Introduction
Livestock, especially ruminants like sheep, contribute to the accumulation of methane emission in the atmosphere due to methanogenesis by archeametanogen in the rumen. Such emission does not only affect the global warming, but it also represents energy loss from the animals, in which the lost can be between 80 and 140 g/kg from the total of digestible energy. Recently, nutritional strategies aimed to mitigate methane emission based on natural substances are preferred over the synthetic ones (Yogianto et al., Reference Yogianto, Sudarman, Wina and Jayanegara2014). Production of volatile fatty acids in the rumen affects the process of methanogenesis depending on their metabolism and microbial activity. Fermentation of crude fibre in the rumen produces acetate and butyrate, results in increasing the formation of methane. On the other hand, fermentation of soluble carbohydrates produced propionate which is accompanied by a decrease in methane production (Bhatta et al., Reference Bhatta, Saravanan, Baruah and Prasad2014). Assuming 9 kg of ruminal CH4 emission per sheep annually (Mbanzamihigo et al., Reference Mbanzamihigo, Fievez, da Costa Gomez, Piattoni, Carlier and Demeyer2002) with a world population of more than 1172 million sheep in the year 2013 (FAOSTAT, 2015), sheep accounts for considerable CH4 emission from enteric fermentation. Enteric CH4 emission by ruminants also represents a loss of 50–150 g/kg of feed gross energy intakes (Singh et al., Reference Singh, Nagpal and Sainj2005). Currently, there is increasing interest in the use of plants and plant by-products and organic farming to reduce enteric ruminal methane emissions and ammonia production. Secondary metabolites (tannins, antioxidants and flavonoids) in pomegranate (Punica granatum L.) peels (PP) and mango (Mangifera indica) leaves (ML) are produced by plants in their intermediary metabolism. Tannins are polyphenolic and important plant secondary metabolites in PP and ML and have an effect on ruminal methane production when included in the diet in significant proportions. Dietary tannins in livestock feeds have both beneficial and detrimental effects on animal performance and health. The valuable effects may comprise better performance and production. The adverse effects have been related to a reduction in palatability and digestibility of diet and growth inhibition of rumen microbes. The positive or negative effects depend on the source and concentration of tannins in the diet (Jayanegara et al., Reference Jayanegara, Leiber and Kreuzer2012). Pomegranate components have attracted attention for their apparent immunomodulatory activity, antibacterial activity, antiatherosclerotic and antioxidative capacities (Sadq et al., Reference Sadq, Ramzi, Hamasalim and Ahmed2016). ML has various biomedical applications including antioxidative, anti-inflammation, anti-allergic, anti-cancer, hepatoprotective and immunomodulatory activities. It is a rich source of various biologically active compounds (Garrido et al., Reference Garrido, Gonzalez, Lemus, Garcia, Lodeiro, Quintero, Delporte, Nunez-Selles and Delgado2004). To the best authors knowledge, a little reports cleared the effects of PP and ML on gas, methane production and antioxidantresponse of Ossimi lambs was found. Therefore, the main aim of this study was to reduce gas production, methane emission and enhancing the immune response of Ossimi sheep by using tannins in PP, ML and its mixture through an in vitro and in vivo assay.
Materials and methods
Pomegranate peel and mango leaf preparation
Pomegranate peel was obtained manually by separation from fruits, while ML was collected from mango trees, rinsed with distilled water and cut into small pieces. Five hundred grams of fresh ML and PP were dried in air circulatory tray drier at 60°C for 6 h (Singh and Sethi, Reference Singh and Sethi2003) to obtain approximately 900 g/kg dry matter (DM), then ground to pass a 1 mm sieve for further chemical analysis as shown in Table 1.
Table 1. Proximate analysis and total tannins in PP and ML (g/kg on dry matter basis)a

PP, pomegranate peel; ML, mango leaves; NDF, neutral detergent fibre; ADF, acid detergent fibre; ME, metabolizable energy.
a All analyses were carried out in triplicate.
b Calculated according to Mirzaei-Aghsaghali et al. (Reference Mirzaei-Aghsaghali, Maheri-Sis, Mansouri, Razeghi, Mirza-Aghazadeh, Cheraghi and Aghajanzadeh-Golshani2011).
c Calculated according to Cheema et al. (Reference Cheema, Sultan, Javaid, Mustafa and Younas2014).
In vitro experiment
A test diet (substrate) was shown in Table 2. The commercial concentrate mixture was composed of cotton seed cake 350 g/kg, wheat bran 330 g/kg, yellow maize 220 g/kg, rice bran 40 g/kg, molasses 30 g/kg and calcium carbonate 30 g/kg. A total of ten treatments were prepared with different replacement levels of PP and ML. The test diets 1, 2 and 3 were prepared by replacing 20, 40 and 60 g/kg of PP with 20, 40 and 60 g/kg of wheat straw based on weight. Diets 4, 5 and 6 contained ML (20, 40 and 60 g/kg). An equal combination of PP and ML (10 + 10, 20 + 20 and 30 + 30 g/kg) was mixed to constitute test diets 7, 8 and 9, in addition to a control diet with zero PP or ML levels.
