INTRODUCTION
Behavioural variant frontotemporal dementia (bvFTD) shares clinical, cognitive, and behavioural features with Alzheimer’s disease (AD), posing a challenge for differential diagnosis between these two conditions. In particular, disturbed executive functions that are classically found in bvFTD can also be impaired in AD, such as working memory, mental flexibility, and planning (Castiglioni et al., Reference Castiglioni, Pelati, Zuffi, Somalvico, Marino, Tentorio and Franceschi2006; Perry & Hodges, Reference Perry and Hodges2000). However, among executive functions, tests of inhibitory control may be useful for the differential diagnosis between bvFTD and AD. Indeed, as disinhibition is a hallmark of bvFTD (Rascovsky et al., Reference Rascovsky, Hodges, Knopman, Mendez, Kramer, Neuhaus, Swieten, Seelaar, Dopper, Onyke, Hillis, Josephs, Boeve, Ketesz, Seeley, Rankin, Johnson, Gorno-Tempini, Rosen, Prioleau-Latham, Lee, Kipps, Lillo, Piguet, Rohrer, Rossor, Warren, Fox, Galasko, Salmon, Black, Mesulam, Weintraud, Dickerson, Diehl-Schmid, Pasquier, Deramecourt, Lebert, Pijinenburg, Chow, Manes, Grafman, Cappa, Freedman, Grossman and Miller2011), a comprehensive assessment of inhibitory control and impulsivity is potentially more accurate in identifying specific symptoms of bvFTD (O’Callaghan, Hodges, & Hornberger, Reference O’Callaghan, Hodges and Hornberger2013a).
Inhibitory control refers to the ability to selectively supress thoughts or behaviours that are not adaptive or appropriate in the current context (Diamond, Reference Diamond2013). Impulsivity is broadly defined as acting prematurely without foresight (Dalley, Everitt, & Robbins, Reference Dalley, Everitt and Robbins2011), and a specific sub-type of impulsivity refers to the tendency to prefer an immediate, but smaller, reward, rather than waiting for a larger, although delayed, reward (Rachlin, Reference Rachlin2000). Impulsivity and inhibitory control are both related to efficient behavioural regulation and to the prefrontal cortex, and may represent the cognitive counterpart of disinhibition (Kim & Lee, Reference Kim and Lee2011).
The value of tasks of inhibitory control for the clinical diagnosis of bvFTD has been previously investigated. The Stroop Test, a classical measure of inhibition of prepotent response, has poor diagnostic value in differentiating bvFTD and AD (Collette et al., Reference Collette, Amieva, Adam, Hogge, Van der Linden, Fabrigoule and Salmon2007; Perry & Hodges, Reference Perry and Hodges2000). Some authors reported that the Hayling Test provides good clinical differentiation between bvFTD and AD (Buhl et al., Reference Buhl, Stokholm and Gade2013; Hornberger et al., Reference Hornberger, Savage, Hsieh, Mioshi, Piguet and Hodges2010). A longitudinal study showed that Hayling Test was sensitive to discriminate bvFTD and AD during the first year of follow-up, while executive and memory tests had modest discriminability (Ramanan et al., Reference Ramanan, Bertoux, Flanagan, Irish, Piguet, Hodges and Hornberger2017). However, Hayling Test did not provide accurate discrimination between AD and bvFTD in another study (Flanagan et al., Reference Flanagan, Wong, Dutt, Tu, Bertoux, Irish, Piguet, Rao, Hodges, Ghosh and Hornberger2016). The Swedish version of Hayling Test (Vestberg et al., Reference Vestberg, Nordström, Waldö, Nilsson, Santillo and Nilsson2019) achieved good diagnostic accuracy for bvFTD in contrast to progressive supranuclear palsy (PSP) and semantic dementia (SD), but it was not compared to AD. Interestingly, Matías-Guiu et al. (Reference Matías-Guiu, Cabrera-Martín, Valles-Salgado, Rognoni, Galán, Moreno-Ramos, Carreras and Matías-Guiu2019) found that both AD and bvFTD were characterised by impairment in inhibitory control. Specifically, Hayling Test scores differed only on the type of strategies deployed to inhibit responses, which is measured by scaled scores or by the classification of errors (Matías-Guiu et al., Reference Matías-Guiu, Cabrera-Martín, Valles-Salgado, Rognoni, Galán, Moreno-Ramos, Carreras and Matías-Guiu2019).
