Introduction
Viable seeds stored in soil – soil seed banks – are important features of plant communities that can influence plant recruitment and dynamics (Warr et al., Reference Warr, Thompson and Kent1993). A key principle regarding forest communities is that seed banks typically contain few seeds of species inhabiting mature forests (Warr et al., Reference Warr, Kent and Thompson1994; Bossuyt and Hermy, Reference Bossuyt and Hermy2001). Rather, forest seed banks are normally dominated by early successional species, often short lived (e.g. annual) and ‘ruderal’ (weedy) species with abilities to colonize disturbed areas rapidly (Grime, Reference Grime2001). When disturbance reduces the tree overstorey, these species recruit, produce seed to replenish seed banks, and then often become sparse or absent as forest cover re-establishes (Roberts and Vankat, Reference Roberts and Vankat1991). On the other hand, species of the mature forest often do not rely on soil seed banks. Instead, these species are usually long lived and regenerate via short-lived seed, rendering persistent seed banks of minimal importance to their population dynamics (Chambers et al., Reference Chambers, Vander Wall and Schupp1999). As a result, seed banks of mature forests are generally dominated by species other than those of the extant mature vegetation (Halpern et al., Reference Halpern, Evans and Nielson1999; Bossuyt and Hermy, Reference Bossuyt and Hermy2001).
There are exceptions, however, to the principle that forest seed banks do not contain species of mature communities. For example, Leckie et al. (Reference Leckie, Vellend, Bell, Waterway and Lechowicz2000) found that 76% of taxa in the seed bank occurred in vegetation of an old (>400 years), temperate deciduous forest in Quebec, Canada. Similarly, high congruence between seed bank and vegetation taxa was reported for some European forests (Brown and Oosterhuis, Reference Brown and Oosterhuis1981; Jankowska-Blaszczuk and Grubb, Reference Jankowska-Blaszczuk and Grubb1997; Olano et al., Reference Olano, Caballero, Laskurain, Loidi and Escudero2002; Wódkiewicz and Kwiatkowska-Falińska, Reference Wódkiewicz and Kwiatkowska-Falińska2010). Additional research might help illuminate how common this exception is and what types of forests do contain seed banks that harbour species of mature vegetation.
Another potentially important, but poorly understood, aspect of soil seed banks is spatial variation within landscapes. Some studies suggest that seed-bank density and species composition can vary substantially among sites within landscapes (Thompson, Reference Thompson1978; Ortega et al., Reference Ortega, Levassor and Peco1997; Ashton et al., Reference Ashton, Harris and Thadani1998). This variation might be linked to the presence of different forest community types and correlated with variation in environment (e.g. elevation and soil texture) and vegetation variables (e.g. tree canopy cover) (Thompson, Reference Thompson1978; Ortega et al., Reference Ortega, Levassor and Peco1997; Cummins and Miller, Reference Cummins and Miller2002). Topographic gradients could affect seed banks by influencing the vegetation that provides inputs to seed banks and by affecting seed retention through soil deposition, erosion, decomposition or wind (Ashton et al., Reference Ashton, Harris and Thadani1998). Soil texture can influence seed bank formation by affecting vegetation and trapping different-sized seeds (Chambers, Reference Chambers1995; Benvenuti, Reference Benvenuti2007). While past vegetation influences seed-bank formation by providing seeds that persist in the soil, current vegetation also provides inputs and influences seed banks through other mechanisms. In forests, tree canopy cover can affect seed banks through influences on understorey vegetation and other properties (Abella et al., Reference Abella, Springer and Covington2007; Allen et al., Reference Allen, Chambers and Nowak2008).
There are many practical purposes to understanding soil seed banks for environmental management, including evaluating plant recruitment potential and exotic species abundance (Bakker et al., Reference Bakker, Poschlod, Strykstra, Bekker and Thompson1996). Coniferous forests of western North America, for example, are under active ecological restoration, which benefits from knowledge of seed banks. Beginning in the late 1800s and early 1900s, Euro-American settlement brought new land-use practices to the region, including intentional fire suppression (Covington and Moore, Reference Covington and Moore1994). During this time period and through the 1900s, tree density and fuel loads sharply increased, concomitant with the size and severity of wildfires. Many western coniferous forests, such as those containing Pinus ponderosa and dry mixed conifers, formerly sustained frequent, low-severity fire and not the extensive crown fires occurring today (Covington and Moore, Reference Covington and Moore1994). Consequently, mechanical thinning of trees is a major ecological restoration treatment currently being performed for re-balancing ecosystem biomass to reduce canopy fuels and promote understorey vegetation (Graham et al., Reference Graham, Jain, Matthews, Elliot, Miller and Audin2010). Understanding soil seed banks could facilitate assessing recruitment potential of plant species, including those of mature forest communities and exotic species, following forest management. The objective of this study was to determine soil seed-bank composition on a western North American coniferous forest landscape and relationships to forest community types, vegetation cover and environmental variables.