Table 2. Ingredients and chemical composition of in vitro experiment diets

PP, pomegranate peel; ML, mango leaves; ME, metabolizable energy; CP, crude protein; CF, crude fibre; NDF, neutral detergent fibre; ADF, acid detergent fibre.
a Concentrate mixture composition: cotton seed cake 350 g/kg, wheat bran 330 g/kg, yellow maize 220 g/kg, rice bran 40 g/kg, molasses 30 g/kg and calcium carbonate 30 g/kg.
b Purchased from Misr Feed Additives for animal nutrition, Egypt. Each 3 kg contain: Vitamin A = 12 000 000 IU, D3 = 2 500 000 IU, E = 15 000 mg, Zinc = 60 000 mg, Manganese = 70 000 mg, Iron = 60 000 mg, Copper = 30 000 mg, Iodine = 5000 mg, Selenium = 300 mg, Cobalt = 1000 mg, Cobalt = 1000 mg.
c Calculated according to feed composition table, NRC for sheep, (1985).
Rumen content was collected individually from ten mature cannulated Ossimi lambs before feeding in the morning. The animals were fed twice a day on the aforementioned concentrate mixture with alfalfa hay and wheat straw. After filtration using four layers of cheese-cloth, the pH-value of rumen fluid was measured immediately using a pH meter (Genway, Model 3520, USA). Five millilitres of rumen fluid was added to 1 ml of protein precipitant, and then centrifuged at 3000 rpm (EBA 21, Hettich, Germany) for 15 min. Short-chain fatty acids (SCFAs) were determined according to Hoeltershinken et al. (Reference Hoeltershinken, Pitt, Tammen, Hoffman and Scholez1997). Concentration of ammonia nitrogen (NH3-N) was determined according to AOAC (1995). For rumen bacteria and protozoa counts, the filtrated rumen liquor was stained with methyl-green formalin saline solution as described by Ogimoto and Imai (Reference Ogimoto and Imai1981), then stoked in a dark place until examination. After gentile mixing of fixed samples, one drop was poured on haemocytometer slide, covered with a cover slip and examined under a light microscope. Samples were used after 72 h of incubation.
The in vitro gas test was performed according to Menke and Steingass (Reference Menke and Steingass1988) to determine the optimum level of PP and ML that minimize CH4 production. Fifty millilitres of filtrated rumen fluid was flushed with CO2, then added (1:2, v/v) to buffered mineral solution and incubated in a shaker water bath at 39°C with a continuous flow of CO2. All laboratory handling of rumen fluid was carried out under a continuous flow of CO2. Approximately 200 mg of test diets (substrate) was placed into calibrated glass syringes of 100 mL capacity. Thirty syringes were divided into ten groups; each group composed of five replicate, three syringes each. Thirty millilitres of rumen liquor–buffer solution was injected into the warmed up syringes and incubated at 39°C. The volume of gas production was recorded from the calibrated scale on the syringe. Readings of total gas output were taken after 3, 6, 12, 24, 48 and 72 h of incubation according to Menke and Steingass (Reference Menke and Steingass1988) recommendation.
CH4 emission was determined at the end of incubation to get the full of methane production in samples by gas chromatography (GC-Agilent 6890) with flame ionization detector according to the method of Soliva and Hess (Reference Soliva, Hess, Makkar and Vercoe2007).
In vivo experiment
Animals and experimental design
Forty Ossimi lambs at 6 months of age with an average weight of 29.25 ± 1.39 kg were obtained from experimental farm of the Faculty of Agriculture, Benha University, Egypt. Animals were divided into four groups (ten lambs each) and housed individually in clean and hygienic pens (0.97 m × 2.82 m). Lambs were subjected to the routine vaccination against infectious diseases and deworming programmes just before the onset of the experiment. The four experimental groups were fed diets formulated to meet the requirements of finishing lambs (Table 3) according to NRC of sheep (1985). The control diet was free of PP or ML; other test diets were supplemented with 60 g/kg of PP (T1), 60 g/kg ML (T2), while the diet T3 contained a mixture of 30 g/kg PP plus 30 g/kg ML. These replacement levels were selected according to the results of the in vitro test. The experimental animals were fed twice daily in equal portions at 09:00 and 17:00 h with an additional allowance of 100 g/kg more than the requirements for the period of 2 months. Feed residues were daily collected and weighed to determine the dry matter intake (DMI). Fresh and clean water was supplied at all times. Body weight changes of individual lambs were recorded at weekly intervals in the morning before feeding and feed conversion ratio (FCR) was calculated for each animal at the end of the experimental period.