Such results show that cognitive evaluation of disinhibition is still inconclusive regarding differential diagnosis between AD and bvFTD, highlighting the importance of using alternative approaches to improve differential diagnosis. More recent studies using tasks of delayed discounting tried to overcome the limits of classical inhibitory control tests. The Delay Discounting Task (DDT) requires the participant to make intertemporal choices, deciding between present versus future reward options. This paradigm may be a reliable model for testing impulsivity, and some reports indicate that DDT can differentiate bvFTD from AD (Bertoux, de Souza, Zamith, Dubois, & Bourgeois-Gironde, Reference Bertoux, de Souza, Zamith, Dubois and Bourgeois-Gironde2015; Lebreton et al., Reference Lebreton, Bertoux, Boutet, Lehericy, Dubois, Fossati and Pessiglione2013).
One of the limits of neuropsychological tests used to investigate inhibitory functions is that they usually lack ecological validity and do not recapitulate real-life situations. By contrast, caregiver-completed neuropsychiatric questionnaires of disinhibition and impulsivity are usually made of questions that investigate everyday situations. The Neuropsychiatric Inventory (NPI) is one of the most used tools to assess neuropsychiatric symptoms in dementia. Other scales have also been proposed to evaluate specific behavioural disorders, such as the Frontal Behavioural Inventory, the Frontal Systems Behavior Scale, and the Cambridge Behavioural Inventory (Kertesz, Davidson, & Fox, Reference Kertesz, Davidson and Fox1997; Malloy, Tremont, Grace, & Frakey, Reference Malloy, Tremont, Grace and Frakey2007; Wedderburn et al., Reference Wedderburn, Wear, Brown, Mason, Barker, Hodges and Williams-Gray2008). Even though these are useful tools, they are time-consuming, require training and depend on subjective factors from the respondent. It remains open whether such neuropsychiatric scales might capture the disinhibition-related disorders associated to bvFTD with higher accuracy than existing neuropsychological tests. Some reports showed that the measurement of impulsive behaviour effectively differentiates bvFTD from AD (Grochmal-Bach et al., Reference Grochmal-Bach, Bidzan, Pachalska, Bidzan, Lukaszewska and Pufal2009; Paholpak et al., Reference Paholpak, Carr, Barsuglia, Barrows, Jimenez, Lee and Mendez2016), while cognitive tests may fail to do so ( Buhl, Stokholm, & Gade, Reference Buhl, Stokholm and Gade2013; Collette et al., Reference Collette, Amieva, Adam, Hogge, Van der Linden, Fabrigoule and Salmon2007; Leslie et al., Reference Leslie, Foxe, Daveson, Flannagan, Hodges and Piguet2016; Perry & Hodges, Reference Perry and Hodges2000; Schubert, Leyton, Hodges, & Piguet, Reference Schubert, Leyton, Hodges and Piguet2016). Previous studies (Bertoux et al., Reference Bertoux, de Souza, Zamith, Dubois and Bourgeois-Gironde2015; Lebreton et al., Reference Lebreton, Bertoux, Boutet, Lehericy, Dubois, Fossati and Pessiglione2013) did not include specific behavioural scales to assess disinhibition, but general measures, like the NPI, which may lead to incomplete understanding of the phenomenon.
The current study aims to address these points by contrasting neuropsychological tests of disinhibition with a neuropsychiatric questionnaire of impulsivity (Barratt Impulsiveness Scale) (Malloy-Diniz et al., Reference Malloy-Diniz, Mattos, Leite, Abreu, Coutinho, Paula, Tavaresv, Vasconcelos and Fuentes2010) in order to determine the accuracy of these tools in the differential diagnosis between bvFTD and AD. To the best of our knowledge, this is the first time that the accuracy of such inhibitory control tests is compared with a specific neuropsychiatric measure of impulsivity. We hypothesised that neuropsychological measures should detect inhibitory dysfunction per se but would be not as good in distinguishing bvFTD from AD. By contrast, the neuropsychiatric questionnaire would allow better diagnostic distinction, due to its higher ecological validity capturing the real-life symptomatology of the patients.