Materials and methods
Study area
We collected samples within a 40,000-ha area on the east side of the Spring Mountains, within Spring Mountains National Recreation Area of the Humboldt-Toiyabe National Forest in southern Nevada, USA (Fig. 1). The 5500-km2 Spring Mountains rise to 3632 m above sea level (Charleston Peak) over surrounding desert shrubland 750 m in elevation in the northern Mojave Desert (Niles and Leary, Reference Niles and Leary2007). Along an elevational gradient from low to high, predominant forest types include pinyon–juniper (Pinus monophylla–Juniperus osteosperma), pinyon, ponderosa pine (Pinus ponderosa), mixed conifer (several conifers such as P. ponderosa, Abies concolor and Pinus flexilis) and bristlecone pine (Pinus longaeva). The climate in 2009–2010 at a pinyon site (Lovell Summit, 2010 m elevation) included the following averages: 51 cm yr− 1 of precipitation, − 3°C January daily low temperature and 31°C July daily high temperature (NWCC, 2011). At a higher elevation of 2738 m at a mixed conifer site (Bristlecone Trail), the following averages were reported from an available record in 2009–2010: 70 cm yr− 1 of precipitation, January daily low temperature of − 5°C and July daily high temperature of 23°C. Most precipitation falls in non-summer months, with summer storms from May through August delivering a 2009–2010 average of 9% of total annual precipitation at the pinyon site and 11% at the mixed conifer site. Topography consists of hillslopes dissected by drainages, large canyons between major slopes, and ridges and other convex landforms. Soils are derived from limestone, dolomite and sandstone and include Mollisols, Inceptisols, Aridisols and Alfisols (Lato, Reference Lato2006). Selective harvest of some timber (primarily P. ponderosa) and fuel for charcoal kilns (primarily of pinyon–juniper forest) was concentrated from the mid-1800s to early 1900s, but less harvest has occurred since. Some localized livestock grazing also occurred but is not presently authorized. Major large native herbivores inhabiting the area include mule deer (Odocoileus hemionus) and bighorn sheep (Ovis canadensis). Based on tree coring at study sites, the oldest trees were a minimum of 150 years old and ages of some trees exceeded 400 years (Abella et al., Reference Abella, Hurja, Merkler, Denton and Brewer2012). At the highest elevations, P. longaeva trees of the bristlecone forest type are among the oldest individual plants on Earth, with life spans of several thousand years (Thorne et al., Reference Thorne, Schoenherr, Clements, Young, Barbour, Keeler-Wolf and Schoenherr2007). Thus, our sample sites are old, mature forests typical of this landscape.

Figure 1 Location of study sites classified to forest type in the Spring Mountains National Recreation Area (SMNRA) of southern Nevada, USA. Photos illustrate the range of forest types sampled: (a) pinyon–juniper site at an elevation of 2470 m, (b) mixed conifer at 2826 m, and (c) bristlecone pine at 3048 m.
Sample and data collection
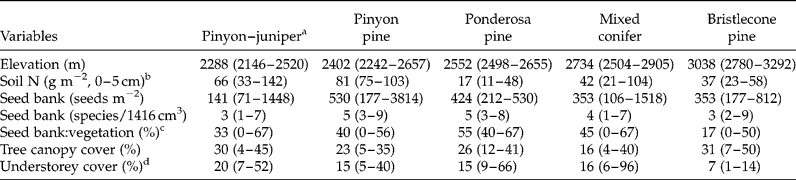
As a framework for data collection, we used a network of Terrestrial Ecological Unit Inventory (TEUI) sites established by the US Forest Service to characterize variability in environmental gradients and vegetation across the landscape (Winthers et al., Reference Winthers, Fallon, Haglund, DeMeo, Nowacki, Tart, Ferwerda, Robertson, Gallegos, Rorick, Cleland and Robbie2005). These sites were established in the centres of mapping units defined by soil scientists on the basis of similarity in climate, soil parent material and vegetation. We collected samples from 36 of 42 TEUI sites (four were not visited due to logistics and two were burned by wildfire) in pinyon–juniper or higher forests on the east side of the mountain range, for which complete soil profile descriptions and vegetation inventories were available. With much of the study area roadless and in designated wilderness (prohibiting mechanized equipment), human disturbance at the 36 sites over the past 50–100 years is not extensive. Sample sites encompassed broad environmental and vegetation gradients across the landscape, ranging in elevation from 2146 to 3292 m, forest type from pinyon–juniper to bristlecone pine, and slope gradient from 8 to 64% (Table 1).
Table 1 Characteristics of coniferous forest types of the Spring Mountains, USA
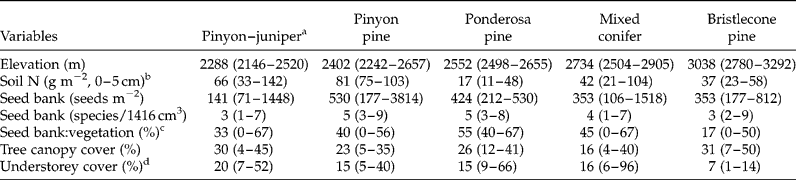
Values are medians (min–max).
a Number of sites, in order of forest type, were 13, 5, 4, 10 and 4.
b Texture of the 0–5 cm soil layer was sandy loam for all forest types except for pinyon pine forest, which was loam.
c Percentage of species in the seed bank that were also detected in vegetation on one or more of the 36 sites.
d Represents the understorey layer of vascular plants (woody and herbaceous) < 5 m in height. The highest cover values are associated with sites containing an abundance of tree seedlings.
We collected seed-bank samples, soil samples and other environmental data at each site from 3 May to 29 May 2009. This was early spring, during or shortly after snowmelt, and thus seed-bank collections represent accumulated seeds and seeds from the previous growing season that persisted through the winter and would be available for germination. We chose this sampling time to occur before current-year seed dispersal (Baskin and Baskin, Reference Baskin and Baskin1998), which can be both in early summer for early flowering species and in late summer post-monsoon (Niles and Leary, Reference Niles and Leary2007). One TEUI plot, 20 m × 20 m (0.04 ha) in size, had been established at each site. We collected seed-bank samples at 15 locations within each plot, with one sample collected every 4 m along the bottom and top plot boundaries and the centreline parallel to these boundaries. We collected samples of the 0–5 cm soil layer, which included mineral soil and could include the duff layer (Oe+a horizon), if present, but not litter (Oi horizon), which we brushed away if present. Samples of 200 cm3 each were collected using a cylinder 7 cm in diameter and were thoroughly mixed to result in a total of 3000 cm3 per plot of seed-bank soil. We collected 0–5 cm mineral soil samples for laboratory analysis using the same methods, and we collected 0–5 cm mineral soil for bulk density analysis from four cores, with one collected from each plot corner.