Table 3. Ingredients and chemical composition of in vivo experiment diets

T1, T2 and T3: diets containing 6% PP, 6% ML or mix levels (3% PP + 3%ML), respectively; PP, pomegranate peel; ML, mango leaves; ME, metabolizable energy; CP, crude protein; CF, crude fibre; NDF, neutral detergent fibre; ADF, acid detergent fibre.
a Concentrate mixture composition: cotton seed cake 350 g/kg, wheat bran 330 g/kg yellow maize 220 g/kg, rice bran 40 g/kg, molasses30 g/kg and calcium carbonate 30 g/kg.
b Purchased from Misr Feed Additives for animal nutrition, Egypt. Each 3 kg contain: Vitamin A = 12 000 000 IU, D3 = 2 500 000 IU, E = 15 000 mg, Zinc = 60 000 mg, Manganese = 70 000 mg, Iron = 60 000 mg, Copper = 30 000 mg, Iodine = 5000 mg, Selenium = 300 mg, Cobalt = 1000 mg, Cobalt = 1000 mg.
c Calculated according to feed composition table, NRC for sheep, (1985).
Gas production and rumen metabolites
The rumen liquor was collected from three lambs in each group after 4 h of morning feed at the monthly interval by oral-gastric tube. The pH-value of rumen fluid, SCFAs, NH3-N and counts of rumen microbes were measured. Total gas production was estimated after 3, 6, 12, 24, 48 and 72 h incubation periods according to Menke and Steingass (Reference Menke and Steingass1988) recommendation, as previously described in the in vitro experiment.
Digestibility trial
After a preliminary period of 3 weeks, a digestibility trial was carried out for each group to determine the digestion of diet DM and nutrients. A stainless steel wire mesh was placed on the floor of pens to separate the faecal matter from urine, which was passed into the drainage. Feed consumption was recorded daily and faecal matter was collected in morning for successive 7 days and weighed. Faecal samples (100 g/kg) were taken daily and dried out at 60°C for 48 h, ground and stored at −20°C till analysis. The nutrient digestible component (NDC) of diet was calculated for DM, organic matter (OM), crude protein (CP), neutral detergent fibre (NDF), acid detergent fibre (ADF), ether extract (EE) and nitrogen-free extract (NFE) from each dietary treatment using the equation proposed by McDonald et al. (Reference McDonald, Edward, Greenhalgh and Morgan2002).

Blood metabolites
Blood samples from lambs were withdrawn in plain tubes from the jugular vein at two intervals; after 1 and 2 months of the experiment. To obtain serum, blood samples were centrifuged at 3000 rpm (Sigma 3-18K) for 15 min. Serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) were estimated according to Reitman and Frankels (Reference Reitman and Frankel1957). Total protein (TP) and albumin were measured according to Bradford (Reference Bradford1976) and Gustafsson (Reference Gustafsson1976), respectively. Globulin is calculated by subtracting the measured albumin from the measured TP. Serum malondialdehyde (MDA) level was measured according to the method of Karatepe (Reference Karatepe2004). Serum ATP and serotonin (5HT) was assayed by high-performance liquid chromatography (HPLC) (Agilent series 1200, USA) according to the method of Teerlink et al. (Reference Teerlink, Marcel, Joli and John1993) and Arafa et al. (Reference Arafa, Salem and Ahmed-Farid2010), respectively. Serum reduced glutathione (GSH), oxidized glutathione (GSSG) and nitric oxide (NO, as nitrite and nitrate) were measured by HPLC according to Jayatilleke and Shaw (Reference Jayatilleke and Shaw1993). Serum superoxide dismutase (SOD) activity was assayed at 420 nm on a UV-Vis Shimadzu spectrophotometer (2450) measured by Marklund and Marklund (Reference Marklund and Marklund1974). Catalase activity (CAT) was measured by a spectrophotometric method based on the decomposition of H2O2 (Aebi, Reference Aebi1984).
Chemical analyses
Samples of dried PP, ML, experimental diets and faecal matter were analysed according to AOAC (1995). DM was measured using a hot air circulation oven (Heraeus Ut20, Germany) at 105°C for 4 h (method no. 930.15), ash was measured using Burnout furnace Ney Vulcan D-550, USA (method no. 942.05). CP was determined using Kjeltec® system 2100, FOSS-Sweden (method no. 984.13), and EE with Soxtec® system 2045, FOSS-Sweden (method no. 920.39). NDF and ADF were estimated using Fibretherm® FT12 according to the method of Van Soest et al. (Reference Van Soest, Robertson and Lewis1991). OM was calculated from the difference between DM and ash. For determination of total tannins, about 120 mL of ethanol (800 g/kg) was added to 50 g of dried PP or ML powder and heated for 5 min, then cooled, washed and filtered using filter paper. To the filtrate, 100 mL of ethanol was further added and the mixture was heated for 10 min followed by filtration. The liquid filtrate was partially evaporated at a temperature of 50°C for 30 min. Total tannins were determined by HPLC method (Agilent 1200 series, quaternary pump and UV detector) according to Durgawale et al. (Reference Durgawale, Durgawale and Khanwelkar2016).