METHODS
Fifty-two patients were recruited in two Brazilian centres of Cognitive and Behavioural Neurology, located at Belo Horizonte (University Hospital from Universidade Federal de Minas Gerais) and São Paulo (University Hospital from Universidade de São Paulo). All of them fulfilled consensus diagnostic criteria for probable bvFTD (Rascovsky et al, Reference Rascovsky, Hodges, Knopman, Mendez, Kramer, Neuhaus, Swieten, Seelaar, Dopper, Onyke, Hillis, Josephs, Boeve, Ketesz, Seeley, Rankin, Johnson, Gorno-Tempini, Rosen, Prioleau-Latham, Lee, Kipps, Lillo, Piguet, Rohrer, Rossor, Warren, Fox, Galasko, Salmon, Black, Mesulam, Weintraud, Dickerson, Diehl-Schmid, Pasquier, Deramecourt, Lebert, Pijinenburg, Chow, Manes, Grafman, Cappa, Freedman, Grossman and Miller2011) or AD (McKhann et al., Reference McKhann, Knopman, Chertkow, Hyman, Jack, Kawas, Klunk, Koroshetz, Manly, Mayeux, Mohs, Morris, Rossor, Scheltens, Carrillo, Thies, Weintraud and Phelps2011). The AD group included patients at early and moderate stages of the disease. All bvFTD and AD patients underwent structural brain magnetic resonance imaging (MRI). Brain MRI was performed for diagnosis purpose only. We did not include patients with neuroimaging disclosing focal lesions or severe vascular lesions. To improve diagnostic accuracy, patients were clinically followed for at least 18 month after the diagnostic definition, and all of them showed clinical progression consistent with the diagnosis.
A subset of patients (eight AD and eight bvFTD) underwent lumbar puncture for cerebrospinal fluid (CSF) biomarkers (Aß42, Tau and P-Tau) analyses. For this subgroup, all AD patients had an “AD CSF biomarker profile”, defined by Tau/Aß42 > .52 (Magalhães et al., Reference Magalhães, Figueiró, Fraga, Mateo, Toledo, Carvalho, Caramelli and Gomes2015). None of the bvFTD patients had this biomarker profile. This procedure was adopted to increase the specificity of the clinical diagnosis. One bvFTD patient had a known genetic mutation (TARDBP).
Community-dwelling elderly with no history of neurologic or psychiatric disorders and intact cognitive assessment constituted the control group.
Groups were matched for education level, years of disease and socio-economic status (Table 1). Socio-economic level was controlled by the Brazilian standard classification (Associação Brasileira de Empresas de Pesquisa, 2016). The local ethics committees approved the study (Project CAAE-17850513.2.0000.5149), and all participants gave written informed consent to participate.
Table 1. Demographical and clinical characterisation of subjects
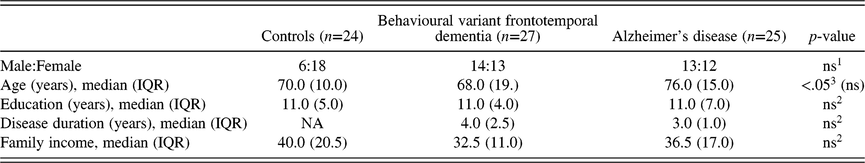
IQR, interquartile range.
1 Chi-square test.
2 Kruskal–Wallis test.
Cognitive Assessment
All participants underwent the same cognitive protocol:
General cognitive measures:
Mini-Mental State Exam (MMSE) (Brucki, Nitrini, Caramelli, Bertolucci, & Okamoto, Reference Brucki, Nitrini, Caramelli, Bertolucci and Okamoto2003): screening for global mental state.
Frontal Assessment Battery (FAB) (Beato, Nitrini, Formigoni, & Caramelli, Reference Beato, Nitrini, Formigoni and Caramelli2007): it evaluates six executive functions: conceptualisation, mental flexibility, programming, sensitivity to interference, inhibitory control, and autonomy.