At the centre of each plot, we recorded location [in Universal Transverse Mercator (UTM) m, North American Datum 1983] and elevation using a Global Positioning System, aspect [linearized to range from 0–2 following Beers et al. (Reference Beers, Dress and Wensel1966)] using a compass, and slope gradient and landform index using a clinometer. Landform index quantifies topographic protection, where low values indicate exposed convex landforms and high values indicate high protection, and was based on the average of eight measurements every 45° to the horizons of surrounding landforms (McNab, Reference McNab1993). From the location, aspect and slope gradient data, we calculated potential direct incident radiation and heat load, following McCune (Reference McCune2007).
Soil samples were air dried, sieved through a 2-mm sieve and analysed for texture (hydrometer method) following Tan (Reference Tan2005); pH and electrical conductivity (1:1 soil:water); available P (Olsen sodium bicarbonate extraction); CaCO3 (manometer method); total C, N and S (dry combustion, CNS analyser); organic C (difference between total and inorganic C); NO3, SO4 and Cl (ion chromatography); and the water-soluble concentrations of Na, K, Mg, Ca, Mn, Fe, Ni, Cu, Zn, Co, B, Mo, Pb and Cd (1:3 soil:water extracts, inductively coupled plasma mass spectroscopy) following Burt (Reference Burt2004). Bulk density was estimated for the bulk density samples by sieving through a 2-mm sieve, oven drying the < 2-mm fraction at 105°C for 24 h, and including volume of coarse fragments >2 mm in the total soil volume. Bulk density was used to convert nutrient concentrations to volumetric contents (Burt, Reference Burt2004).
We obtained soil characterization and vegetation data sets for each TEUI plot from the US Forest Service (Humboldt-Toiyabe National Forest, Las Vegas, Nevada, USA). Based on a soil pit described by soil scientists using standard procedures (Soil Survey Division Staff, 1993), we obtained data on A- and B-horizon thickness, pH and texture; and on depth to a root-restricting layer (e.g. bedrock or an indurated horizon). We obtained data on areal cover of each understorey vascular plant species rooted in each plot and canopy cover of overstorey trees (>5 m in height) in total and by species (Abella et al., Reference Abella, Hurja, Merkler, Denton and Brewer2012). Vegetation data were collected in summer 2004 to 2006 and thus represent vegetation before seed-bank sampling.
Seed-bank processing
Seed-bank samples were processed within a week of finishing sample collection by placing eight subsamples each of 177 cm3 (translating to a 2-cm thick layer) of seed-bank soil per site in separate 4-litre cylindrical pots. Pots were filled two-thirds full with sterile potting soil (United Industries Co., St. Louis, Missouri, USA), and the seed-bank soil was placed on top of the potting soil. Pots were randomly arranged on a bench in a greenhouse and were kept moist by daily watering. The start of the emergence period (June) corresponded to the natural photoperiod for germination, so no supplemental lighting was provided in the greenhouse. Seedlings were counted and pulled every 2 weeks when identified to the finest taxonomic level possible using local flora descriptions (Niles and Leary, Reference Niles and Leary2007) as soon as plant parts required for accurate identification appeared. Of 488 total seedlings emerging, 427 (88%) were identified to genus or species, with the remainder identified only to growth form (forb, graminoid or shrub). A forb comprised 53 of 61 (87%) of the unidentified seedlings, with the remainder being two unknown seedlings of a fern and six of a graminoid. Unidentified seedlings were included in total seed densities but not in species richness or compositional data. Pots containing only potting soil were established to check for greenhouse seed contamination, which was not detected. Nomenclature, growth form and native/exotic status follow the National Resources Conservation Service database (NRCS, 2011).
Data analysis
We converted seedling counts from the eight subsamples per site into a single seeds m− 2 (0–5 cm depth) estimate per site. Species richness, based on the cumulative number of species in the 1416 cm3 volume of the eight subsamples/site, was calculated for each site. Sample volume was identical across all sites to be compared, so we report richness as species/volume, as is customary for seed-bank studies and analogous to species/area in vegetation studies (Gotelli and Colwell, Reference Gotelli and Colwell2001). We compared median seed density and species richness among forest types using Kruskal–Wallis tests (SAS Institute, 2009). We further computed the proportion of seed bank species at each plot that were detected in vegetation of at least one plot.
We constructed a matrix of seed-bank measures (density, richness and proportion of species in vegetation), together with the 2 location (UTM x, y), 6 topographic (elevation, transformed aspect, slope gradient, landform index, potential direct incident radiation and heat load), 40 soil (our 0–5 cm soil lab measures including bulk density and coarse fragment content, and the TEUI A and B horizon thickness, pH, % sand, silt, and clay, and depth to root restriction) and 8 tree variables (total tree canopy cover and canopy cover of seven individual tree species). To derive a smaller number of composite variables portraying variability within the environmental data, we performed a principal components analysis (cross-products matrix calculated from correlation) on the location, topographic and soil variables using PC-ORD (McCune and Mefford, Reference McCune and Mefford1999). We then calculated Spearman correlation coefficients between all seed bank, environmental (including the first three principal components) and vegetation variables (SAS Institute, 2009).
To portray species composition, we calculated species × site matrices of relative seed density (speciesi /sum all species) on a site basis and relative understorey plant cover. The seed bank matrix was weakly structured and a significant non-metric multidimensional scaling ordination was not derived (P>0.40 all three axes, PC-ORD's slow and thorough mode). Thus, we used Bray–Curtis ordination (Sørensen distance, variance–regression endpoint selection, and city-block axis projection geometry and residual distances) for both the seed bank and vegetation matrices in PC-ORD (McCune and Mefford, Reference McCune and Mefford1999). We used joint plots to identify relationships of the species and environmental variables to gradients in community composition.