Statistical analysis
Data were analysed using the Statistical Analysis System (SAS, 2004) Computer Program, using the General Linear Model (GLM) procedure for analysing the data with equal subclass numbers. The statistical model was applied as follows:
In vitro experiment (Model 1)
Where: Yij is the observation of measured traits; μ is the overall mean; Ti is the fixed effect of i th treatment in the diet (i = PP, ML and Mix); Lj is the fixed effect of j th levels of treatment in the diet (j = 20, 40, 60 g/kg); TLij is the fixed effect of interaction between treatment and levels; eij is a random error associated with the individual observation.
In vivo experiment (Model 2)
Where: Yij is the observation of measured traits; μ is the overall mean; Ti is the fixed effect of i th treatment group (i = control, PP, ML and Mix); and eij is a random error associated with the individual observation.
Blood metabolites (Model 3)
Where: Yij is the observation of measured traits; μ is the overall mean; Ti is the fixed effect of i th treatment in the diet (i = PP, ML and Mix); S j is the fixed effect of j th sample month (j = first month and second month); TSij is the fixed effect of interaction between treatment and sample month; eij is a random error associated with the individual observation.
The significant differences between means of treatments were measured according to Duncan (Reference Duncan1955).
Results
Tannins in PP and ML and their proximate composition
Data of proximate analysis and tannins content of PP and ML are summarized in Table 1. PP in general contained less CP and NFE than ML, while the contents of EE, CF, NDF, ADF and ash were higher than that of ML. Overall, total tannins concentration in PP and ML was high, PP contained more tannins than ML.
The in vitro experiment
The mean values of total gas and methane production were presented in Table 4. The lowest CH4 values (P < 0.05) were for test diets contained 60 g/kg of PP, 60 g/kg of ML and the mix diet of 30 g/kg PP plus 30 g/kg ML. With these levels, there was insignificant (P > 0.05) reduction in SCFAs, NH3-N, rumen bacteria and protozoa counts were detected. pH values were not affected by PP or ML dietary replacement.
Table 4. Total gas, methane production after incubation periods and fermentation metabolites of the in vitro experiment

SEM, standard error of mean; PP, pomegranate peel; ML, mango leaf; SEM, standard error of mean; SCFAs, short-chain fatty acids; NH3-N, ammoniacal nitrogen.
All data are the results of a measurement performed in triplicate.
a Short-chain fatty acids.
In vivo experiment
Results in Table 5 show the effect of replacement of PP, ML or their mixture on DMI, body weight gain (BWG), FCR and apparent digestibility of DM, OM and nutrients. Lambs fed on a diet containing 60 g/kg PP (T1) had significantly lower DMI (P < 0.05) than that of the control group while the total BWG was comparable to other groups. FCR mean values of animals fed 60 g/kg PP were better than that of all test groups. There were no significant differences between means of the NDC of diet DM and nutrients between experimental groups except for CP. There was significant (P < 0.05) reduction in CP digestibility in lambs fed 60 g/kg PP comparatively with other groups.
Table 5. Live performance and nutrient digestible component of experimental lambs the in vivo experiment

T1, T2 and T3: diets containing 6% PP, 6% ML or mix levels (3% PP + 3%ML), respectively; SEM, standard error of mean; DMI, dry matter intake; BWG, body weight gain; FCR, feed conversion ratio; NDF, neutral detergent fibre; ADF, acid detergent fibre; NFE, nitrogen-free extract.
Total gas production with the results of rumen parameters is demonstrated in Table 6. Result values of total gas production were significantly lower (P < 0.05) in lambs fed ration contained 60 g/kg PP than that of the control group. Rumen parameters (pH value, SCFAs, bacteria and protozoa count) were not affected by the replacement of PP or ML in the diet. NH3-N values were reduced (P < 0.05) in animals fed PP or ML when compared with the control group.
Table 6. Total gas production and rumen metabolites of lambs fed the experimental diet in vivo experiment

T1, T2 and T3: diets containing 6% PP, 6% ML or mix levels (3% PP + 3%ML), respectively; SEM, standard error of mean; SCFAs, short-chain fatty acids; NH3-N, ammoniacal nitrogen.
a Short-chain fatty acids.
Blood metabolites
Data in Table 7 present the effect of PP, ML and their combinations on lamb's liver function (ALT, AST) and protein production (TP, albumin and globulin) during 2 months experimental period. Inclusion of PP, ML or their combination in the diet for lambs did not show any pathological changes in liver function and protein production that is markedly by normal level of serum ALT, AST, TP, albumin and globulin. Replacement of PP, ML or their combination showed significant (P < 0.05) decrease in MDA, NO and GSSG values and increase GSH, endogenous antioxidant enzymes (CAT, SOD), cell energy (ATP) and neurotransmitter stimulate growth factor (5-HT) in comparison with the control group. Blood sample month (first or second month) had no significant effect on blood metabolites means except for GSH, CAT and ATP level was higher in the second month than the first month of the feeding period. Interaction between treatment and sample month effects was not significant.