Figure Memory Tests (FMT) from the Brief Cognitive Battery (Nitrini et al., Reference Nitrini, Caramelli, Porto, Charchat-Fichman, Formigoni, Carthery-Goulart, Otero and Prandini2007): 10 figures are presented to the subject, who is asked to name them. After that, the figures are removed and the participant is required to recall them (incidental memory). Then, the figures are presented again, and the subject is requested to memorise them. Two recall tasks are then performed (immediate memory and learning). After 5 min, late memory and recognition are tested.
Verbal fluencies (“FAS” and “Animals”) (Machado et al., Reference Machado, Fichman, Santos, Carvalho, Fialho, Koenig, Fernandes, Lourenço, de Paiva Paradela and Caramelli2009), requiring the maximal production of words, starting with specific letters (“F”, “A” and “S”) or into some category (“Animals”), within 1-min time limit.
Forwards and Backwards Digit Span (Wechsler, Reference Wechsler1997): a sequence of numbers is read to the participant, who is required to repeat it forward and backwards.
Specific inhibitory and impulsivity control measures:
Stroop-Victoria (Strauss, Sherman, & Spreen, Reference Strauss, Sherman and Spreen2006): This version has 24 items on each of three tasks (naming the colour of dots, of neutral words, and of colour words printed in contrasting colours). Stroop Test is a classical paradigm designed to evaluate the capacity to suppress a prepotent answer in saying colours instead of reading it.
Hayling (Siqueira, Scherer, Reppold, & Fonseca, Reference Siqueira, Scherer, Reppold and Fonseca2010): In Part A, incomplete sentences are read and the participant is required to complete them as fast as possible. In Part B, incomplete sentences are also read, and the participant is required to complete them as fast as possible but sentences must lack coherence. Thus, Hayling Test evaluates the capacity to inhibit a prepotent verbal answer when completing phrases meaninglessly. For the purpose of this work, the parameters of time in Part A and B are extracted. In Part B, specifically, error score and scaled score (PQt and PQl, respectively) are also obtained.
Five Digits Test (FDT) (de Paula, Oliveira, Querino, & Malloy-Diniz, Reference de Paula, Oliveira, Querino and Malloy-Diniz2017): The test has four parts, like a “numerical Stroop task”. Each part contains 50 stimuli (numbers or points) distributed in 10 rows with 5 stimuli per column. Parts One and Two evaluate processing speed and attention by asking the participant to read numbers and to count numbers, respectively. Parts Three and Four evaluate inhibitory control (suppressing a prepotent answer by counting numbers rather than saying the exhibited number), and cognitive flexibility (alternating two different rules in the same task). FDT was chosen because it minimises the influence of low education level (de Paula et al., Reference de Paula, Oliveira, Querino and Malloy-Diniz2017).
Delay Discounting Task (DDT)
The DDT was performed using a computerised version of the original questionnaire (Kirby, Petry, & Bickel, Reference Kirby, Petry and Bickel1999), created using PowerPoint. Participants were required to choose either an amount of money available today or a larger amount available in the future. The amount of delay (days) and the sum of money varied. A total of 27 forced-choices were presented to participants one at a time, and delayed versus immediate reward options were randomised to occur on either the left or right side of the screen in equal proportions. Figure 1 illustrates examples of 2 out of the 27 forced-choices. Participants were not awarded any actual monetary payments based on their performance, but they were encouraged to approach the choices as if real money was at stake.
Fig. 1. Examples of the delay-discounting task.
A hyperbolic discount parameter (k) is inferred from subjects’ choices on the DDT. Delay discounting is the tendency to discount the present value of a reward as delay to the reward increases, calculated by the equation: PV = FV/(1 – kt), where: PV= Present Value; FV= Future Value; t = time; and, k = slope. A higher k value is consistent with a steeper discounting of future rewards, indicating a higher degree of impulsivity.
The 27 options from DDT comprised nine items in three groups: small ($15–$25), medium ($35–$55) and large ($75–$85) values, which refers to the size of the delayed reward. Thus, four parameters are extracted from DDT: a general k for all the 27 items, and three separate k values for small, medium, and large items. Based on values available in our task, k values could vary from .000158 to .25.