Results
Seed-bank characteristics
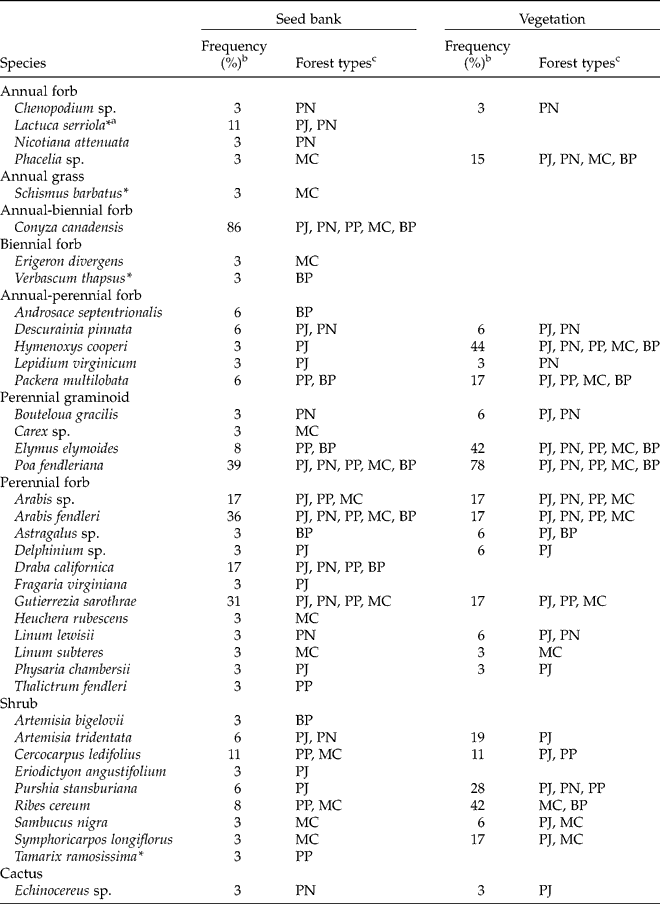
Native perennial taxa dominated the seed bank. A total of 39 taxa emerged, consisting of 5 (13%) annual, 1 (3%) annual-biennial, 2 (5%) biennial, 5 (13%) annual-perennial, and 26 (67%) perennial taxa (Table 2). Twenty-four (62%) of the 39 taxa were forbs, 5 (13%) were graminoids, 9 (23%) were shrubs and 1 (3%) was a cactus. The most frequent species, detected at >10% of sites, were Conyza canadensis, Poa fendleriana, Arabis fendleri, Gutierrezia sarothrae, Draba californica, Arabis spp., Cercocarpus ledifolius and Lactuca serriola. Only four exotic species emerged, with only one (L. serriola, detected at four sites) emerging from more than one site.
Table 2 Species composition of the soil seed bank and relationship to vegetation in coniferous forests of the Spring Mountains, USA
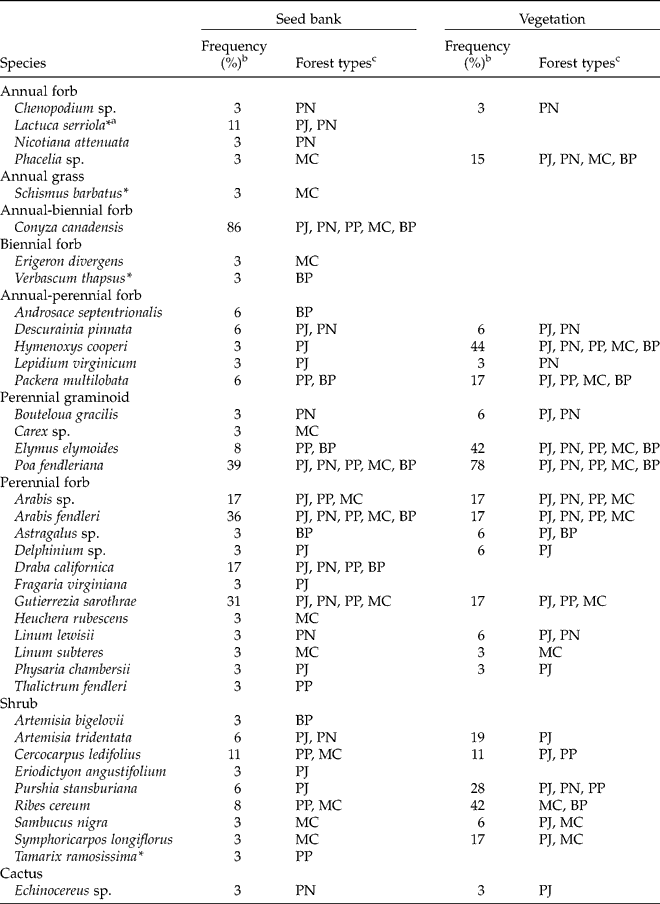
a Asterisks denote exotic species.
b Out of 36 sites.
c Forest types in which a species was detected in the seed bank of at least one site or in the vegetation of at least one site. From low to high elevation: PJ, pinyon–juniper; PN, pinyon pine; PP, ponderosa pine; MC, mixed conifer; and BP, bristlecone pine.
Seed density among sites ranged from 71 to 3814 seeds m− 2, with 1518 seeds m− 2 being the second most dense site, and richness from 1 to 9. Across all sites, seed density averaged 479 (SD = 662) and species richness 4.3 species per site (SD = 2.1). Half of the sites exhibited densities in the range of 106–282 seeds m− 2.