Table 7. Effects of PP, ML and MIX on lambs liver function, oxidative stress markers, endogenous antioxidant enzymes, cell energy and neurotransmitters in serum
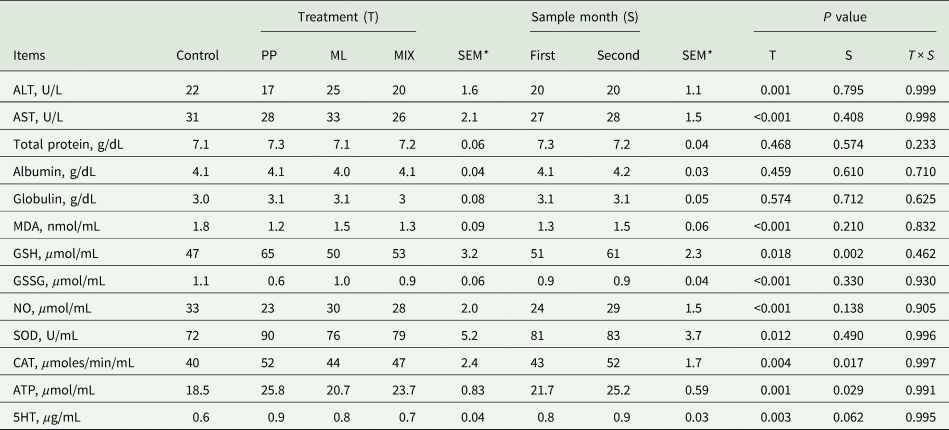
All data are the results of a measurement performed in triplicate.
PP, pomegranate peel; ML, mango leaf; SEM, standard error of mean; ALT, alanine transaminase; AST, aspartate transaminase; MDA, malondialdehyde; GSH, reduced glutathione; GSSG, oxidized glutathione; NO, nitric oxide; SOD, superoxide dismutase; CAT, catalase; ATP, adenosine triphosphate; 5HT, 5 hydroxytryptamine (serotonine).
Discussion
The obtained chemical composition values of PP were in line with previous studies (Mirzaei-Aghsaghali et al., Reference Mirzaei-Aghsaghali, Maheri-Sis, Mansouri, Razeghi, Mirza-Aghazadeh, Cheraghi and Aghajanzadeh-Golshani2011; Shabtay et al., Reference Shabtay, Eitam, Tadmor, Orlov, Meir, Weinberg, Weinberg, Chen, Brosh, Izhaki and Kerem2008). For ML, the nutritive contents were in the range of another study (Cheema et al., Reference Cheema, Sultan, Javaid, Mustafa and Younas2014). Differences in the chemical composition of PP and ML may be attributed to the different geographical distribution of plant species, climate, maturity and growing conditions (Cheema et al., Reference Cheema, Sultan, Javaid, Mustafa and Younas2014). Despite the high fibre and low protein contents in PP and ML, they can provide a good source of nutrients for feeding ruminants. Total tannins in PP and ML were found to be 68.25 and 27.21 mg/g DM, respectively. Tannins were in this study more abundant in PP than ML (27.21 mg/g DM) that was near the level (21.3 g/kg DM) determined by Cheema et al. (Reference Cheema, Sultan, Javaid, Mustafa and Younas2014).
In this study, the in vitro test for methane measurement was conducted to select the optimum level which induces maximum reduction of total gas and CH4 concentration without any detrimental effect on rumen health. With the gradual increase of PP or ML levels, there was a gradual suppression of total gas and CH4 production. From three levels of PP, ML or a combination of them, it was found that the maximum dietary incorporation of 60 g/kg PP or ML and the combined level (30 g/kg PP + 30 g/kg ML) reduced total gas and CH4 emission effectively in comparison with the control (zero PP or ML) or other test levels (20, 40 and 60 g/kg of PP and ML). Results in this study were in the line of previous in vitro study (Bhatta et al., Reference Bhatta, Saravanan, Baruah and Prasad2014) who indicated that total gas and CH4 concentration decreases as the level of tannins and period of incubation increases. Tannins can reduce CH4 emission through a reduction in fibre digestion which decreases H2 production, inhibition of the growth of methanogens (Tavendale et al., Reference Tavendale, Meagher, Pacheco, Walker, Attwood and Sivakumaran2005). The slight decrease in SCFAs concentrations in this study may be attributed to the slight depression in fermentative activity from fibre degradation which could be a sequel of depressed methanogenesis (Jayanegara et al., Reference Jayanegara, Leiber and Kreuzer2012). It was observed that the addition of condensed tannin decreased SCFAs levels in an in vitro fermentation study of Bhatta et al. (Reference Bhatta, Baruah, Saravanan, Suresh and Sampath2013). In all levels of PP or ML, a decrease in NH3-N was observed. This reduction in NH3-N may be attributed to binding of tannins with proteins; forming undegradable tannin–protein complexes by proteases (Makkar, Reference Makkar2003).