Impulsivity Questionnaire
Symptoms of impulsivity were assessed with the Brazilian version of the Barratt Impulsiveness Scale 11th version (BIS-11) (Malloy-Diniz et al., Reference Malloy-Diniz, Mattos, Leite, Abreu, Coutinho, Paula, Tavaresv, Vasconcelos and Fuentes2010). The BIS-11 is a self-report scale comprising 30 items. The participant is required to analyse each item and classify the frequency of that behaviour, using a Likert scale, ranging from never (1 point) to very frequently (4 points), with the minimum score of 30 and the maximum of 120. A higher score means a higher degree of impulsivity. BIS assesses three main dimensions of impulsivity: motor (acting without thinking), attentional (a deficiency of focus on the ongoing task) and non-planning (orientation to the present rather than to the future) (Malloy-Diniz et al., Reference Malloy-Diniz, Paula, Vasconcelos, Almondes, Pessoa, Faria, Coutinho, Costa, Duran, Coutinho, Corrêa, Fuentes, Abreu and Mattos2015). Similar to previous studies, the BIS-11 was adapted as a caregiver-report version, in order to avoid a possible anosognosia effect in the patients (O’Callaghan, Naismith, Hodges, Lewis, & Hornberger, Reference O’Callaghan, Naismith, Hodges, Lewis and Hornberger2013b). For the BIS-11, a cut-off of 82 was established to determine impairment as this value is 2 SD superior to the mean score from the Brazilian norms (Malloy-Diniz et al., Reference Malloy-Diniz, Paula, Vasconcelos, Almondes, Pessoa, Faria, Coutinho, Costa, Duran, Coutinho, Corrêa, Fuentes, Abreu and Mattos2015). Controls completed the BIS-11 original version.
Statistics
Descriptive and comparative analyses were performed. Normality was checked with Shapiro-Wilk Test and homogeneity of variances with Levene’s Test. Categorical variables were analysed by chi-square, and socio-demographic variables were analysed by Kruskall-Wallis followed by Mann-Whitney pairwise post-hoc comparison.
As age was statistically different between AD and bvFTD, it was necessary to control “age affects” in the results. Data were logarithmic transformed, and an ANCOVA analysis was deployed, with Bonferroni post-hoc comparisons. Still, age had no influence on any of the results. In order to guarantee precision, correlation score was also generated for all three groups and each group separately (data not shown) in a way to check any possible association between age and other variables. Once again, results were not significant, reassuring that age played no role in the results found.
In the DDT, within-group analyses were performed by the Wilcoxon method in order to evaluate magnitude effects. Receiver operator characteristics (ROC) curve analyses were carried out to test diagnostic accuracy. Analyses were performed with Statistical Package for the Social Sciences (SPSS) 22.0 and MedCalc 17.1 softwares.
RESULTS
The final groups consisted of 27 bvFTD patients, 25 AD patients, and 24 healthy controls. Table 1 describes socio-demographic data. bvFTD patients were younger than AD, but age did not differ between clinical groups and controls. Other socio-demographic variables (schooling and socio-economic level) were similar across groups. AD and bvFTD patients had similar symptom duration (years of disease).
Detailed results for all cognitive measures are presented on Table 2. Cognitive and behavioural measures of impulsivity and cognitive control are here presented in detail. Considering neuropsychological measures, there were statistically significant differences between clinical groups and healthy controls, with bvFTD and AD scoring worse than controls on most of measures (Table 2). There were no significant differences between bvFTD and AD for most of measures of executive function. In the “Flexibility” domain from the FDT, AD performed significantly worse than bvFTD (bvFTD mean 43.9 SD: 18.7; AD mean 84.1 SD: 45.1; F(2, 52) = 8,624.9, p < .002, d = −1.19), and the switching time in the same test (bvFTD mean 74.4 SD: 23.1; AD mean 115.7 SD: 50.7; F (2, 52) = 10,892.2, p < .005, d = −1.03), with strong effect size for both. AD patients also performed worse than bvFTD in the delayed recall from the Figure Memory Test (FMT).