Environment and vegetation relationships
The first three principal components portrayed 42% of the variation in the environmental data set, and many different variables exhibited high loadings on components. For example, coarse fragment content and sand had the largest positive loadings and a suite of soil nutrients (e.g. S, N and organic C) the largest negative loadings on component 1 (21% of variance). Component 2 (12% of variance) exhibited large positive loadings of topographic variables (e.g. landform index and slope gradient) and large negative loadings of several soil variables such as CaCO3.
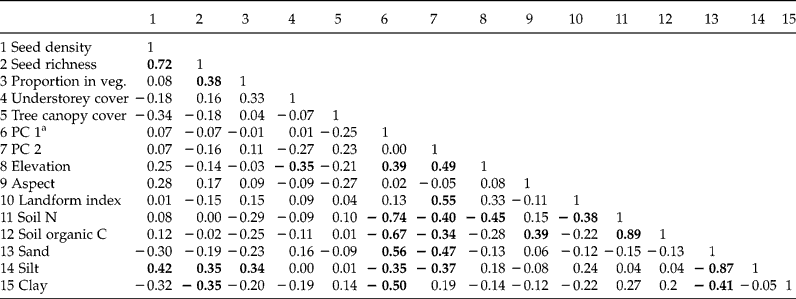
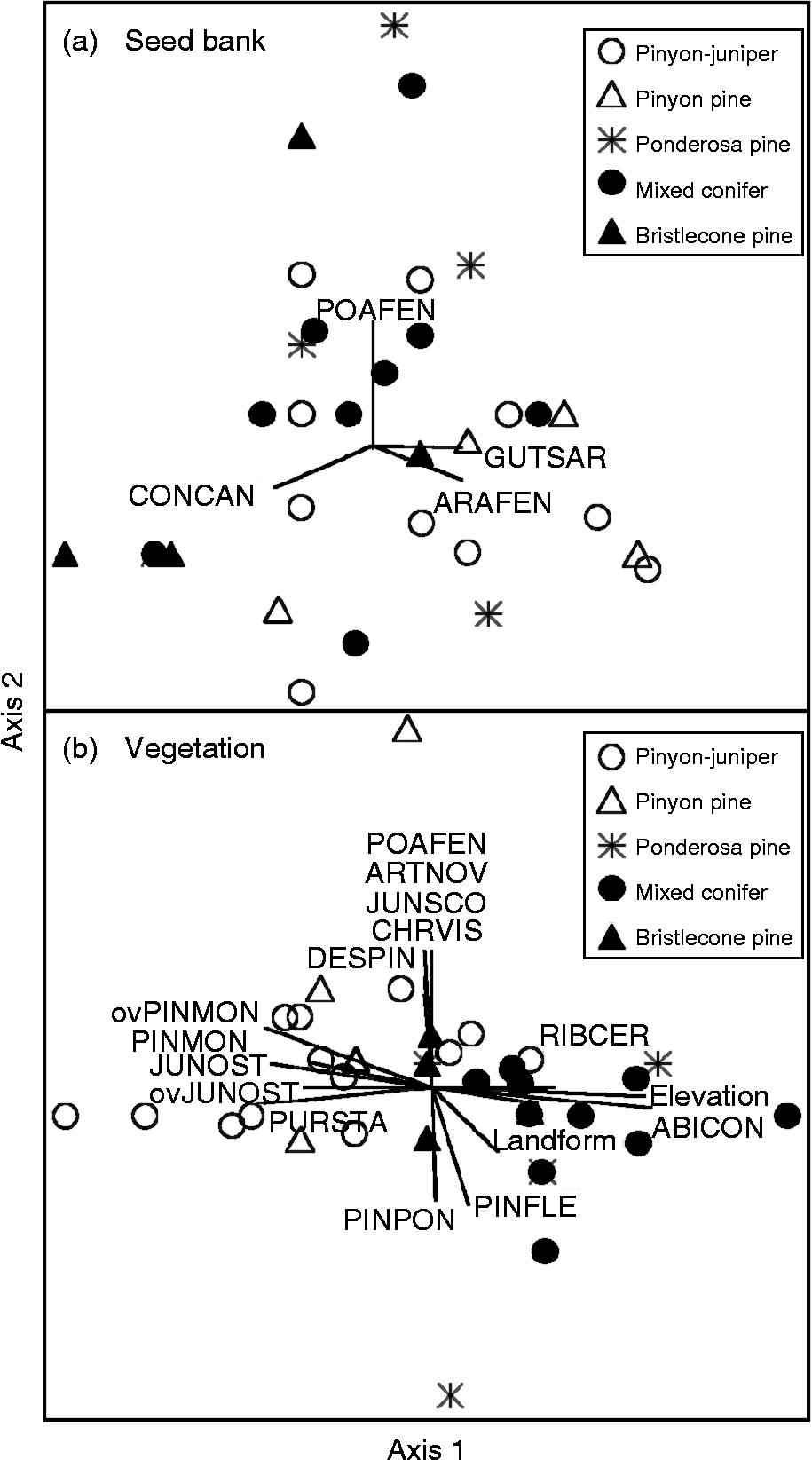
Relationships of seed-bank measures with environmental (including principal components) and vegetation variables were not strong. Considering seed-bank density and richness and the proportion of seed-bank species in the vegetation, no Spearman rho of these measures with any environmental or vegetation variable exceeded |0.42|. Soil silt was the environmental variable most strongly correlated with seed-bank measures (Table 3). Similarly, median seed-bank density (Kruskal–Wallis χ2 statistic = 5.61; P = 0.231, df = 4) and species richness (χ2 = 3.68; P = 0.451) did not differ among forest types. Seed density ranges overlapped and medians spanned 141–530 seeds m− 2 (Table 1). A pinyon forest site contained the highest seed-bank density (3814 seeds m− 2) among sites, but this forest type also contained sites exhibiting among the lowest seed densities. The ordination further suggested that seed-bank species composition showed little tendency to group according to forest type, and no environmental variables exhibited an r 2 value greater than the 0.25 cutoff with either ordination axis (Fig. 2a). This differed from vegetation species composition aboveground, which grouped by low- and high-elevation forest types (Fig. 2b).