With increasing PP or ML levels, the count of bacteria and protozoa was slightly reduced. In this trend, Manasri et al. (Reference Manasri, Wanapat and Navanukraw2012) have proven that plants containing tannins have antimicrobial properties. This inhibitory effect could be attributed to the affinity of tannins to form complexes with the cell wall and membrane of bacteria causing morphological changes of the cell wall and the extracellular enzymes secreted (Jones et al., Reference Jones, McAllister, Cheng and Muir1994). Ruminal pH values varied from 6.4 to 6.9, which were within the optimum ranges (6–7) for maintaining a normal cellulolytic organism. The effects of tannins on ruminal pH were variably reported among studies; either no effect (Dschaak et al., Reference Dschaak, Williams, Holt, Eun, Young and Min2011) or increase (Jami et al., Reference Jami, Nikbachat, Yosef, Miron and Mizrahi2012).
Regarding lambs performance, the total DMI of animals fed 60 g/kg PP (T1) was the lowest followed by T3, T2 and the control group, respectively. Similar results obtained by Archimede et al. (Reference Archimede, Rira, Barde, Labirin, Marie-Magdeleine, Calif, Periacarpin, Fleury, Rochette, Morgavi and Doreau2016) who indicated that DMI was reduced with tannin-rich plants in the diet. This finding agrees with a previous study (Ngwa et al., Reference Ngwa, Nsahlai and Bonsi2003) who observed that tannins usually decreased palatability and preference of plants by cattle, sheep and goats. The reduction in palatability was referred to: firstly, a reaction between tannins and salivary mucoproteins, or through a direct negative impact on the taste receptors, annoying an astringent sensation (Frutos et al., Reference Frutos, Raso, Hervás, Mantecón, Pérez and Giráldez2004). Secondly, impaired digestion of DM in the rumen prolongs the emptying of the digestive tract, providing signals to the nerve centres. The third mechanism is based on the identification of negative post-prandial actions mediated by microbial fermentation following the ingestion of tannins (Waghorn, Reference Waghorn1996). There are some exceptions to tannin effect on DMI, as in some cases, there was an increase (Puchala et al., Reference Puchala, Min, Goetsch and Ahlu2005) or insignificant reduction in voluntary feed intake in sheep (McSweeney et al., Reference McSweeney, Kennedy and John1988). Variations in DMI due to the presence of tannins in the diet could be elucidated through its concentration in the diet; low tannin content exhibits a positive effect on DMI (Aerts et al., Reference Aerts, Barry and McNabb1999), while higher levels in the diet reduced dramatically DMI (Frutos et al., Reference Frutos, Raso, Hervás, Mantecón, Pérez and Giráldez2004).
Although the final BWG of all animals in this study showed no differences between treatments, there was insignificant (P > 0.05) improvement of FCR for T1 (60 g/kg PP) and T3 (30 g/kg PP + 30 g/kg ML). These beneficial FCR values were due to lower DMI and efficient utilization of nutrients for groups T1 and T3 compared to other treatments. In this line, the final body weight did not have any change when lambs fed PP at levels (20, 40 and 60 g/kg PP). DM and nutrients retention in all treatments showed no change relative to the control group. Only CP digestibility was reduced (P < 0.05) for lambs fed 60 g/kg PP (T1) comparatively with other treatment groups. This finding was attributed to the formation of tannin–protein complex which reduces plant protein degradation to NH3 in the rumen. Low pH-value in abomasum promotes disassociation of tannin–protein complexes and enhances the proteins flow for digestion in the small intestine (Frutos et al., Reference Frutos, Raso, Hervás, Mantecón, Pérez and Giráldez2004). However, the reduction in apparent digestibility of CP in this study indicates that the tannin–protein complexes did not undergo complete disassociation in the abomasum, thereby lowering the digestion of CP in the total tract. Similar results observed by Beauchemin et al. (Reference Beauchemin, McGinn, Martinez and McAllister2007) who concluded that tannin extract had no effect on DM, energy or fibre (ADF and NDF) digestibility, but apparent digestibility of CP decreased linearly. Also Archimede et al. (Reference Archimede, Rira, Barde, Labirin, Marie-Magdeleine, Calif, Periacarpin, Fleury, Rochette, Morgavi and Doreau2016) revealed that using tannin-rich plants in sheep diet did not affect apparent OM digestibility. In the study of McNabb et al. (Reference McNabb, Waghorn, Peters and Barry1996), up to 45 g/kg condensed tannins in the diet improved production efficiency in ruminants by binding and protection of dietary proteins from microbial degradation in the rumen and consequently increasing the flow of essential amino acids and non-ammonia nitrogen for intestinal absorption. Therefore, improvement of FCR with less DMI in this study could be attributed to the increment of intestinal supply of metabolizable proteins (Waghorn, Reference Waghorn1996), carbohydrates (Makkar et al., Reference Makkar, Blümmel and Becker1995) and fats (Patra and Saxena, Reference Patra and Saxena2011) for maximum absorption. With 60 g/kg PP, ML or their mix (30 g/kg PP + 30 g/kg ML), there was no adverse effect either on DM or nutrients utilization by lambs. This favourable effect might be the result of added valuable nutrients in PP and ML as a feed ingredient in the diet. In this line, findings of Jami et al. (Reference Jami, Nikbachat, Yosef, Miron and Mizrahi2012) proved that tannins in PP had no large negative effects on digestibility of nutrients which was reflected on improved performance of dairy cows. Shabtay et al. (Reference Shabtay, Eitam, Tadmor, Orlov, Meir, Weinberg, Weinberg, Chen, Brosh, Izhaki and Kerem2008) found that the tannin-rich PP intake up to 200 g/kg of the total DM intake does not possess deleterious or positive effects on fattening ration intake of feedlot calves.