Table 2. Neuropsychological and behavioural results

AD, Alzheimer’s disease; BIS-11, Barratt Impulsiveness Scale 11th version; bvFTD, behavioural variant Frontotemporal Dementia; CTR, Healthy Controls; N/A, not applicable, SD, standard deviation.
ANCOVA results controlling for age with Bonferroni post-hoc test.
Level of significance: *p<.05.
Regarding the DDT, there were no differences among groups for all parameters. The analyses within group, however, showed that the control group present k values for small rewards statistically higher than the k values for both the large and medium rewards. In contrast, for the bvFTD and AD groups, the k values for the small rewards were only significantly higher than the k values for the large rewards.
Impulsivity measures (BIS-11) revealed significant differences between groups. bvFTD patients were more impulsive than both AD and controls (bvFTD mean 76.1 SD 9.5; AD mean 62.9 SD: 13.5 ; F (2, 54) = 8.599, p = .007, d = 1.15), with strong effect sizes.
Considering Brazilian norms for the BIS-11 (Malloy-Diniz et al., Reference Malloy-Diniz, Paula, Vasconcelos, Almondes, Pessoa, Faria, Coutinho, Costa, Duran, Coutinho, Corrêa, Fuentes, Abreu and Mattos2015), controls’ mean score was at the 45th percentile, AD scores were at the 55th percentile and bvFTD mean score were within the 90th–95th percentile.
The FDT Flexibility score and BIS-11 Total score were analysed in a ROC curve procedure to establish the diagnostic accuracy of bvFTD versus AD (Table 3, Figure 2). For the BIS-11, a cut-off of 68 achieved 68.2% sensitivity and 80% specificity. FDT Flexibility achieved 86.4% sensitivity and 76.5% specificity with a cut-off of 54 s. The composition of a unique score with all three or even two instruments did not improve the diagnostic accuracy significantly.
Table 3. Results for receiver operator characteristics curve analysis


Fig. 2. ROC curve for BIS-11 and FDT.
DISCUSSION
Our results show for the first time that a specific caregiver-completed questionnaire of impulsivity is more accurate and reliable in distinguishing bvFTD from AD than neuropsychological tests of inhibitory control.
In more detail, the behavioural scale (BIS-11) provided better diagnostic accuracy than cognitive measures of inhibitory control, including the DDT, Stroop, and Hayling. Some studies reported differences between bvFTD and AD on inhibitory cognitive tests (Buhl et al., Reference Buhl, Stokholm and Gade2013; Collette et al., Reference Collette, Amieva, Adam, Hogge, Van der Linden, Fabrigoule and Salmon2007), while others did not (Flanagan et al., Reference Flanagan, Wong, Dutt, Tu, Bertoux, Irish, Piguet, Rao, Hodges, Ghosh and Hornberger2016; Hornberger et al., Reference Hornberger, Savage, Hsieh, Mioshi, Piguet and Hodges2010; Matías-Guiu et al., Reference Matías-Guiu, Cabrera-Martín, Valles-Salgado, Rognoni, Galán, Moreno-Ramos, Carreras and Matías-Guiu2019). Our current results corroborate the latter findings by showing no difference on the Hayling Test. Some previous studies reported differences only on qualitative measures of the Hayling Test (Matías-Guiu et al, Reference Matías-Guiu, Cabrera-Martín, Valles-Salgado, Rognoni, Galán, Moreno-Ramos, Carreras and Matías-Guiu2019). In a longitudinal study, Leslie et al. (Reference Leslie, Foxe, Daveson, Flannagan, Hodges and Piguet2016) found that the scores on the Hayling Test were not different between AD and bvFTD patients at the baseline assessment, but bvFTD patients had worse performance than AD after 1 year of follow-up (Leslie et al., Reference Leslie, Foxe, Daveson, Flannagan, Hodges and Piguet2016). This result was not confirmed in another longitudinal study (Ramanan et al., Reference Ramanan, Bertoux, Flanagan, Irish, Piguet, Hodges and Hornberger2017). Together, these data indicate that the Hayling Test may lack accuracy for differential diagnosis of neurodegenerative diseases. Similarly, there was no difference in Stroop between patient groups, which is in agreement with previous reports (Heflin et al., Reference Heflin, Laluz, Jang, Ketelle, Miller and Kramer2011).