Table 3 Spearman correlation coefficients (rho) for seed-bank measures and representative environmental and vegetation variables of coniferous forests of the Spring Mountains, USA. Bold denotes coefficients significant at P<0.05

a PC, Principal component.
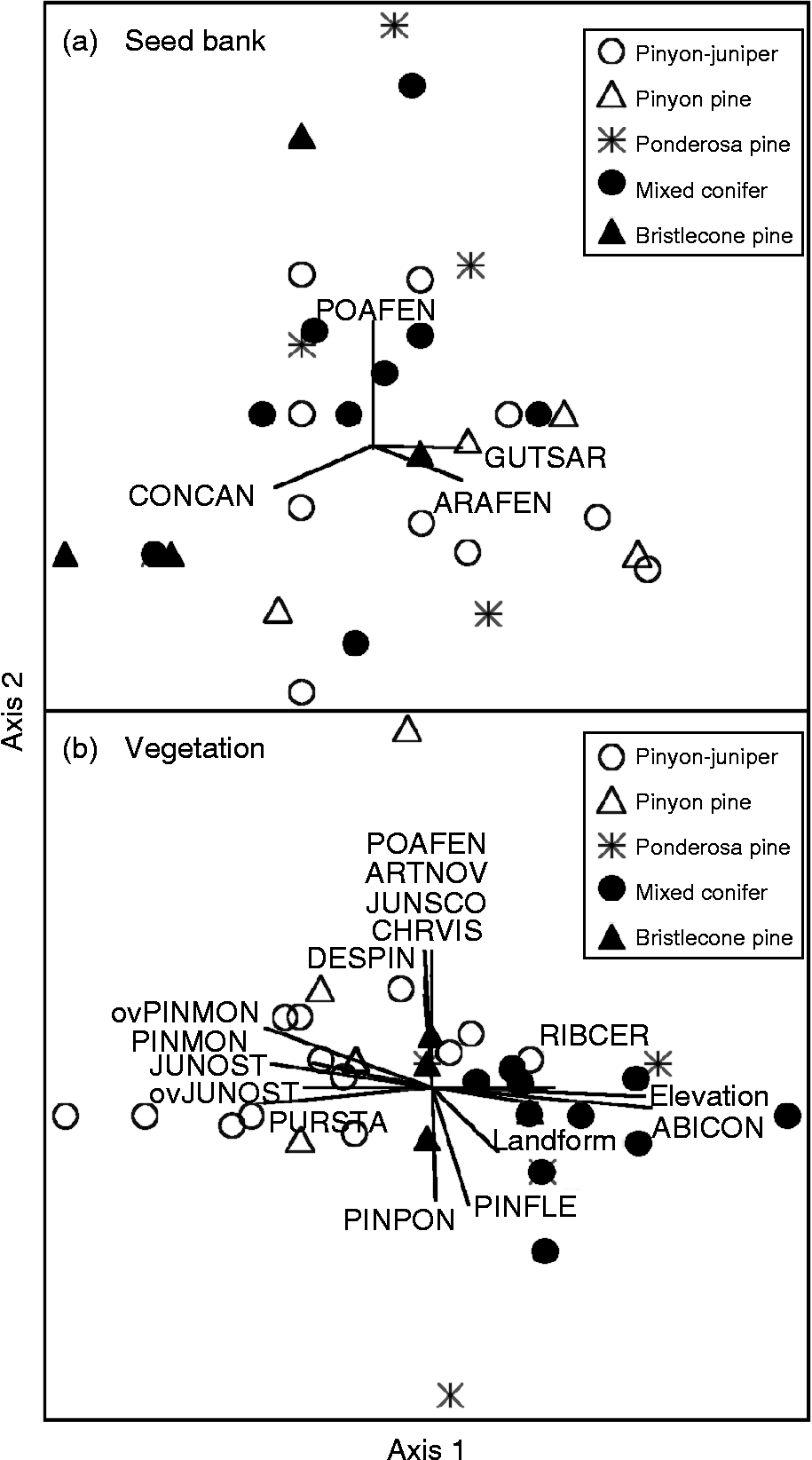
Figure 2 Species composition of (a) the soil seed bank and (b) the vegetation among forest types in coniferous forests of the Spring Mountains, USA. Vectors display species and environmental variables that exhibited r 2 values ≥ 0.25 with compositional patterns, with vector lengths proportional to strengths of relationships. In (a), axis 1 extracted 53% of variability and axis 2, 26%; and in (b), axis 1 extracted 42% and axis 2, 17%. Abbreviations of seed-bank species for (a): ARAFEN, Arabis fendleri; CONCAN, Conyza Canadensis; GUTSAR, Gutierrezia sarothrae; POAFEN, Poa fendleriana. Abbreviations of vegetation species for (b): ABICON, Abies concolor; ARTNOV, Artemisia nova; CHRVIS, Chrysothamnus viscidiflorus; DESPIN, Descurainia pinnata; JUNOST, Juniperus osteosperma (‘ov’ prefix denotes overstorey as opposed to understorey trees); JUNSCO, Juniperus scopulorum; PINFLE, Pinus flexilis; PINMON, Pinus monophylla; PINPON, Pinus ponderosa; POAFEN, Poa fendleriana; PURSTA, Purshia stansburiana; RIBCER, Ribes cereum.
Seed bank:vegetation relationship
A high proportion (62%) of taxa in the seed bank occurred in aboveground vegetation (Table 2). Congruence of seed bank and vegetation occurrence was especially common (71%, 22 of 31 taxa) for native perennial taxa or those capable of perennial life spans (annual–perennial). Native perennial seed-bank taxa also frequent aboveground included Poa fendleriana, Arabis sp., Gutierrezia sarothrae, Cercocarpus ledifolius, Elymus elymoides and Ribes cereum.