Pomegranate peel or ML replacement in the diet reduced CH4 and total gas production linearly in comparison with the control group similarly as described in the in vitro experiment. Due to the binding of tannins to dietary protein, and also to a reduction in the activity of a large proportion of microflora in the rumen as well as binding to carbohydrates, a reduction in total gas emission is resulted (Sadq et al., Reference Sadq, Ramzi, Hamasalim and Ahmed2016). PP replacement in the diet of sheep at 60 g/kg induced maximum reduction of CH4 emissions (166.2 g/kg) compared with the control. Similar results were obtained by Hess et al. (Reference Hess, Tiemann, Noto, Carulla and Kreuzer2006) in sheep fed diet with added tannins where CH4 emissions were reduced by 130 g/kg without major losses in feeding value of the diet. Animut et al. (Reference Animut, Puchala, Goetsch, Patra, Sahlu, Varel and Wells2008) reported that the goat's diet containing tannins decreased CH4 emissions effectively. Most feasible conditions responsible for reduced CH4 production are changes in the number and/or activity of bacteria other than ones highly fibrolytic, including methanogens (Field et al., Reference Field, Kortekaas and Lettinga1989) and protozoa (Schönhusen et al., Reference Schönhusen, Zitnan, Kuhla, Jentsch, Derno and Voigt2003). Tannins reduce CH4 emission through several ways; (1) reduction in fibre digestion which decreases H2 production by prevention or at least interfering with the attachment of rumen microorganisms to plant cell walls for further degradation (McAllister et al., Reference McAllister, Bae, Jones and Cheng1994). (2) Direct inhibitory effect of methanogens and declined digestibility of proteins and carbohydrates (Jayanegara et al., Reference Jayanegara, Leiber and Kreuzer2012), and (3) reduction of protozoa population where part of the methanogens are associated symbiotically on the surface or inside the micro-fauna (Vogels et al., Reference Vogels, Hoppe and Stumm1980). Also TVFAs level was reduced (P < 0.05) by PP or ML supply in the diet, while no change in SCFAs concentration between experimental groups. This reduction in TVFAs might be due to lowered fibre degradation in the rumen by adhering of tannins to plant cell wall hindering microbial attack. According to Bhatta et al. (Reference Bhatta, Saravanan, Baruah and Prasad2014), tannins in PP or ML exhibited effectively CH4 reduction but did not cause a real inhibition of the TVFAs concentration that would indicate that CH4 suppression was primarily due to antimethanogenic activity rather than lowered fibre digestibility. In this trend, no significant reduction in SCFAs concentration in this study was observed. The authors indicated that feeding tannin sometimes results in a decrease in ammonia concentration, enhancing the efficient use of TVFAs for microbial protein synthesis. In contrast, the results of other studies indicated no difference in TVFAs level between groups (Min et al., Reference Min, Barry, McNabb and Kemp1998; Puchala et al., Reference Puchala, Min, Goetsch and Ahlu2005). As a consequence of reduced CP digestibility, NH3-N was reduced (P < 0.05) in all treatment groups compared with control. Min et al. (Reference Min, Barry, McNabb and Kemp1998) noted that ewes fed forage containing condensed tannin had lower ruminal ammonia than ewes grazing tannin-free forage. The undegradable tannin–protein complex by microflora decreases the level of ruminal NH3-N (Makkar, Reference Makkar2003). Tannins can bind to proteins and carbohydrates, leading also to a reduction in ruminal gas production and methane emission (Makkar et al., Reference Makkar, Blümmel and Becker1995). Due to a combination of these activities, tannins can be associated with improvements in animal growth and productivity and consequentially minimization of effects on the environment (Silanikova et al., Reference Silanikove, Tagari and Shkolnik1993).