The DDT also failed to detect differences between bvFTD and AD, in contrast to previous reports (Bertoux et al., Reference Bertoux, de Souza, Zamith, Dubois and Bourgeois-Gironde2015; Lebreton et al., Reference Lebreton, Bertoux, Boutet, Lehericy, Dubois, Fossati and Pessiglione2013). The reasons for the present result remain unclear but may be due to cultural specificities related to Brazilian background. Further studies are required to explore intercultural variability in DDT. The magnitude effect in the DDT refers to the finding that discount rates decrease as the amount of reward increases, as subjects are more willing to wait for a larger reward (Green Myerson, Holt, Slevin, & Estle, Reference Green, Myerson, Holt, Slevin and Estle2004). All groups exhibited magnitude effect on the DDT, showing largest k values for the smallest rewards. Nonetheless, AD and bvFTD patients’ differed only between large and small rewards, while controls had different scores between small rewards and both large and medium ones. This suggests that controls discounted small rewards more steeply compared to the larger rewards. This finding proposes that patients were overall less sensitive to magnitude effects, compared to controls. The reason for this is not completely clear, but executive deficits may account for flaws in decision-making process and discounting (Ballard et al., Reference Ballard, Kim, Liatsis, Aydogan, Cohen and McClure2017).
By contrast to the neuropsychological findings, the neuropsychiatric results showed that there were significant differences between AD and bvFTD on the BIS-11. This was further corroborated by the ROC analysis, with good accuracy for the differential diagnosis between bvFTD and AD. The BIS-11, which is quick to complete and has high ecological validity, emerges therefore as a good clinical resource to distinguish bvFTD from AD based on the impulsive symptoms. This result is similar to a previous report comparing BIS-11 scores in bvFTD versus Parkinson Disease, with bvFTD patients presenting a significant higher degree of impulsivity (O’Callaghan et al., Reference O’Callaghan, Naismith, Hodges, Lewis and Hornberger2013b).
Our results clearly highlight the limits of using cognitive measures of inhibitory control for the differential diagnosis between bvFTD and AD, in accordance to previous reports (Flanagan et al., Reference Flanagan, Wong, Dutt, Tu, Bertoux, Irish, Piguet, Rao, Hodges, Ghosh and Hornberger2016; Ramanan et al., Reference Ramanan, Bertoux, Flanagan, Irish, Piguet, Hodges and Hornberger2017). Most tests of inhibitory control were not specifically designed for neurodegenerative conditions. For instance, the DDT was initially developed to study addiction (Kirby et al., Reference Kirby, Petry and Bickel1999), while the Stroop was originally designed for cognitive screening in young adults (Heflin et al., Reference Heflin, Laluz, Jang, Ketelle, Miller and Kramer2011).
It is also important to consider that inhibitory control is a set of complex cognitive functions (Dalley et al., Reference Dalley, Everitt and Robbins2011; Hampshire & Sharp, Reference Hampshire and Sharp2015). Indeed, distinct cognitive abilities are required for efficient inhibitory control. For instance, the DDT may engage diverse cognitive operations, such as prospective memory and emotional processing. Therefore, different cognitive deficits may underlie impulsive behaviour. bvFTD and AD patients may fail in tests of inhibitory control due to deficits in different sub-processes, which are not specifically tapped by most standard tests. Hence, the design of new tests of inhibitory control for the diagnosis of bvFTD should consider more specific sub-processes of this ability. The development of new cognitive tests for the diagnosis of bvFTD should also take into account its clinical variability, with patients exhibiting either disinhibited, apathetic or mixed profiles (Lansdall et al., Reference Lansdall, Coyle-Gilchrist, Jones, Vázquez Rodríguez, Wilcox, Wehmann, Dick, Robbins and Rowe2017; O’Connor et al., Reference O’Connor, Landin-Romero, Clemson, Kaizik, Daveson, Hodges, Hsieh, Piguet and Mioshi2017). Furthermore, next studies should include more “ecological” measures of behavioural disorders, such as social cognition tasks (e.g., theory of mind test) and multitasking, in order to increase the diagnostic accuracy of early bvFTD. The optimal diagnosis of bvFTD requires a set of tests tapping into the different possible behavioural aspects of the disease. It must be stressed that the diagnosis of dementia is a complex procedure. Cognitive investigation is one part of the diagnostic framework, and all neuropsychological findings should be interpreted in the light of medical context.