Considering the ten most frequent species aboveground, five occurred in the seed bank. These species were Poa fendleriana (the most frequent species in vegetation, inhabiting 78% of sites), Hymenoxys cooperi (44%), Ribes cereum (42%), Elymus elymoides (42%) and Purshia stansburiana (28%). The five other species not detected in the seed bank were conifer trees, including Abies concolor (47%), Pinus monophylla (44%), Pinus longaeva (31%) and Juniperus osteosperma (28%), in addition to the perennial grass Achnatherum hymenoides (28%).
Discussion
Representation of mature forest species
The seed bank was dominated by native perennial species that also occurred in vegetation of these old forests. This finding is uncommon but is consistent with a limited number of other studies of forest seed banks, such as Leckie et al. (Reference Leckie, Vellend, Bell, Waterway and Lechowicz2000) in a temperate deciduous forest of Quebec, Canada, and Brown and Oosterhuis (Reference Brown and Oosterhuis1981) in old coppice woods of eastern England. Potential commonalities in factors related to the high representation of mature forest species among these studies and ours remain unresolved. However, Leckie et al. (Reference Leckie, Vellend, Bell, Waterway and Lechowicz2000) hypothesized that a contributing factor to high representation of mature forest species in their study was that the forest had never been harvested, as opposed to old secondary forests that earlier had been harvested and disturbed by humans. With the exception of selective logging of largely Pinus ponderosa at some (though not all) sites, our study sites can likely also be classified as primary forest. This lack of past acute disturbance (e.g. clear cutting, clearing for agriculture) might partly explain the observed scarcity of ruderal species in seed banks.
There are some possibilities as to why species of mature forests were well represented in the seed bank in our study. For instance, while the forests are much moister than the surrounding arid Mojave Desert, they are still semi-arid. Thus, seed banks might be more important to re-establish populations following droughts than in more temperate forests. Extensive die-offs of perennial species have been recorded in low-elevation ecosystems of the Mojave Desert during recent decades (Hereford et al., Reference Hereford, Webb and Longpre2006), but it is unclear if similar die-offs occurred at the higher elevations and whether drought-related mortality provides sufficient evolutionary pressure to select for seed banks. With tree canopy cover at a maximum of 50% (Table 1), these forests are more open than many temperate forests. This openness might make germination from soil seed banks a viable recruitment strategy in these forests compared to more closed-canopy forests. Some of the major perennial species in the seed bank are intolerant or only moderately tolerant of shade, suggesting that the forest openness was important for their persistence. For example, the three most frequent shrub species – Cercocarpus ledifolius, Ribes cereum and Purshia stansburiana – in soil seed banks are, at most, moderately shade tolerant (FEIS, 2011). The possibility that incomplete canopy closure in these forests facilitated greater use of seed banks by mature forest species warrants additional research.
The sparseness of exotic and ruderal species partly meant that mature forest species proportionally dominated seed banks and could also be related to several factors. The aboveground vegetation at the sites was not dominated by exotic or annual species, which might have limited inputs to the seed bank. Livestock grazing does not occur in the study area and is known to promote short-lived plants in other forests (Bakker et al., Reference Bakker, Rudebusch and Moore2010). Similarly, Allen et al. (Reference Allen, Chambers and Nowak2008) found that burning a Great Basin Desert pinyon–juniper woodland site to the north of our study area reduced soil seed-bank density of the shrub Artemisia tridentata (a species also found in our samples) by fourfold and increased the annual forb Descurainia pinnata fourfold. These results are consistent with the observation that fire frequently increases abundance of annual plants in western coniferous forests (Laughlin and Fulé, Reference Laughlin and Fulé2008). Our study sites are not known to have burned for at least decades, potentially contributing to the low abundance of short-lived species. This mountain range also is isolated, surrounded by desert land and contains extensive roadless area, which might serve to reduce seed dispersal from surrounding lands.
Species of the mature vegetation that were conspicuously absent from the seed bank were the conifer tree species, consistent with small seed banks of conifer trees reported by previous studies. For example, using the extraction method in Idaho mixed conifer forests, Kramer and Johnson (Reference Kramer and Johnson1987) extracted 2038 seeds of seven conifer tree species from samples, but only one seed was viable. Viable seeds of Pinus ponderosa were detected in the spring seed-bank sampling of Washington conifer forests by Pratt et al. (Reference Pratt, Black and Zamora1984) and the study of northern Arizona P. ponderosa forests by Abella et al. (Reference Abella, Springer and Covington2007), but at low densities averaging < 20 seeds m− 2. Many conifer tree species have short-lived seeds with low dormancy, often germinating in the fall of dispersal or the following spring, thus not forming a persistent seed bank (Chambers et al., Reference Chambers, Vander Wall and Schupp1999). Some species of Juniperus, present in the pinyon–juniper forests of our study area, exhibit longer lived seed, but no Juniperus seed was detected in seed banks. It is possible that germination requirements were not met for these species if their seeds were present (Chambers et al., Reference Chambers, Vander Wall and Schupp1999).
