45 but females seem to be more susceptible to lipoperoxidation than males. Considering that females with prenatal malnutrition and consumption of sucrose 5% in adulthood show a great visceral fat and adiposity index,Reference Cervantes-Rodriguez, Martinez-Gomez and Cuevas 10 possibly they had a high fat accumulation in the liver accompanied by high concentrations of serum MDA levels. Although the FAT accumulation in the liver of males was lower.
Whereas the association between a high-fat diet and increased oxidative stress is well known,Reference Kozakowski, Gietka, Leszczynska and Majos 34 little or nothing has been mentioned regarding to the impact of consumption of carbohydrates. Our present findings provide compelling evidence that the female offspring from dams fed low protein during pregnancy are more prone to accumulate fat in the liver that could increase the hepatic and systemic oxidative stress following sucrose intake at adulthood.
Acknowledgments
The authors acknowledge the assistance of Dr. Arturo Estrada Torres in the statistical analysis.
Financial Support
This study was supported by the Centro Tlaxcala de Biología de la Conducta and the Posgrado en Ciencias Biológicas.
Conflicts of Interest
None.
Ethical Standards
The Research Ethics Committee from the Universidad Autónoma de Tlaxcala approved all the experimental procedures in accordance with the Mexican Guidelines for Animal Care (NOM-062-ZOO-1999).