An important element for future studies is to consider the anatomical specificities of bvFTD patients. bvFTD has marked behavioural variability according to anatomical substrates. For instance, patients with predominant ventromedial involvement may preferentially display symptoms related to disinhibition, while patients with dorsolateral, medial, and cingulate involvement may have an “apathetic” profile (Le Ber et al., Reference Le Ber, Guedj, Gabelle, Verpillat, Volteau, Thomas-Anterion, Decousus, Hannequin, Véra, Lacomblez, Camuzat, Didier, Puel, Lotterie, Golfier, Bernard, Vercelletto, Magne, Sellal, Namer, Michel, Pasquier, Salachas, Bochet, Brice, Habert and Dubois2006; Massimo et al., Reference Massimo, Powers, Moore, Vesely, Avants, Gee, Libon and Grossman2009; Zamboni, Huey, Krueger, Nichelli, & Grafman, Reference Zamboni, Huey, Krueger, Nichelli and Grafman2008). Moreover, basal ganglia-thalamocortical circuits are differently affected in neurodegenerative diseases, leading to distinct behavioural presentations (Ducharme, Price, & Dickerson, Reference Ducharme, Price and Dickerson2018; O’Callaghan et al., Reference O’Callaghan, Hodges and Hornberger2013a, Reference O’Callaghan, Naismith, Hodges, Lewis and Hornberger2013b). This study did not include quantitative neuroimaging analysis which could have shed light on how different patterns of anatomical involvement explain the variation in cognitive and behavioural tests.
Despite these interesting findings, our study has some caveats. (1) The diagnosis was established under clinical basis, and pathological confirmation was not available for any patient. However, patients were selected according to consensual criteria, and all patients had a minimal follow-up of 18 month and had clinical progression consistent with the diagnosis. (2) Disease severity was not assessed by specific scales. However, we can consider that bvFTD and AD patients had equivalent staging, as they had similar disease duration and similar performance on general cognitive measures (e.g., FAB). All bvFTD and AD patients were from mild to moderate stages; none of them were at a severe stage. (3) Although BIS-11 results seem very interesting and promising, they must be seen with caution as they may just reflect caregivers’ awareness of behavioural symptoms suggestive of bvFTD. Anyway, this behavioural scale might be helpful to discriminate these patients, and further studies are warranted to evaluate its diagnostic accuracy at early stages of neurodegenerative diseases.
In conclusion, this study highlights the dissociation between cognitive tests of prefrontal functions and behavioural disorders related to these same regions, the “frontal paradox” (Burgess, Alderman, Volle, Benoit, & Gilbert, Reference Burgess, Alderman, Volle, Benoit and Gilbert2009; Volle et al., Reference Volle, Costello, Coates, McGuire, Towgood, Gilbert, Kinkingnehun, McNeil, Greenwood, Papps, den Broeck and Burgess2011). The present study reinforces this observation as bvFTD patients presented higher scores of impulsive behaviour than AD and controls, while no differences were observed in tasks assessing inhibitory control. There is a need to develop objective cognitive measures of disinhibited behaviour for clinical use. The gap between behaviour and cognition in bvFTD remains a clinical challenge.
ACKNOWLEDGEMENTS
All the authors certify that they have no affiliations with or involvement in any organisation or entity with any financial or non-financial interest or receive any grant in the subject matter or materials discussed in this manuscript. ALT, LCS and PC are supported by Brazilian National Council for Scientific and Technological Development (CNPq – bolsa de produtividade em pesquisa). This study was partially funded by CNPq (402853/2012-1).
CONFLICT OF INTEREST
The authors have nothing to disclose.