Comparison to other western conifer forests
A comparison of our results to a sampling of the previously published literature reveals that the composition of life-history traits in soil seed banks in western North American coniferous forests is highly variable. In mature mixed conifer forests in Idaho, nearly 50% of seed-bank species were perennial forbs, but three genera accounted for 50% of the viable seeds (Ceanothus, Physocarpus and Carex; Kramer and Johnson, Reference Kramer and Johnson1987). Wienk et al. (Reference Wienk, Sieg and McPherson2004) also noted that perennial species dominated soil seed banks in South Dakota P. ponderosa forests. Pratt et al. (Reference Pratt, Black and Zamora1984) observed that 44% of species in a P. ponderosa forest soil seed bank were perennial forbs, but annual forb seeds comprised 45% of the density of seeds and woody species less than 1%. Sixty-three per cent of species in the seed bank were also present in the aboveground vegetation. Koniak and Everett (Reference Koniak and Everett1982) reported that 89% of emerging plants in a California Pinus monophylla woodland were annuals. In contrast, Allen et al. (Reference Allen, Chambers and Nowak2008) reported a majority of perennial species (including many Artemisia tridentata seeds) in pre-fire seed banks in a Nevada P. monophylla woodland. In meadows of the Cascade Mountains, ruderal species were dominant and only approximately 30% of species in the aboveground vegetation were also in the seed bank (Lang and Halpern, Reference Lang and Halpern2007). Korb et al. (Reference Korb, Springer, Powers and Moore2005) also noted large amounts of ruderals in seed banks of three P. ponderosa sites in northern Arizona. Abella et al. (Reference Abella, Springer and Covington2007) reported that 40% of species in soil seed banks of a wide range of P. ponderosa sites were annuals and 33% were perennials, a much lower percentage of perennial species than in the present study. Resolving reasons for the apparent large variability in plant life forms dominating seed banks among different landscapes within this coniferous forest region warrants additional research.
Environment and vegetation
Seed-bank characteristics did not display strong relationships with environmental variables, vegetation cover or forest community types. Soil texture, shown experimentally to be important for seed retention (Chambers, Reference Chambers1995; Benvenuti, Reference Benvenuti2007), exhibited the strongest correlation with seed density of any environmental variable, but the correlation was still weak at ≤ 0.42. Despite sampling sites that spanned an elevation range of 2146–3292 m, elevation was not related to any seed-bank characteristic, contrasting with some previous studies of European soil seed banks (Ortega et al., Reference Ortega, Levassor and Peco1997; Cummins and Miller, Reference Cummins and Miller2002). The Spring Mountains are also topographically diverse (Niles and Leary, Reference Niles and Leary2007), but no topographic measures were correlated with seed banks. Our study did not systematically sample along topographic gradients in the way that Ashton et al. (Reference Ashton, Harris and Thadani1998) did. They found that seed banks differed from valleys to ridge tops in eastern USA deciduous forests. Using a systematic gradient design might be useful to explore potential finer-scale variation in seed banks in our study area. Lack of relationship between seed banks and site-level tree canopy cover contrasts sharply with a previous study 250 km to the south-east of ours, on a P. ponderosa forest landscape, where seed density was threefold greater below open compared to dense canopies (Abella and Springer, Reference Abella and Springer2008). Some previous studies in western USA coniferous forests found that, contrasting with our results, seed banks differed among ecosystem and forest types within landscapes (Kramer and Johnson, Reference Kramer and Johnson1987; Abella et al., Reference Abella, Springer and Covington2007). While vegetation in our study segregated by forest type, which would be expected to result in seed bank differences via seed inputs, the rarity of most species within the seed-bank data made composition of sites mostly individualistic. With 50% of sites within the narrow range of 106–282 seeds m− 2, spatial variability in seed banks also was low. This low variation would temper relationships to environmental and vegetation variables and likely accounts for the low bivariate correlations and the lack of difference in seed banks among forest types.
Considerations for forest management
Soil seed banks are a central consideration for ecosystem management strategies and forecasting potential plant recruitment after disturbance (Bossuyt and Hermy, Reference Bossuyt and Hermy2001). Findings of this study suggest several considerations for forest management to understand plant regeneration potential, disturbance regimes and exotic species management. Land managers have considered fuels and fire potential to be hazardous on our study landscape, and thus are thinning trees to decrease fuel loads (Graham et al., Reference Graham, Jain, Matthews, Elliot, Miller and Audin2010). This also creates opportunities for understorey plant recruitment. Our results suggest that while the seed bank is not large, more than half of the species detected in the seed bank are present in existing vegetation and the seed bank may be important to population dynamics following tree thinning. The seed bank was especially rich in perennial species and contained seeds of such predominant species as Poa fendleriana, Arabis fendleri, Draba californica and Cercocarpus ledifolius, representing a range of grass, forb and shrub growth forms. Examining successional pathways, such as after wildfires and tree thinning, may be useful in future research to evaluate the correspondence of seed banks with actual successional patterns (Wienk et al., Reference Wienk, Sieg and McPherson2004). The general structure of the seed bank (forb- and shrub-dominated with a component of graminoids) mirrored and reinforced the physiognomy of the vegetation. This physiognomy could have important implications for disturbance itself, especially fire, which can behave differently in shrub–forb versus grass fuels (Graham et al., Reference Graham, Jain, Matthews, Elliot, Miller and Audin2010). An additional important finding of this study was that exotic species were sparse in the seed bank. Only four exotic species were detected and all occurred at ≤ 11% of sites. Sample sites were not located along roadsides, on wildfires or on other heavily disturbed sites, which might have potential to contain greater exotic seed-bank densities. Much of the landscape, however, is currently in a relatively undisturbed state, represented by our sample sites where exotic species in the vegetation were also sparse. Results suggest that soil seed banks were dominated by native perennial species characteristic of mature forests.
Acknowledgements
We thank Wally Covington and the Ecological Restoration Institute (ERI) at Northern Arizona University (NAU) for funding this study; Jim Hurja (Humboldt-Toiyabe National Forest) for providing access to TEUI sites and vegetation data; Brad Blake and Phil Patterson and the NAU research greenhouse for providing space to assay seed bank samples; Mark Daniels (ERI) for helping with seedling inventories; Sharon Altman (University of Nevada Las Vegas) for formatting figures; and the Associate Editor, two anonymous reviewers and Sharon Altman for reviewing the manuscript.