Dietary supply of PP or ML did not affect the counts of bacteria or protozoa as well as ruminal pH-value in this study. This result is supported by the meta-analysis study (Jayanegara et al., Reference Puchala, Min, Goetsch and Ahlu2012) in which tannins did not significantly reduce log protozoa population in both in vitro and in vivo experiments. Pilajun and Wanapat (Reference Pilajun and Wanapat2013) also reported that diet supplementation with tannins-rich plants did not affect the microbial population in swamp buffalo. In other studies, plants containing tannins and saponins had antimicrobial properties (Manasri et al., Reference Manasri, Wanapat and Navanukraw2012). Variations in results of this work with those obtained by other studies could be referred to the different sources of diets, dose of tannins, animal type, feeding programme and environmental conditions (Anantasook et al., Reference Anantasook, Wanapat, Cherdthong and Gunun2013).
Data did not show any pathological response with PP, ML and their combined replacement compared with the control group. ML was found to contain various biomedical substances of antioxidative, anti-inflammation, anti-allergic, anti-cancer, hepatoprotective and immunomodulatory activities (Garrido et al., Reference Garrido, Gonzalez, Lemus, Garcia, Lodeiro, Quintero, Delporte, Nunez-Selles and Delgado2004). Also the presence of photochemical constituents especially flavonoids and tannins in PP set animal at normal homeostasis markedly by normal liver function (ALT, AST, TP, albumin and globulin) and normal cell structure markedly by normal level of MDA, NO, GSSG and GSH. These results are in agreement with those obtained by Abdel-Moneim et al. (Reference Abdel-Moneim, Dkhil and Al-Quraishy2011) who found that PP and ML did not show any pathological alteration in liver and kidney functions. Recent studies showed the beneficial effects of ML on the liver through mitigating serum enzymes and the decreasing liver ALT and AST (Ojekale et al., Reference Ojekale, Ojiako, Saibu, Lala and Olodude2007).
Data of SOD and CAT revealed improvement in the endogenous defence mechanism in animal fed diet supplemented with PP or ML owing to their antioxidant activities. Similarly, studies of Parmar and Kar (Reference Parmar and Kar2008) demonstrated that PP decreased lipid peroxidation in cardiac, hepatic and renal tissues. ML extract contains many polyphenols and mangiferin which is one of the biologically active cytoprotective compounds prevents mitochondrial stress and iron-induced oxidative damage in the liver (Kawpoomhae et al., Reference Kawpoomhae, Sukma, Ngawhirunpat, Opanasopit and Sripattanaporn2010). In this study, a considerable increase in the ATP level in lambs fed diets T1, T2 and T3. This indicates that tannins are able to protect the mitochondria from the damaging effects by scavenging the reactive oxygen species. These findings were similar to those obtained by Gulcin et al. (Reference Gulcin, Huyut, Elmastas and Aboul-Enein2010) who reported that tannic acid is the effective natural antioxidant component for food preservation.
Serotonin (5-HT) is a tryptamine, monoamine neurotransmitter synthesized in serotonergic neurons and in the gastrointestinal tract. According to the fundamental activity of 5-HT, its influence in the secretion of pituitary growth hormone is by inhibiting the production of hypothalamic somatostatin (Lesch, Reference Lesch2001). Phytochemical constituent of PP and ML showed an increased level of 5-HT that may be due to the synergetic effect of flavonoid mixture in the plant. GSH, CAT and ATP level was higher in the second month of feeding period than the first month that may be due to increasing of metabolism activity.
Conclusion
Replacement of 60 g/kg PP, 60 g/kg ML or their mix (30 g/kg PP + 30 g/kg ML) in sheep diet reduced total gas emission. Also, it enhanced the growth performance, digestibility and rumen functions, as well as it decreased the oxidative stress markers, and improvement in the antioxidant defence mechanisms of fattening Ossimi lambs.
Acknowledgements
The authors are grateful to Professor Fawzy Abo-Donia, Professor of Animal Nutrition, and Dr Mahmoud El-Attrouny for their assistance in carrying out the experiment and the statistical analysis. Thanks are also due to the central laboratory of Animal Production Department and Agricultural Research Centre, Sheep Farm, Faculty of Agriculture at Moshtohor, Benha University, Egypt.
Financial support
This work was supported by the central laboratory of Animal Production Department and Agricultural Research Centre, Sheep Farm, Faculty of Agriculture at Moshtohor, Benha University, Egypt.
Conflict of interest
The authors declare no conflict of interests.
Ethical standards
Humane animal care and handling procedures were conducted in accordance with the Animal Care Committee of the Department of Animal Production, Faculty of Agriculture at Moshtohor, Benha University and with the instructions from the Ministry of Agriculture in Egypt.










